Clinical Prognosis of Lung Cancer in Patients with Moderate Chronic Kidney Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Diagnosis of Lung Cancer
2.3. Definition of Moderate Chronic Kidney Disease
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients with Lung Cancer in CKD
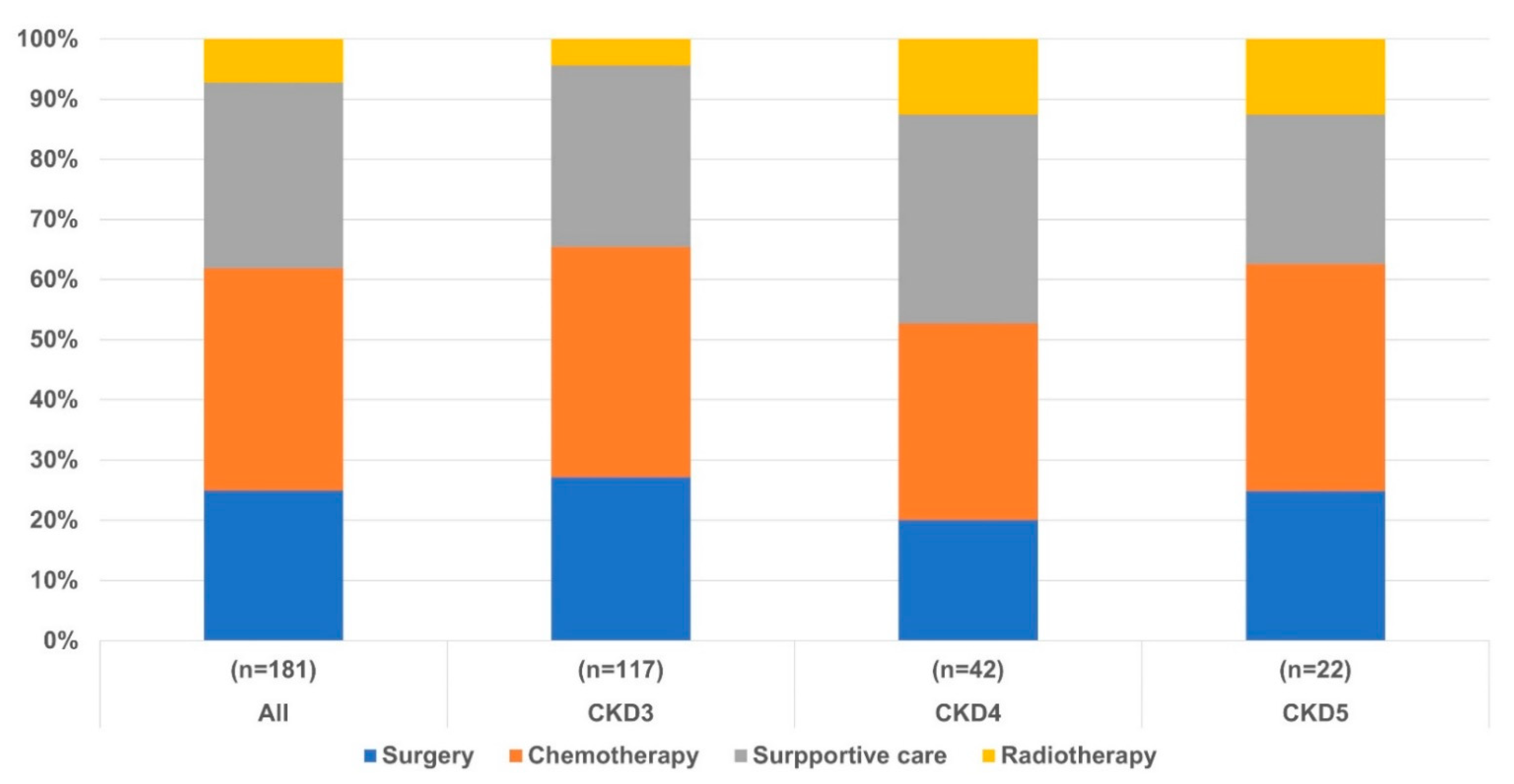
3.2. Clinical Outcomes in Patients with Lung Cancer According to CKD Stage
3.3. Clinical Outcomes of Patients with Lung Cancer in CKD
3.4. Prognostic Factors of Patients with Lung Cancer in CKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. Ca Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. Impact of comorbidity on lung cancer survival. Int. J. Cancer 2003, 103, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Yancik, R. Cancer burden in the aged: An epidemiologic and demographic overview. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 1273–1283. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, R.D. Changes in renal function with aging. Drugs Aging 1992, 2, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.-H.; Hung, K.-Y.; Huang, H.-L.; Chen, J.-H.; Sung, P.-K.; Huang, K.-C. Cancer-specific mortality in chronic kidney disease: Longitudinal follow-up of a large cohort. Clin. J. Am. Soc. Nephrol. 2011, 6, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Sung, J.Y.; Chang, J.H.; Kim, S.; Lee, H.H.; Park, Y.H.; Chung, W.; Oh, K.-H.; Jung, J.Y. Chronic kidney disease in cancer patients: An independent predictor of cancer-specific mortality. Am. J. Nephrol. 2011, 33, 121–130. [Google Scholar] [CrossRef]
- Patel, P.; Henry, L.L.; Ganti, A.K.; Potti, A. Clinical course of lung cancer in patients with chronic kidney disease. Lung Cancer 2004, 43, 297–300. [Google Scholar] [CrossRef]
- Lu, M.S.; Chen, M.F.; Lin, C.C.; Tseng, Y.H.; Huang, Y.K.; Liu, H.P.; Tsai, Y.H. Is chronic kidney disease an adverse factor in lung cancer clinical outcome? A propensity score matching study. Thorac. Cancer 2017, 8, 106–113. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Chen, J.-Y.; Lee, H.-S.; Wu, J.-T.; Hsu, C.-K.; Hsu, Y.-C. Association of chronic kidney disease with mortality risk in patients with lung cancer: A nationwide Taiwan population-based cohort study. BMJ Open 2018, 8, e019661. [Google Scholar] [CrossRef]
- Detterbeck, F.C. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J. Thorac. Cardiovasc. Surg. 2018, 155, 356–359. [Google Scholar] [CrossRef]
- Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin. Exp. Nephrol. 2019, 23, 1. [Google Scholar] [CrossRef]
- Nishino, M.; Jackman, D.M.; Hatabu, H.; Yeap, B.Y.; Cioffredi, L.A.; Yap, J.T.; Van den Abbeele, A.D. New response evaluation criteria in solid tumors: Revised RECIST guideline (version1. 1). AJR Am. J. Roentgenol. 2010, 195, W221–W228. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Goldstraw, P. The IASLC lung cancer staging project: The new database to inform the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2014, 9, 1618–1624. [Google Scholar] [CrossRef]
- Chansky, K.; Detterbeck, F.C.; Nicholson, A.G.; Rusch, V.W.; Vallières, E.; Groome, P.; Kennedy, C.; Krasnik, M.; Peake, M.; Shemanski, L. The IASLC lung cancer staging project: External validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2017, 12, 1109–1121. [Google Scholar] [CrossRef]
- Wahbah, M.; Boroumand, N.; Castro, C.; El-Zeky, F.; Eltorky, M. Changing trends in the distribution of the histologic types of lung cancer: A review of 4439 cases. Ann. Diagn. Pathol. 2007, 11, 89–96. [Google Scholar] [CrossRef]
- Takahama, M.; Yamamoto, R.; Nakajima, R.; Tsukioka, T.; Tada, H. Pulmonary resection for lung cancer patients on chronic hemodialysis: Clinical outcome and long-term results after operation. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 150–153. [Google Scholar] [CrossRef][Green Version]
- Hashim, D.; Carioli, G.; Malvezzi, M.; Bertuccio, P.; Waxman, S.; Negri, E.; La Vecchia, C.; Boffetta, P. Cancer mortality in the oldest old: A global overview. Aging 2020, 12, 16744. [Google Scholar] [CrossRef]
- Marcus, M.W.; Chen, Y.; Duffy, S.W.; Field, J.K. Impact of comorbidity on lung cancer mortality-a report from the Liverpool Lung Project. Oncol. Lett. 2015, 9, 1902–1906. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Cipriano, L.E.; Romanus, D.; Earle, C.C.; Neville, B.A.; Halpern, E.F.; Gazelle, G.S.; McMahon, P.M. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health 2011, 14, 41–52. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.-K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.-M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.-C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015, 5, 2892. [Google Scholar] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Obuchi, T.; Hamanaka, W.; Yoshida, Y.; Yanagisawa, J.; Hamatake, D.; Shiraishi, T.; Iwasaki, A. Clinical outcome after pulmonary resection for lung cancer patients on hemodialysis. Ann. Thorac. Surg. 2009, 88, 1745–1748. [Google Scholar] [CrossRef]



| Characteristics | All Patients (n = 181) | CKD 3 (n = 117) | CKD 4 (n = 42) | CKD 5 (n = 22) | p-Value |
|---|---|---|---|---|---|
| Age, year | 75 (70–81) | 75 (71–81) | 79 (71–82) | 73 (64–78) | 0.040 |
| Male | 157 (87%) | 104 (89%) | 35 (83%) | 18 (82%) | 0.507 |
| Body mass index, kg/m2 | 23.0 (21.4–25.1) | 23.4 (22.0–25.7) | 22.0 (19.8–24.9) | 22.0 (20.0–24.0) | 0.007 |
| BUN, mg/dL | 24.6 (19.1–31.8) | 22.0 (17.5–25.6) | 32.0 (28.0–45.4) | 44.6 (25.9–54.1) | <0.001 |
| Creatine, mg/dL | 1.68 (01.45–2.29) | 1.5 (1.4–1.68) | 2.4 (1.8–3.2) | 6.3 (5.0–8.3) | <0.001 |
| Creatine clearance, mL/minute/1.73 m2 | 35.9 (23.8–45.0) | 40.9 (36–48) | 23.0 (17.3–26.4) | 8.9 (6.8–8.3) | <0.001 |
| Charlson comorbidities index | 10.0 (8.0–11.0) | 10.0 (8.0–11.0) | 10.0 (7.8–11.0) | 10.0 (6.8–11.3) | 0.920 |
| Comorbidity | |||||
| Hypertension | 123 (68%) | 82 (70%) | 29 (69%) | 12 (55%) | 0.353 |
| Diabetes | 85 (47%) | 50 (43%) | 22 (52%) | 13 (59%) | 0.268 |
| Chronic airway disease | 50 (28%) | 33 (28%) | 13 (31%) | 4 (18%) | 0.540 |
| Heart disease | 33 (18%) | 17 (15%) | 9 (21%) | 7(32%) | 0.130 |
| Other cancer | 33 (18%) | 22 (19%) | 8 (19%) | 3 (14%) | 0.837 |
| Old cerebral disease | 22 (12%) | 12 (12%) | 5 (12%) | 3 (14%) | 0.975 |
| Pathologic type | |||||
| NSCLC | 150 (83%) | 99 (85%) | 33 (79%) | 18 (82%) | 0.665 |
| Adenocarcinoma | 61 (41%) | 38 (38%) | 12 (36%) | 11 (61%) | 0.203 |
| Squamous cell carcinoma | 68 (45%) | 50 (51%) | 13 (39%) | 5 (28%) | 0.124 |
| Large cell carcinoma | 8 (5%) | 5 (5.1%) | 3 (10%) | 0 | 0.415 |
| Sarcoma | 2 (1%) | 1 (1.0%) | 1 (3.0%) | 0 | 0.625 |
| Undifferentiated NSCLC | 11 (7%) | 5 (5.1%) | 4 (12%) | 2 (11%) | 0.388 |
| SCLC | 31 (17%) | 18 (15%) | 9 (21%) | 4 (18%) | 0.665 |
| EGFR (n = 107) | 18 (17%) | 10 (9%) | 4 (4%) | 4 (4%) | 0.387 |
| ALK (n = 54) | 1 (2%) | 0 | 0 | 1 (1.9%) | <0.001 |
| PD-1 (n = 49) | 26 (53%) | 20(41%) | 3 (6%) | 3 (6%) | 0.017 |
| NSCLC stage | |||||
| Stage I | 35 (23%) | 21 (21%) | 7 (21%) | 7 (39%) | 0.251 |
| Stage II | 16 (11%) | 10 (10%) | 6 (18%) | 0 | 0.126 |
| Stage III | 34 (23%) | 23 (23%) | 6 (18%) | 5 (28%) | 0.717 |
| Stage IV | 65 (43%) | 45 (46%) | 14 (42%) | 6 (33%) | 0.630 |
| SCLC stage | |||||
| Limited stage | 8 (26%) | 4 (22%) | 1 (11%) | 3 (75%) | 0.045 |
| Extensive stage | 23 (74%) | 14 (78%) | 8 (89%) | 1 (25%) | 0.045 |
| Complete blood cell count | |||||
| Platelet, 10³/μL | 249 (194–302) | 260 (207–310) | 230 (176–287) | 187 (146–260) | 0.001 |
| AMC, /μL | 500 (400–649) | 500 (400–640) | 523(395–677) | 463 (358–660) | 0.595 |
| PLR | 162 (117–243) | 171 (118–246) | 176 (121–271) | 117 (90–193) | 0.086 |
| NLR | 3.37 (2.45–5.10) | 3.37 (2.46–4.76) | 3.71 (2.29–6.37) | 3.19 (2.65–5.31) | 0.677 |
| All Patients | CKD 3 | CKD 4 | CKD 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 181) | (n = 117) | (n = 42) | (n = 22) | p-Value | ||||||
| PFS (months) | 4.9 (2.7–106) | 4.7 (2.9–7.8) | 4.9 (0.8–10.6) | 7.2 (2.7–18.2) | 0.456 | |||||
| Death | 151 (83%) | 93 (80%) | 38 (90%) | 20 (90%) | 0.156 | |||||
| OS (months) | 5year survival | 10.2 (3.3–28.3) | 17 (9%) | 11.1 (4.2–31.3) | 12 (10%) | 6.0 (1.8–16.3) | 2 (5%) | 4.7 (2.1–40.1) | 3 (14%) | |
| Age ≥ 75 years | 6.1 (2.1–18.5) | 3 (3%) | 6.5 (2.4–18.6) | 2 (3.7%) | 4.1 (1.8–14.2) | 0 | 4.5 (2.1–57.4) | 1 (14%) | 0.694 | |
| < 75 years | 13.8 (4.7–40.8) | 14 (17%) | 22.6 (8.5–50.0) | 12 (19%) | 10.0 (1.6–22.8) | 2 (13%) | 4.8 (1.8–39.7) | 2 (13%) | 0.044 | |
| Pathologic type | ||||||||||
| NSCLC | 11.1 (4.0–35.0) | 17 (11%) | 15.6 (5.7–39.5) | 12 (12%) | 9.5 (3.6–25.6) | 2 (6%) | 4.7 (2.7–45.2) | 3 (16%) | 0.122 | |
| ADC | 21.7 (8.1–50.8) | 13 (21%) | 17.7 (9.8–53.5) | 8 (21%) | 31.4 (8.6–57.9) | 2 (17%) | 11.5 (4.5–60.9) | 3 (27%) | 0.686 | |
| Sqcc | 8.8 (3.3–26.2) | 4 (6%) | 12.6 (4.2–28.8) | 4 (8%) | 4.0 (1.6–9.5) | 0 | 3.7 (0.2–22.7) | 0 | 0.001 | |
| SCLC | 3.4 (1.0–8.4) | 0 | 4.4 (1.1–8.5) | 0 | 1.7 (0.9–3.6) | 0 | 3.0 (1.5–33.1) | 0 | 0.194 | |
| NSCLC stage | ||||||||||
| Stage I | 51.9 (20.0–71.5) | 12 (34%) | 53.5 (29.2–98.5) | 9 (43%) | 34.5 (10.4–58.9) | 1 (14%) | 41.2 (5.3–62.2) | 2 (29%) | 0.186 | |
| Stage II | 14.5 (5.0–40.0) | 0 | 22.3 (6.6–46.3) | 0 | 10.0 (3.3–29.4) | 0 | - | - | 0.151 | |
| Stage III | 11.1 (4.4–31.3) | 3 (9%) | 20.6 (9.1–50.0) | 3 (13%) | 6.1 (1.8–10.6) | 0 | 3.7 (0.7–18.9) | 0 | 0.002 | |
| Stage IV | 6.6 (30.1–14.4) | 2 (3%) | 8.2 (3.3–15.1) | 0 | 5.9 (2.1–16.7) | 1 (7%) | 3.8 (1.5–18.8) | 1 (17%) | 0.875 | |
| SCLC stage | ||||||||||
| Limited stage | 4.1 (1.5–21.4) | 0 | 4.1 (1.2–5.5) | 0 | 3.23 | 0 | 25.6 (0.9–39.7) | 0 | 0.295 | |
| Extensive stage | 2.1 (0.9–8.2) | 0 | 4.4 (1.1–9.3) | 0 | 1.4 (0.9–3.5) | 0 | 2.13 | 0 | 0.421 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Kim, S.H.; Choi, H.; Shin, T.R.; Kim, H.I.; Jang, S.H.; Hong, J.Y.; Lee, M.G.; Chung, S.; Hyun, I.G.; et al. Clinical Prognosis of Lung Cancer in Patients with Moderate Chronic Kidney Disease. Cancers 2022, 14, 4786. https://doi.org/10.3390/cancers14194786
Kim T, Kim SH, Choi H, Shin TR, Kim HI, Jang SH, Hong JY, Lee MG, Chung S, Hyun IG, et al. Clinical Prognosis of Lung Cancer in Patients with Moderate Chronic Kidney Disease. Cancers. 2022; 14(19):4786. https://doi.org/10.3390/cancers14194786
Chicago/Turabian StyleKim, Taehee, Sang Hyuk Kim, Hayoung Choi, Tae Rim Shin, Hwan Il Kim, Seung Hun Jang, Ji Young Hong, Myung Goo Lee, Soojie Chung, In Gyu Hyun, and et al. 2022. "Clinical Prognosis of Lung Cancer in Patients with Moderate Chronic Kidney Disease" Cancers 14, no. 19: 4786. https://doi.org/10.3390/cancers14194786
APA StyleKim, T., Kim, S. H., Choi, H., Shin, T. R., Kim, H. I., Jang, S. H., Hong, J. Y., Lee, M. G., Chung, S., Hyun, I. G., & Sim, Y. S. (2022). Clinical Prognosis of Lung Cancer in Patients with Moderate Chronic Kidney Disease. Cancers, 14(19), 4786. https://doi.org/10.3390/cancers14194786





