FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact
Abstract
Simple Summary
Abstract
1. Introduction
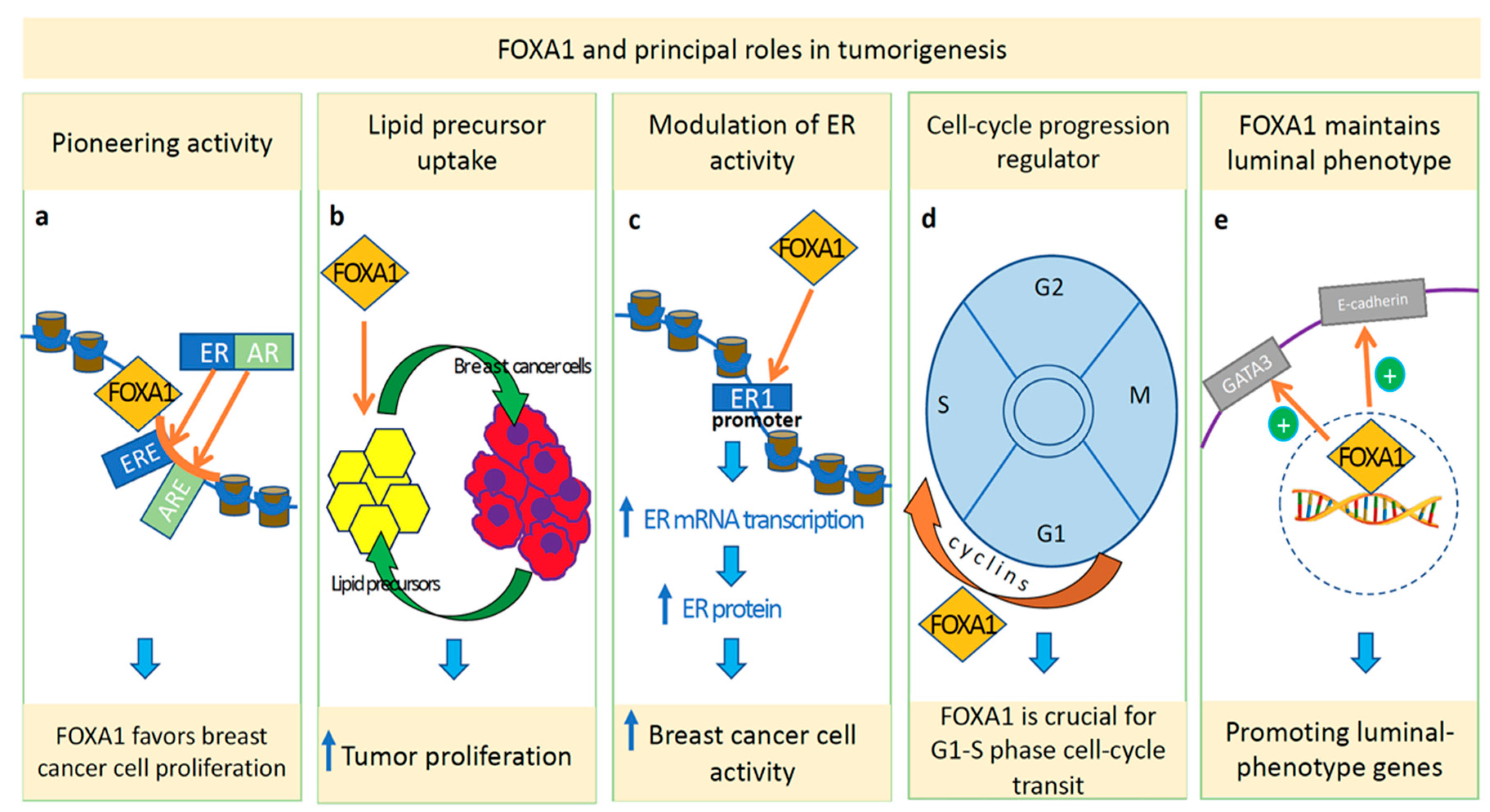
2. FOXA1: Principal Roles in Normal and Tumor Tissues
3. Evaluation of FOXA1 Expression: Immunohistochemistry and Gene Expression Analysis
4. Prognostic and Predictive Roles of FOXA1
4.1. Luminal Breast Cancer
4.2. Non-Luminal Breast Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of Breast Cancer, a Paradigm of the “Common Soil” Hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Y.; Liu, Y.; Kao, L.-P.; Wang, X.; Skerry, B.; Li, Z. FOXA1 Defines Cancer Cell Specificity. Sci. Adv. 2016, 2, e1501473. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Z.; Xu, T.; Zhang, J.-Y.; Zhu, Y.; Chen, W.-X.; Zhong, S.-L.; Zhao, J.-H.; Tang, J.-H. FOXA1: A Promising Prognostic Marker in Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rangel, N.; Fortunati, N.; Osella-Abate, S.; Annaratone, L.; Isella, C.; Catalano, M.G.; Rinella, L.; Metovic, J.; Boldorini, R.; Balmativola, D.; et al. FOXA1 and AR in Invasive Breast Cancer: New Findings on Their Co-Expression and Impact on Prognosis in ER-Positive Patients. BMC Cancer 2018, 18, 703. [Google Scholar] [CrossRef]
- Naderi, A.; Meyer, M.; Dowhan, D.H. Cross-Regulation between FOXA1 and ErbB2 Signaling in Estrogen Receptor-Negative Breast Cancer. Neoplasia 2012, 14, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Wang, H.-S.; Liu, N.; Ge, L.-C. Bisphenol A Stimulates the Epithelial Mesenchymal Transition of Estrogen Negative Breast Cancer Cells via FOXA1 Signals. Arch. Biochem. Biophys. 2015, 585, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, A.; Paredes, J.; Sousa, B.; Milanezi, F.; Carneiro, V.; Bastos, J.; Costa, S.; Vieira, D.; Lopes, N.; Lam, E.W.; et al. Expression of FOXA1 and GATA-3 in Breast Cancer: The Prognostic Significance in Hormone Receptor-Negative Tumours. Breast Cancer Res. 2009, 11, R40. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.L.; Macarthur, S.; Ross-Innes, C.S.; Tilley, W.D.; Neal, D.E.; Mills, I.G.; Carroll, J.S. Androgen Receptor Driven Transcription in Molecular Apocrine Breast Cancer Is Mediated by FoxA1. EMBO J. 2011, 30, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.H.; Grayson, D.R.; Darnell, J.E. Multiple Hepatocyte-Enriched Nuclear Factors Function in the Regulation of Transthyretin and Alpha 1-Antitrypsin Genes. Mol. Cell. Biol. 1989, 9, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Gredler, M.L.; Patterson, S.E.; Seifert, A.W.; Cohn, M.J. Foxa1 and Foxa2 Orchestrate Development of the Urethral Tube and Division of the Embryonic Cloaca through an Autoregulatory Loop with Shh. Dev. Biol. 2020, 465, 23–30. [Google Scholar] [CrossRef]
- Bach, D.-H.; Long, N.P.; Luu, T.-T.-T.; Anh, N.H.; Kwon, S.W.; Lee, S.K. The Dominant Role of Forkhead Box Proteins in Cancer. Int. J. Mol. Sci. 2018, 19, E3279. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Xiu, Y.-L.; Chen, X.; Sun, K.-X.; Chen, S.; Wu, D.-D.; Liu, B.-L.; Zhao, Y. The Transcription Factor FOXA1 Induces Epithelial Ovarian Cancer Tumorigenesis and Progression. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017, 39, 1010428317706210. [Google Scholar] [CrossRef] [PubMed]
- Mirosevich, J.; Gao, N.; Gupta, A.; Shappell, S.B.; Jove, R.; Matusik, R.J. Expression and Role of Foxa Proteins in Prostate Cancer. Prostate 2006, 66, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Augello, M.A.; Hickey, T.E.; Knudsen, K.E. FOXA1: Master of Steroid Receptor Function in Cancer. EMBO J. 2011, 30, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; Bose, S.; Williamson, E.A.; Miller, C.W.; Karlan, B.Y.; Koeffler, H.P. FOXA1: Growth Inhibitor and a Favorable Prognostic Factor in Human Breast Cancer. Int. J. Cancer 2007, 120, 1013–1022. [Google Scholar] [CrossRef]
- He, S.; Zhang, J.; Zhang, W.; Chen, F.; Luo, R. FOXA1 Inhibits Hepatocellular Carcinoma Progression by Suppressing PIK3R1 Expression in Male Patients. J. Exp. Clin. Cancer Res. CR 2017, 36, 175. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Pereira, R.; De Angelis, C.; Veeraraghavan, J.; Nanda, S.; Qin, L.; Cataldo, M.L.; Sethunath, V.; Mehravaran, S.; Gutierrez, C.; et al. FOXA1 Upregulation Promotes Enhancer and Transcriptional Reprogramming in Endocrine-Resistant Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 26823–26834. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.L.; Carroll, J.S. FoxA1 Is a Key Mediator of Hormonal Response in Breast and Prostate Cancer. Front. Endocrinol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Jozwik, K.M.; Carroll, J.S. Pioneer Factors in Hormone-Dependent Cancers. Nat. Rev. Cancer 2012, 12, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.A.; Zhao, J.C.; Fong, K.-W.; Kim, J.; Li, S.; Song, C.; Song, B.; Zheng, B.; He, C.; Yu, J. FOXA1 Potentiates Lineage-Specific Enhancer Activation through Modulating TET1 Expression and Function. Nucleic Acids Res. 2016, 44, 8153–8164. [Google Scholar] [CrossRef]
- Bernardo, G.M.; Keri, R.A. FOXA1: A Transcription Factor with Parallel Functions in Development and Cancer. Biosci. Rep. 2012, 32, 113–130. [Google Scholar] [CrossRef]
- Carroll, J.S.; Brown, M. Estrogen Receptor Target Gene: An Evolving Concept. Mol. Endocrinol. 2006, 20, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S.; Carroll, J.S. Pioneer Transcription Factors: Establishing Competence for Gene Expression. Genes Dev. 2011, 25, 2227–2241. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.M.; Lozada, K.L.; Miedler, J.D.; Harburg, G.; Hewitt, S.C.; Mosley, J.D.; Godwin, A.K.; Korach, K.S.; Visvader, J.E.; Kaestner, K.H.; et al. FOXA1 Is an Essential Determinant of ERalpha Expression and Mammary Ductal Morphogenesis. Development 2010, 137, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.M.; Bebek, G.; Ginther, C.L.; Sizemore, S.T.; Lozada, K.L.; Miedler, J.D.; Anderson, L.A.; Godwin, A.K.; Abdul-Karim, F.W.; Slamon, D.J.; et al. FOXA1 Represses the Molecular Phenotype of Basal Breast Cancer Cells. Oncogene 2013, 32, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Takaku, M.; Grimm, S.A.; De Kumar, B.; Bennett, B.D.; Wade, P.A. Cancer-Specific Mutation of GATA3 Disrupts the Transcriptional Regulatory Network Governed by Estrogen Receptor Alpha, FOXA1 and GATA3. Nucleic Acids Res. 2020, 48, 4756–4768. [Google Scholar] [CrossRef] [PubMed]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 Maintains the Differentiation of the Luminal Cell Fate in the Mammary Gland. Cell 2006, 127, 1041–1055. [Google Scholar] [CrossRef]
- Ghosh, S.; Gu, F.; Wang, C.-M.; Lin, C.-L.; Liu, J.; Wang, H.; Ravdin, P.; Hu, Y.; Huang, T.H.M.; Li, R. Genome-Wide DNA Methylation Profiling Reveals Parity-Associated Hypermethylation of FOXA1. Breast Cancer Res. Treat. 2014, 147, 653–659. [Google Scholar] [CrossRef]
- Slebe, F.; Rojo, F.; Vinaixa, M.; García-Rocha, M.; Testoni, G.; Guiu, M.; Planet, E.; Samino, S.; Arenas, E.J.; Beltran, A.; et al. FoxA and LIPG Endothelial Lipase Control the Uptake of Extracellular Lipids for Breast Cancer Growth. Nat. Commun. 2016, 7, 11199. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Holmes, K.A.; Ross-Innes, C.S.; Schmidt, D.; Carroll, J.S. FOXA1 Is a Key Determinant of Estrogen Receptor Function and Endocrine Response. Nat. Genet. 2011, 43, 27–33. [Google Scholar] [CrossRef]
- Castaneda, M.; Hollander, P.D.; Mani, S.A. Forkhead Box Transcription Factors: Double-Edged Swords in Cancer. Cancer Res. 2022, 82, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Eeckhoute, J.; Carroll, J.S.; Geistlinger, T.R.; Torres-Arzayus, M.I.; Brown, M. A Cell-Type-Specific Transcriptional Network Required for Estrogen Regulation of Cyclin D1 and Cell Cycle Progression in Breast Cancer. Genes Dev. 2006, 20, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Wu, D.; Chen, H.; Chen, Z.; Thomas-Ahner, J.M.; Zynger, D.L.; Eeckhoute, J.; Yu, J.; Luo, J.; et al. Definition of a FoxA1 Cistrome That Is Crucial for G1 to S-Phase Cell-Cycle Transit in Castration-Resistant Prostate Cancer. Cancer Res. 2011, 71, 6738–6748. [Google Scholar] [CrossRef] [PubMed]
- Anzai, E.; Hirata, K.; Shibazaki, M.; Yamada, C.; Morii, M.; Honda, T.; Yamaguchi, N.; Yamaguchi, N. FOXA1 Induces E-Cadherin Expression at the Protein Level via Suppression of Slug in Epithelial Breast Cancer Cells. Biol. Pharm. Bull. 2017, 40, 1483–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambrosone, C.B.; Higgins, M.J. Relationships between Breast Feeding and Breast Cancer Subtypes: Lessons Learned from Studies in Humans and in Mice. Cancer Res. 2020, 80, 4871–4877. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Huang, W.; Zhao, X.; Huang, X.; Chen, Y.; Yu, L.; Tan, Y. Increased FOXA1 Levels Induce Apoptosis and Inhibit Proliferation in FOXA1-Low Expressing Basal Breast Cancer Cells. Am. J. Cancer Res. 2022, 12, 2641–2658. [Google Scholar]
- Sribenja, S.; Maguire, O.; Attwood, K.; Buas, M.F.; Palmer, J.R.; Ambrosone, C.B.; Higgins, M.J. Deletion of Foxa1 in the Mouse Mammary Gland Results in Abnormal Accumulation of Luminal Progenitor Cells: A Link between Reproductive Factors and ER-/TNBC Breast Cancer? Am. J. Cancer Res. 2021, 11, 3263–3270. [Google Scholar]
- Badve, S.; Turbin, D.; Thorat, M.A.; Morimiya, A.; Nielsen, T.O.; Perou, C.M.; Dunn, S.; Huntsman, D.G.; Nakshatri, H. FOXA1 Expression in Breast Cancer—Correlation with Luminal Subtype A and Survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4415–4421. [Google Scholar] [CrossRef]
- Niemeier, L.A.; Dabbs, D.J.; Beriwal, S.; Striebel, J.M.; Bhargava, R. Androgen Receptor in Breast Cancer: Expression in Estrogen Receptor-Positive Tumors and in Estrogen Receptor-Negative Tumors with Apocrine Differentiation. Mod. Pathol. 2010, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Guiu, S.; Charon-Barra, C.; Vernerey, D.; Fumoleau, P.; Campone, M.; Spielmann, M.; Roché, H.; Mesleard, C.; Arnould, L.; Lemonnier, J.; et al. Coexpression of Androgen Receptor and FOXA1 in Nonmetastatic Triple-Negative Breast Cancer: Ancillary Study from PACS08 Trial. Future Oncol. 2015, 11, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.A.; Wolf, I.; O’Kelly, J.; Bose, S.; Tanosaki, S.; Koeffler, H.P. BRCA1 and FOXA1 Proteins Coregulate the Expression of the Cell Cycle-Dependent Kinase Inhibitor P27(Kip1). Oncogene 2006, 25, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Washington, M.K.; Crawford, H.C. Loss of FOXA1/2 Is Essential for the Epithelial-to-Mesenchymal Transition in Pancreatic Cancer. Cancer Res. 2010, 70, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Hughes-Davies, L. A Functionally Significant Cross-Talk between Androgen Receptor and ErbB2 Pathways in Estrogen Receptor Negative Breast Cancer. Neoplasia 2008, 10, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.G.B.; Madden, S.F.; Brennan, K.; Hopkins, A.M. A Transcriptional Link between HER2, JAM-A and FOXA1 in Breast Cancer. Cells 2022, 11, 735. [Google Scholar] [CrossRef]
- Angus, S.P.; Stuhlmiller, T.J.; Mehta, G.; Bevill, S.M.; Goulet, D.R.; Olivares-Quintero, J.F.; East, M.P.; Tanioka, M.; Zawistowski, J.S.; Singh, D.; et al. FOXA1 and Adaptive Response Determinants to HER2 Targeted Therapy in TBCRC 036. NPJ Breast Cancer 2021, 7, 51. [Google Scholar] [CrossRef]
- Chen, Q.-X.; Yang, Y.-Z.; Liang, Z.-Z.; Chen, J.-L.; Li, Y.-L.; Huang, Z.-Y.; Weng, Z.-J.; Zhang, X.-F.; Guan, J.-X.; Tang, L.-Y.; et al. Time-Varying Effects of FOXA1 on Breast Cancer Prognosis. Breast Cancer Res. Treat. 2021, 187, 867–875. [Google Scholar] [CrossRef]
- Horimoto, Y.; Sasahara, N.; Sasaki, R.; Hlaing, M.T.; Sakaguchi, A.; Saeki, H.; Arakawa, A.; Himuro, T.; Saito, M. High FOXA1 Protein Expression Might Predict Late Recurrence in Patients with Estrogen-Positive and HER2-Negative Breast Cancer. Breast Cancer Res. Treat. 2020, 183, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Ma, J.; Shi, C.-T.; Han, W.; Gao, X.-J.; Zhou, M.-H.; Ding, H.-Z.; Wang, H.-N. Roles and Correlation of FOXA1 and ZIC1 in Breast Cancer. Curr. Probl. Cancer 2020, 44, 100559. [Google Scholar] [CrossRef]
- Byun, J.S.; Singhal, S.K.; Park, S.; Yi, D.I.; Yan, T.; Caban, A.; Jones, A.; Mukhopadhyay, P.; Gil, S.M.; Hewitt, S.M.; et al. Racial Differences in the Association Between Luminal Master Regulator Gene Expression Levels and Breast Cancer Survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1905–1914. [Google Scholar] [CrossRef]
- Cheng, T.-Y.D.; Yao, S.; Omilian, A.R.; Khoury, T.; Buas, M.F.; Payne-Ondracek, R.; Sribenja, S.; Bshara, W.; Hong, C.-C.; Bandera, E.V.; et al. FOXA1 Protein Expression in ER+ and ER- Breast Cancer in Relation to Parity and Breastfeeding in Black and White Women. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2020, 29, 379–385. [Google Scholar] [CrossRef]
- Nelson, L.J.; Wright, H.J.; Dinh, N.B.; Nguyen, K.D.; Razorenova, O.V.; Heinemann, F.S. Src Kinase Is Biphosphorylated at Y416/Y527 and Activates the CUB-Domain Containing Protein 1/Protein Kinase C δ Pathway in a Subset of Triple-Negative Breast Cancers. Am. J. Pathol. 2020, 190, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Saponaro, C.; Vagheggini, A.; Opinto, G.; Centonze, M.; Vicenti, C.; Popescu, O.; Pastena, M.; Giotta, F.; Silvestris, N. Should Tumor Infiltrating Lymphocytes, Androgen Receptor, and FOXA1 Expression Predict the Clinical Outcome in Triple Negative Breast Cancer Patients? Cancers 2019, 11, E1393. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Chen, X.; Li, T.; Zhang, J.; Jin, G.; Cai, D.; Huang, Z. FOXA1 Is Prognostic of Triple Negative Breast Cancers by Transcriptionally Suppressing SOD2 and IL6. Int. J. Biol. Sci. 2019, 15, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- De Lara, S.; Nyqvist, J.; Werner Rönnerman, E.; Helou, K.; Kenne Sarenmalm, E.; Einbeigi, Z.; Karlsson, P.; Parris, T.Z.; Kovács, A. The Prognostic Relevance of FOXA1 and Nestin Expression in Breast Cancer Metastases: A Retrospective Study of 164 Cases during a 10-Year Period (2004–2014). BMC Cancer 2019, 19, 187. [Google Scholar] [CrossRef]
- Kutasovic, J.R.; McCart Reed, A.E.; Males, R.; Sim, S.; Saunus, J.M.; Dalley, A.; McEvoy, C.R.; Dedina, L.; Miller, G.; Peyton, S.; et al. Breast Cancer Metastasis to Gynaecological Organs: A Clinico-Pathological and Molecular Profiling Study. J. Pathol. Clin. Res. 2019, 5, 25–39. [Google Scholar] [CrossRef]
- Schrijver, W.; Schuurman, K.; van Rossum, A.; Droog, M.; Jeronimo, C.; Salta, S.; Henrique, R.; Wesseling, J.; Moelans, C.; Linn, S.C.; et al. FOXA1 Levels Are Decreased in Pleural Breast Cancer Metastases after Adjuvant Endocrine Therapy, and This Is Associated with Poor Outcome. Mol. Oncol. 2018, 12, 1884–1894. [Google Scholar] [CrossRef]
- Guiu, S.; Mollevi, C.; Charon-Barra, C.; Boissière, F.; Crapez, E.; Chartron, E.; Lamy, P.-J.; Gutowski, M.; Bourgier, C.; Romieu, G.; et al. Prognostic Value of Androgen Receptor and FOXA1 Co-Expression in Non-Metastatic Triple Negative Breast Cancer and Correlation with Other Biomarkers. Br. J. Cancer 2018, 119, 76–79. [Google Scholar] [CrossRef]
- Mai, R.; Zhou, S.; Zhou, S.; Zhong, W.; Hong, L.; Wang, Y.; Lu, S.; Pan, J.; Huang, Y.; Su, M.; et al. Transcriptome Analyses Reveal FOXA1 Dysregulation in Mammary and Extramammary Paget’s Disease. Hum. Pathol. 2018, 77, 152–158. [Google Scholar] [CrossRef]
- Mori, H.; Chen, J.Q.; Cardiff, R.D.; Pénzváltó, Z.; Hubbard, N.E.; Schuetter, L.; Hovey, R.C.; Trott, J.F.; Borowsky, A.D. Pathobiology of the 129:Stat1−/− Mouse Model of Human Age-Related ER-Positive Breast Cancer with an Immune Infiltrate-Excluded Phenotype. Breast Cancer Res. 2017, 19, 102. [Google Scholar] [CrossRef]
- Humphries, M.P.; Sundara Rajan, S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of Male Breast Cancer: A Descriptive Biomarker Study from a Large Patient Series. Sci. Rep. 2017, 7, 45293. [Google Scholar] [CrossRef]
- Davis, D.G.; Siddiqui, M.T.; Oprea-Ilies, G.; Stevens, K.; Osunkoya, A.O.; Cohen, C.; Li, X.B. GATA-3 and FOXA1 Expression Is Useful to Differentiate Breast Carcinoma from Other Carcinomas. Hum. Pathol. 2016, 47, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tokunaga, E.; Yamashita, N.; Sagara, Y.; Ohi, Y.; Taguchi, K.; Ohno, S.; Okano, S.; Oda, Y.; Maehara, Y. The Relationship between the Expression of FOXA1 and GATA3 and the Efficacy of Neoadjuvant Endocrine Therapy. Breast Cancer Tokyo Jpn. 2017, 24, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and Verification of the PAM50-Based Prosigna Breast Cancer Gene Signature Assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Picornell, A.C.; Echavarria, I.; Alvarez, E.; López-Tarruella, S.; Jerez, Y.; Hoadley, K.; Parker, J.S.; Del Monte-Millán, M.; Ramos-Medina, R.; Gayarre, J.; et al. Breast Cancer PAM50 Signature: Correlation and Concordance between RNA-Seq and Digital Multiplexed Gene Expression Technologies in a Triple Negative Breast Cancer Series. BMC Genom. 2019, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Lai, Y.; Xu, J.; Huang, J. Prognostic Value of FOXA1 in Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2016, 27, 35–43. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Tokunaga, E.; Yamashita, N.; Akiyoshi, S.; Okada, S.; Nakashima, Y.; Aishima, S.; Morita, M.; Kakeji, Y.; Maehara, Y. Impact of FOXA1 Expression on the Prognosis of Patients with Hormone Receptor-Positive Breast Cancer. Ann. Surg. Oncol. 2012, 19, 1145–1152. [Google Scholar] [CrossRef]
- Park, S.; Koh, E.; Koo, J.S.; Kim, S.I.; Park, B.-W.; Kim, K.-S. Lack of Both Androgen Receptor and Forkhead Box A1 (FOXA1) Expression Is a Poor Prognostic Factor in Estrogen Receptor-Positive Breast Cancers. Oncotarget 2017, 8, 82940–82955. [Google Scholar] [CrossRef][Green Version]
- Ijichi, N.; Shigekawa, T.; Ikeda, K.; Horie-Inoue, K.; Shimizu, C.; Saji, S.; Aogi, K.; Tsuda, H.; Osaki, A.; Saeki, T.; et al. Association of Double-Positive FOXA1 and FOXP1 Immunoreactivities with Favorable Prognosis of Tamoxifen-Treated Breast Cancer Patients. Horm. Cancer 2012, 3, 147–159. [Google Scholar] [CrossRef]
- Mehta, R.J.; Jain, R.K.; Leung, S.; Choo, J.; Nielsen, T.; Huntsman, D.; Nakshatri, H.; Badve, S. FOXA1 Is an Independent Prognostic Marker for ER-Positive Breast Cancer. Breast Cancer Res. Treat. 2012, 131, 881–890. [Google Scholar] [CrossRef]
- Ademuyiwa, F.O.; Thorat, M.A.; Jain, R.K.; Nakshatri, H.; Badve, S. Expression of Forkhead-Box Protein A1, a Marker of Luminal A Type Breast Cancer, Parallels Low Oncotype DX 21-Gene Recurrence Scores. Mod. Pathol. 2010, 23, 270–275. [Google Scholar] [CrossRef]
- Fu, X.; Jeselsohn, R.; Pereira, R.; Hollingsworth, E.F.; Creighton, C.J.; Li, F.; Shea, M.; Nardone, A.; De Angelis, C.; Heiser, L.M.; et al. FOXA1 Overexpression Mediates Endocrine Resistance by Altering the ER Transcriptome and IL-8 Expression in ER-Positive Breast Cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6600–E6609. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Ardasheva, A.; Mahmud, Z.; Coombes, R.C.; Yagüe, E. FOXA1 Is a Determinant of Drug Resistance in Breast Cancer Cells. Breast Cancer Res. Treat. 2021, 186, 317–326. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Wei, T.; Xiao, Y.-T.; Sheng, H.; Su, H.; Hollern, D.P.; Zhang, X.; Ma, J.; Wen, S.; et al. FOXA1 Overexpression Suppresses Interferon Signaling and Immune Response in Cancer. J. Clin. Investig. 2021, 131, 147025. [Google Scholar] [CrossRef]
- Horimoto, Y.; Arakawa, A.; Harada-Shoji, N.; Sonoue, H.; Yoshida, Y.; Himuro, T.; Igari, F.; Tokuda, E.; Mamat, O.; Tanabe, M.; et al. Low FOXA1 Expression Predicts Good Response to Neo-Adjuvant Chemotherapy Resulting in Good Outcomes for Luminal HER2-Negative Breast Cancer Cases. Br. J. Cancer 2015, 112, 345–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, C.; Wei, Q.; Guo, J.; Zhou, J.C.; Mei, J.; Jiang, Z.N.; Shen, J.G.; Wang, L.B. FOXA1 Expression Significantly Predict Response to Chemotherapy in Estrogen Receptor-Positive Breast Cancer Patients. Ann. Surg. Oncol. 2015, 22, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Dagher, E.; Royer, V.; Buchet, P.; Abadie, J.; Loussouarn, D.; Campone, M.; Nguyen, F. Androgen Receptor and FOXA1 Coexpression Define a “Luminal-AR” Subtype of Feline Mammary Carcinomas, Spontaneous Models of Breast Cancer. BMC Cancer 2019, 19, 1267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Lee, W.-W.; Wang, C.-Y.; Chao, T.-H.; Chen, Y.; Chen, J.H. Regulatory Mechanisms Controlling Human E-Cadherin Gene Expression. Oncogene 2005, 24, 8277–8290. [Google Scholar] [CrossRef] [PubMed]
- Nakashoji, A.; Matsui, A.; Nagayama, A.; Iwata, Y.; Sasahara, M.; Murata, Y. Clinical Predictors of Pathological Complete Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Oncol. Lett. 2017, 14, 4135–4141. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.G.B.; Madden, S.F.; Richards, C.E.; Vellanki, S.H.; Jahns, H.; Hudson, L.; Fay, J.; O’Farrell, N.; Sheehan, K.; Jirström, K.; et al. Human Epidermal Growth Factor Receptor-3 Expression Is Regulated at Transcriptional Level in Breast Cancer Settings by Junctional Adhesion Molecule-A via a Pathway Involving Beta-Catenin and FOXA1. Cancers 2021, 13, 871. [Google Scholar] [CrossRef] [PubMed]
| Study | Clone | Species | Dilution | Manufacturer |
|---|---|---|---|---|
| Rangel et al. [4] | Monoclonal, 2F83 | Mouse | prediluted | Ventana-Roche |
| Chen et al. [46] | Monoclonal, EPR10881, ab170933 | Rabbit | 1:100 | Abcam |
| Horimoto et al. [47] | Polyclonal, ab23738 | Rabbit | NR * | Abcam |
| Zhang et al. [48] | Monoclonal | Rabbit | 1:100 | Bioss, China |
| Byun et al. [49] | Polyclonal, ab23738 | Rabbit | 1:10,000 | Abcam |
| Cheng et al. [50] | Monoclonal, sc-101058, Q6 | Mouse | NR | Santa Cruz Biotechnology |
| Nelson et al. [51] | Monoclonal, ab173287 | Rabbit | 1:4000 | Abcam |
| Mangia et al. [52] | Monoclonal, 2F83 | Mouse | 1:200 | Merck Millipore |
| Dai et al. [53] | NR | NR | 1:100 | Abcam |
| De Lara et al. [54] | Monoclonal, 2F83 | Mouse | 1:100 | CELL MARQUE |
| Kutasovic et al. [55] | Monoclonal, 2F83, ab40868 | Mouse | 1:100 | Abcam |
| Schrijver et al. [56] | Monoclonal, WMAB-2F83 | Mouse | 1: 100,000 | Seven Hills Bioreagents |
| Guiu et al. [57] | Polyclonal, HNF-3α/β (C-20) | Goat | NR | Santa Cruz |
| Mai et al. [58] | Monoclonal | Mouse | 1:50 | Sigma |
| Mori et al. [59] | Monoclonal, EPR10881 | Rabbit | NR | Abcam |
| Humphries et al. [60] | Monoclonal, ab55718 | Mouse | 1:500 | Abcam |
| Davis et al. [61] | Monoclonal, 2F83 | Mouse | 1:100 | Millipore |
| Tanaka et al. [62] | Monoclonal | Mouse | 1:500 | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metovic, J.; Borella, F.; D’Alonzo, M.; Biglia, N.; Mangherini, L.; Tampieri, C.; Bertero, L.; Cassoni, P.; Castellano, I. FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact. Cancers 2022, 14, 4699. https://doi.org/10.3390/cancers14194699
Metovic J, Borella F, D’Alonzo M, Biglia N, Mangherini L, Tampieri C, Bertero L, Cassoni P, Castellano I. FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact. Cancers. 2022; 14(19):4699. https://doi.org/10.3390/cancers14194699
Chicago/Turabian StyleMetovic, Jasna, Fulvio Borella, Marta D’Alonzo, Nicoletta Biglia, Luca Mangherini, Cristian Tampieri, Luca Bertero, Paola Cassoni, and Isabella Castellano. 2022. "FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact" Cancers 14, no. 19: 4699. https://doi.org/10.3390/cancers14194699
APA StyleMetovic, J., Borella, F., D’Alonzo, M., Biglia, N., Mangherini, L., Tampieri, C., Bertero, L., Cassoni, P., & Castellano, I. (2022). FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact. Cancers, 14(19), 4699. https://doi.org/10.3390/cancers14194699










