Simple Summary
According to reports from more than a decade ago, the proportion of primary salivary gland-type carcinoma (SGC) among all lung cancers is only 0.1–1.0% and the 5-year overall survival rate is more than 60%. However, previous reported studies mostly had small sample sizes due to the low proportion of primary SGC in lung cancer. The characteristics of SGC proportion and prognosis have not yet been elucidated. The aim of this study was to elucidate the clinical and prognostic characteristics of primary SGC. This study found that lung SGC has the best prognosis among adenocarcinoma, squamous cell carcinoma, and SGC. In addition, lobectomy can further improve the prognosis of SGCs.
Abstract
This study aimed to explore the clinical and prognostic characteristics of primary salivary gland-type carcinoma (SGC). The entire cohort from the Surveillance, Epidemiology, and End Results database was used to calculate the SGC proportion. In total, 253,096 eligible patients, including 165,715 adenocarcinomas (ADCs), 87,062 squamous cell carcinomas (SCCs), and 319 SGCs, were selected to perform survival analyses. The data of 42 SGC patients from our hospital showed postoperative survival. Overall survival (OS) curves for different histological and surgical types were presented. The proportion of primary SGCs was 0.8 per 1000 patients. Patients with age ≤ 64 years old had a much higher proportion of SGC than those patients with age >64 years old. After adjusting for other confounders, among ADCs, SCCs, and SGC, SGCs had the best prognosis (HR 0.361, p < 0.001). Moreover, the 5-year OS rates of SGC patients were 55% and 7% in the group with surgery or without surgery, respectively (p < 0.001). The data of 42 patients from our hospital also showed a good survival of SGCs. Lobectomy improved the survival of SGCs significantly (adjusted HR 0.439, p = 0.016). In conclusion, pulmonary SGCs had the best prognosis among ADCs, SCCs, and SGCs. In addition, lobectomy could further improve the prognostic outcomes of SGCs.
1. Introduction
Lung cancer is still the leading cause of cancer mortality worldwide, although the morbidity rate has decreased [1,2]. In all lung malignancies, non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) account for about 85% and 15%, respectively [3]. Salivary gland-type carcinomas (SGCs) originate from the salivary glands located in the upper aerodigestive tract, such as parotid, submandibular, and sublingual [4,5,6]. SGCs mainly include mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (ACC), and epithelial-myoepithelial carcinoma (EMC) [7].
According to reports from more than a decade ago, however, the proportion of the primary SGCs is only 0.1–1.0% in all lung cancers [8,9]. Previous studies suggested that lung cancer patients for SGC had satisfactory outcomes, of which the 5-year overall survival (OS) rate was over 60% [7,10]. Due to the low proportion of primary SGCs in lung cancer, previously reported studies mostly had small sample sizes [7,10,11]. Thus, the information about the proportion of primary SGCs in all lung malignancies needs to be updated. Some research has analyzed postoperative survival [7,10]; however, the role of surgery in the prognosis of SGCs needs to be further explored. In addition, it is unclear what the better prognostic outcomes in SGCs and other NSCLCs are. Therefore, it is important to study the surgical significance of SGCs and compare differences in survival between SGC and other NSCLCs, such as adenocarcinoma (ADC) and squamous cell carcinoma (SCC). The aim of this study was to investigate the clinical and prognostic characteristics of patients with SGCs.
2. Materials and Methods
2.1. Patients
The Ethics Committee of Shanghai Pulmonary Hospital approved this study (IRB number K22-209). Cases were diagnosed as lung malignancy in the Surveillance, Epidemiology, and End Results (SEER) database, which contains clinicopathological and survival data of cancer patients from 18 registries [12]. Patients diagnosed as primary SGC in Shanghai Pulmonary Hospital were also included in this study. All patient records were anonymized before analysis. The information about eligible patients was used to perform proportion and prognostic analyses. The selection criteria of patients are presented in Figure 1. The data for a total of 593,662 patients between 2004 and 2018 was used in the proportion calculation, and the data of 253,096 cases between 2004 and 2015 was used for survival analyses. In addition, we collected 42 SGC patients with surgery between 2011 and 2018 in Shanghai Pulmonary Hospital. These 42 patients did not have distant metastasis of the lymph nodes or other organs. Information collected from the SEER database included race/ethnicity, sex, age at diagnosis, tumor location, marital status, treatment approach (including surgical treatment, radiotherapy, and chemotherapy), tumor size, tumor differentiation, histological subtype, tumor node metastasis (TNM) stage, survival time, and definitive survival status. According to the staging-related information and the guidelines of the 8th TNM staging system [13], the 6th TNM stage was re-translated to the 8th edition. Cases from 2016 to 2018 were excluded from further analyses due to a lack of details required for retranslation from the sixth into the eighth edition staging.
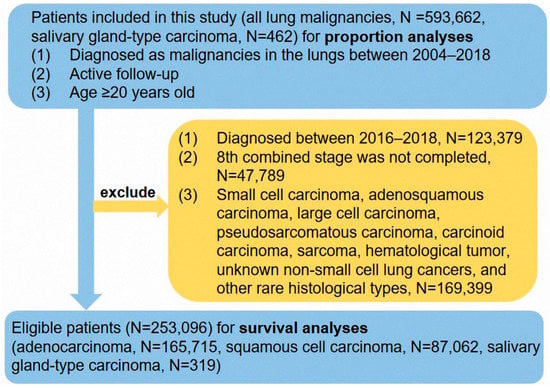
Figure 1.
The flow chart of this study.
2.2. Follow-Up
The follow-up duration of the cases for the survival analyses ranged from 0.0 to 179.0 months, with a median of 13.0 months. The follow-up information of the SEER database was updated in November 2020. The patients included in the survival analyses had definitive survival status, death or alive. OS, which was the duration from the date of diagnosis to death, was regarded as our observational endpoint.
2.3. Statistical Analysis
The main statistical analyses were performed using SPSS statistics 25.0 software (IBM SPSS Inc., Chicago, IL, USA), R 4.1.2 software (R Fundamental for Data Science, Vienna, Austria) (https://www.r-project.org/, accessed on 1 December 2021), and GraphPad Prism 9 (https://www.graphpad.com/scientific-software/prism/, accessed on 7 December 2021). Categorical variables were compared using Pearson’s Chi-square test. The analysis of linear regression was used to identify the relation between the proportion of SGC and the year of diagnosis. Risk ratios (RRs), hazard ratios (HRs), and 95% confidence intervals (CIs) were calculated using logistic regression analysis and Cox regression analysis, respectively (the regression method was Enter selection). Kaplan–Meier survival analysis and the Log-rank test were used to draw and compare the survival curves. The multivariable Cox proportional-hazards model was used to calculate the average value of each covariate and estimate the adjusted survival curves of different surgical types [12]. Statistical tests were considered statistically significant with a two-sided p value < 0.05.
3. Results
3.1. Patient Characteristics
In the cohort for the proportion analyses, the majority of the patients were over 64 years old (N = 402,390, 67.8%). The majority of the patients were male (N = 312,706, 52.7%), and 480,358 were Caucasian patients (80.9%). In total, 462 patients were diagnosed with SGCs, including 270 MECs, 181 ACCs, and 11 EMCs.
After case selection, 253,096 eligible patients, including 319 SGCs, 165,715 ADCs, and 87,062 SCCs, were included in the survival analyses. There were 122 ACCs, 193 MEC, and 4 EMCs in all 319 SGCs. The majority of cases were stage IV (N = 119,382, 47.2%), and the rate of stage IIA was low at 2.2% (N = 5676). The tumor differentiation of 104,001 patients was undifferentiated grade (41.1%), and 71,327 patients had a poor grade (28.2%). In total, 69.0% of the patients were confirmed not to undergo an operation (N = 174,529). In total, 108,109 patients (42.7%) received chemotherapy, and 98,998 patients (39.1%) underwent radiotherapy. Of note, 50,359 patients did not undergo any treatment. Other detailed information on the patient characteristics from the SEER database and our hospital is shown in Table 1 and Table 2, respectively.

Table 1.
Clinical and pathological characteristics of lung cancer patients from the Surveillance Epidemiology and End Results database between 2004 and 2015.

Table 2.
Detailed information about pulmonary salivary gland-type carcinoma in Shanghai Pulmonary Hospital.
3.2. The Analyses of Proportion
In the entire cohort of 593,662 patients, 462 cases were diagnosed as primary SGCs. The proportion was 0.8 (95% CI 0.3–1.3) per 1000 patients, which was essentially unchanged from 2004 to 2018 (Figure 2, 0.7 [95% CI 0.4–1.0] per 1000 persons in 2004; 1.1 [95% CI 0.8–1.4] per 1000 persons in 2018). The results of the linear regression also revealed that the proportion of SGCs was not changed by the year of diagnosis (r = 0.432, p = 0.108). The RR (diagnosis in 2018 vs. diagnosis in 2004) was 1.492 (95% CI 0.916–2.430, p = 0.108), adjusted for sex, age, and race. The results of the multivariable logistic regression revealed that patients with age ≤ 64 years had a much higher proportion of SGC than patients with age > 64 years (adjusted RR = 0.341, 95% CI 0.283–0.410, p < 0.001, Figure 2), and sex and race/ethnicity did not have an impact on the proportion of primary SGCs (all p > 0.05).
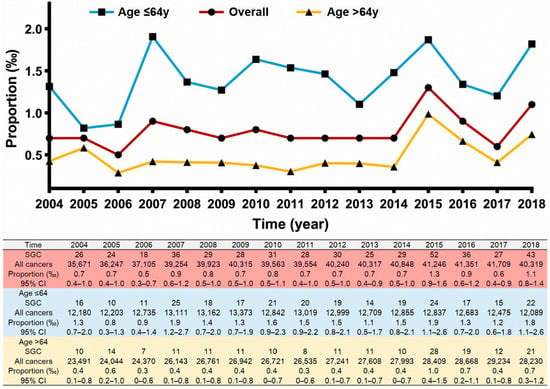
Figure 2.
The crude incidence rate of salivary gland-type carcinoma over time in the 593,662 lung cancer patients.
3.3. Survival Analysis of Different Histological Types
The median survival time of 253,092 patients was 13.0 months (ranging from 0.0 to 179.0 months). Moreover, the 1-, 3-, and 5-year OS rates of this cohort were 38%, 25%, and 9%, respectively. The unadjusted 5-year OS rate was best in patients with SGCs (41%) and worst in patients with SCCs (7%). The median survival time was 121.0 months (95% CI 83.8–158.2 months) in patients with SGCs, 14.0 months in ADC patients, and 12.0 months in SCC patients, which indicated that SGC patients had satisfactory outcomes (Figure 3A). Multivariable Cox regression analysis was used to identify the prognostic role of SGCs in the different NSCLCs (Table 3). After adjusting for other confounders, among patients with ADCs, SCCs, and SGCs, SGCs had the best prognosis (adjusted HR 0.513, 95% CI 0.437–0.603, p < 0.001) and SCCs had the worst survival (adjusted HR 1.119,95% CI 1.108–1.130, p < 0.001). Furthermore, the entire primary SGC cohort had much better survival than patients with stage IB ADCs or stage I SCCs (Figure 3B).
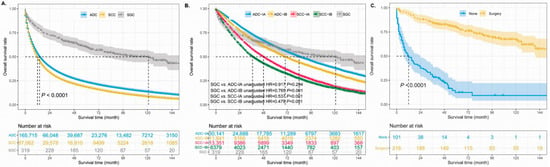
Figure 3.
The unadjusted survival curves of different histological types (A,B). The unadjusted survival curves for SGC patients according to whether they received surgery (C). ADC: adenocarcinoma, SCC: squamous cell carcinoma, SGC: salivary gland-type carcinoma.

Table 3.
Univariable and multivariable Cox proportional hazard regression analyses for prognostic factors in lung cancer patients with ADC, SCC, and SGC.
3.4. Surgical Significance in Primary SGCs
To further investigate the surgical significance for the prognosis of primary SGCs, we first combined patients with lobectomy, sub-lobectomy, and pneumonectomy into one group. Two patients were excluded from the analyses because they lacked detailed information about whether they had undergone surgery. The survival curves showed that SGC patients who underwent surgery had much better survival than patients who did not undergo surgery (Figure 3C, p < 0.001). The median survival time in the SGC patients without surgery was 12.0 months (95% CI 5.1–18.9 months); however, the median survival time was not reached in cases with surgery. There were 65 death events in the surgery group, which accounted for 30.1%. However, 81.2% of the patients in the group without surgery reached the end of life. The 5-year OS rate of the patients was 55% (Figure 4A) and 7% in the group with surgery and without surgery, respectively (unadjusted HR = 0.137, 95% CI 0.096–0.195, p < 0.001). In addition, there were 42 SGC patients and only 5 patients with recurrent or metastatic diseases between 2012 and 2017 in our hospital (Table 2). The duration of follow-up ranged from 39 to 129 months (median, 70.5 months). The data of our hospital recorded that all pulmonary SGC patients who underwent operations were alive (follow-up update on 23 March 2022). The 5-year disease-free survival rate was 88.0% in the Shanghai Pulmonary Hospital cohort (Figure 4B).
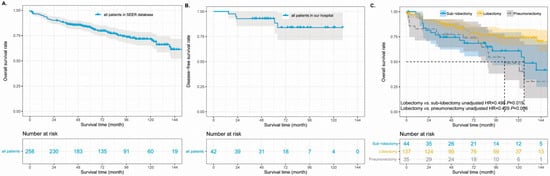
Figure 4.
The unadjusted survival curves of for patients who underwent surgery in the SEER database (A) and Shanghai Pulmonary Hospital (B). The adjusted survival curves of different surgical types (C). SEER: Surveillance, Epidemiology, and End Results.
A prognostic analysis for postoperative patients was further performed. In the SEER database, of 216 SGC patients who underwent surgery, 5 patients were excluded from prognostic analysis because of their distant metastasis of lymph nodes or other organs. After adjusting for other confounders, the multivariable analysis identified poor to undifferentiated grade, age > 64 years, N1 classification, and tumor size of 3.1–5.0 cm as independent risk factors for postoperative prognosis (Table 4). Lobectomy, as an independent protective factor, improved the survival outcomes of SGCs significantly (adjusted HR = 0.439, 95% CI 0.225–0.856). The 5-year OS rates were 45% vs. 65% in the cases of sub-lobectomy and lobectomy, respectively (Figure 4C, overall p = 0.005).

Table 4.
Univariable and multivariable Cox proportional hazard regression analyses of postoperative prognostic factors in salivary gland-type carcinoma patients.
4. Discussion
In the present study, the data of 593,662 patients was used to perform proportion analyses. We found that the proportion of primary SGCs in the lung was 0.8 per 1000 patients based on the SEER database between 2004 and 2018, which is consistent with previous reports [4,8]. The results also revealed that the proportion of SGCs did not change over time. Moreover, the incidence rate differed by age group. Patients with age ≤ 64 years had a higher proportion of primary SGCs than cases with age > 64 years. Moreover, sex and race/ethnicity did not have an impact on the incidence rate of primary SGCs. To further explore the prognosis of SGCs, we compared the survival among ADCs, SCCs, and SGCs. In the NSCLCs, previous reports about the comparison of prognoses among ADCs, SCCs, and SGCs are lacking. The multivariable Cox regression uncovered that SGC patients had the best survival, and SCC patients had the worst survival among the three classifications of patients. In the SGC group with operation, we investigated the surgical significance for prognosis. We confirmed that older patients had a poorer prognosis than younger patients, and lobectomy could improve the survival outcomes. In addition, the prognosis for the group of ACCs and EMCs was poorer than MECs. After adjusting for other confounders, grade, age, surgical types, N classification, and tumor size were identified as independent factors affecting survival. Given the surgery patients, especially lobectomy, have satisfactory survival outcomes, we propose that primary SGC patients without metastatic disease are recommended for surgery. Furthermore, lobectomy is more optimal than others.
The impact of the grading of tumors on the prognosis of primary SGCs remains unclear. A previous report found that high-grade SGCs were likely to present lymph-node metastasis [14]. In addition, some studies showed that high-grade primary SGCs had much poorer survival than low- to intermediate-grade cases [7,15]. Our results in the present study, however, revealed that poor- to undifferentiated-grade SGCs had a poorer prognosis than those with well to moderate grades. The findings from Hsieh CC et al. showed that the grade of tumor did not affect the survival of pulmonary MECs [16]. The results in the above studies are not consistent. On the one hand, there were other studies about pulmonary SGCs; however, survival analyses were lacking [17,18]. Moreover, the sample size of primary SGCs was small, and the information about tumor grading was not available in some cases [17]. On the other hand, many high-grade neoplasms can show the presence of low-grade areas even though they do not form the bulk of the tumor [19]. Thus, the prognostic significance of tumor grading in pulmonary SGCs needs to be further investigated.
Surgery, especially lobectomy, should be recommended for eligible patients of primary SGCs. A study from Kim BG et al. showed that surgical resection could improve survival of primary SGCs compared with bronchoscopic intervention based on the analyses of information from 181 patients [20]. Another study by Zhu F et al. also revealed that patients who underwent operation had satisfactory survival outcomes [7]. In the present study, we found similar results to the abovementioned two studies. Regrettably, the above two studies did not compare the effect of different surgical approaches on prognosis with pulmonary SGCs. We found that patients who underwent lobectomy had improved survival compared to sub-lobectomy. In addition, in the univariable analysis, the N1 and N2 classifications were regarded as factors affecting survival. After multivariable analysis, however, only one of the above two factors, N1 classification, could independently affect prognosis. N2 classification is a prognostic risk indicator but did not present statistical significance in the multivariable analysis. The reason for this phenomenon might be that the number of patients with N2 was too small. Therefore, we think that the resection of lymph nodes during operation for pulmonary SGCs still needs to be focused on.
In addition, the histological type of ACC served as a negative factor influencing survival compared with MECs. The findings of the research by Wang Y et al. showed that OS in ACC patients was lower than that in MEC patients [21]. This study included 108 MEC patients and 183 ACC patients. The eligible patients of their research mainly underwent tracheobronchial resection. Patients who underwent lobectomy operations were the majority in another study from Zhu Y et al. [7]. They agreed on the results of the above research that MECs had better survival than ACCs; however, they did not perform multivariable analysis to adjust for confounders, including age, sex, TNM stage, and tumor differentiation. Thus, the results of the research from Zhu Y et al. might be challenged, as they were affected by the failure to consider other confounding factors. The present study included 63 ACCs, 147 MECs, and 1 EMC in the SEER cohort with surgery. In the univariable analysis, we found similar results to the above two studies. As the sample size was small, the comparison of survival in the different histological subtypes did not have statistical significance after multivariable Cox regression. Based on this, we still believe that the prognosis of MEC may show a better trend than that of ACC. In addition, prognostic research on EMC, as the least common tumor in SGCs, needs to be put on the agenda.
There was an interesting phenomenon in the results in that marital status had a prognostic effect on NSCLS patients. Married patients had a better survival than non-married patients, which is in accordance with some studies involving other malignant tumors [22,23]. Several possible reasons may explain the relationship between marital status and survival outcomes. First, given that married patients are more likely to receive support from family members, they may be diagnosed much earlier and be inclined to receive treatment, leading to a satisfactory survival rate. Second, mental support is urgently needed for cancer patients in addition to physical care. Married patients often receive their spouses’ help throughout diagnosis and treatment. However, unpleasant and upsetting relationships generate depression, which may have acted as a predictor of disease progression and motility of malignant tumors in a meta-analysis [24]. Without support from spouses, unmarried patients are prone to suffering from greater emotional pressure and worse socioeconomic situations, which may be associated with poor prognosis among patients with NSCLC.
Notably, patients with stage I SCCs had a poor prognosis. In previous studies, some studies compared the prognosis of ADCs and SCCs and found that the prognosis of ADCs is better than that of SCCs [25,26]. In one report, non-cancer-related deaths in patients with SCCs mainly included pneumonia, chronic obstructive pulmonary disease, and sepsis [25]. Moreover, this study found that SCC patients had a higher non-cancer-related mortality rate than ADC patients [25]. The observational end point of our study was OS, which might result in a lower OS rate in SCC patients because of its high non-cancer mortality rate. In addition, considering that this study was a retrospective study, the data on staging in the SEER database might not be detailed and accurate enough, which would cause staging differences to a certain extent, so that relatively advanced patients enter the cohort with stage I. However, ADC patients have a better prognosis than SCCs overall. Therefore, even if it is early stage SCC, we should pay attention to its prognosis and give necessary postoperative adjuvant therapy and follow-up management.
This study has several limitations. First, some important information was not detailed, such as the completeness of resection, chemotherapy sequence, and immunohistochemistry, as we could not obtain it from the SEER database. Second, although the data we used was obtained from a large population-based cohort, the sample size of pulmonary SGCs was still small. For example, we categorized patients with wedge resection or segmental resection into sub-lobe resection because of the small sample of these two kinds of patients. Third, we excluded patients with age < 20 years to ensure that all patients were not juveniles. However, given that age in the SEER database is not a continuous value, it is grouped at five-year intervals (such as 15–19 and 20–24), so patients aged 18–19 could only be excluded from this study. This action might affect the results of statistical analyses. Finally, given that this study was a retrospective study, it was impossible to avoid selection bias. Therefore, more studies are necessary to further validate our findings.
5. Conclusions
Primary SGCs in all lung cancers were rare, of which the incidence rate was 0.8 per 1000 patients between 2004 and 2018. Moreover, patients with age ≤ 64 years had a much higher proportion of SGC than patients with age > 64 years. Fortunately, pulmonary SGCs had satisfactory survival, and they had the best prognosis among ADCs, SCCs, and SGCs. In addition, surgery, particularly lobectomy, could further improve the prognostic outcomes of SGCs. However, more research is required to confirm these findings.
Author Contributions
Conceptualization, L.-L.W., Z.-X.L. and D.X.; Data curation, J.-Y.Q. and W.-K.L.; Formal analysis, L.-L.W., J.-Y.Q. and Z.-X.L.; Funding acquisition, Z.-X.L. and D.X.; Investigation, Y.Z.; Methodology, C.-W.L.; Resources, K.L.; Software, C.-W.L.; Supervision, K.L.; Validation, Y.Z.; Writing—original draft, L.-L.W. and W.-K.L.; Writing—review and editing, D.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Shanghai ShenKang Hospital Development Centre (SHDC22020218), Science and Technology Commission of Shanghai Municipality (21Y11913400), and Shanghai Pulmonary Hospital Foundation (fkxr1904 and FK1936). The funding bodies played no role in the design of the study; the collection, analysis, and interpretation of the data; nor the writing of the manuscript.
Institutional Review Board Statement
The Ethics Committee of Shanghai Pulmonary Hospital approved this study (IRB number K22-209), and the human data was in accordance with the Declaration of Helsinki in the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any researchers interested in this study should contact the correspondence author Dong Xie to request the data.
Acknowledgments
We thank the software SEER*Stat, which was produced by Information Management Services, Inc., in consultation with the Surveillance Research Program of the Division of Cancer Control and Population Sciences, National Cancer Institute. The SEER*Stat Web site is located at: seer.cancer.gov/seerstat. We took the proper permissions to access the SEER database and used SEER*Stat (version 8.3.9) to acquire the database (Incidence-SEER Research Plus Data, 17 Registries, Nov 2020 Sub [2000–2018]) to select eligible cases. Besides, L. L. W. sincerely thank T. H. R. and G. W. M. for instructing clinical knowledge, surgery, and research in thoracic oncology. L. L. W. also sincerely thanks the singer Leslie Cheung, whose songs brought him much encouragement to persevere on his research path. All authors appreciated statistical suggestions from Yi-Han Guo, who is from the Department of Scientific Research, Shaanxi Academy of Social Sciences, Shaanxi, China.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
NSCLC: non-small cell lung cancer, SCLC: small cell lung cancer, SGC: salivary gland-type carcinomas, MEC: mucoepidermoid carcinoma, ACC: adenoid cystic carcinoma, EMC: epithelial-myoepithelial carcinoma, OS: overall survival, ADC: adenocarcinoma, SCC: squamous cell carcinoma, SEER: Surveillance, Epidemiology, and End Results, TNM: tumor, node, metastasis, RR: Risk ratios, HR: hazard ratios, CI: confidence intervals.
References
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Muir, C.; Weiland, L. Upper aerodigestive tract cancers. Cancer 1995, 75 (Suppl. S1), 147–153. [Google Scholar] [CrossRef]
- Boukheris, H.; Curtis, R.E.; Land, C.E.; Dores, G.M. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: A population-based study in the United States. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2899–2906. [Google Scholar] [CrossRef]
- Jiang, W.-M.; Wu, L.-L.; Wei, H.-Y.; Ma, Q.-L.; Zhang, Q. A Parsimonious Prognostic Model and Heat Map for Predicting Survival Following Adjuvant Radiotherapy in Parotid Gland Carcinoma with Lymph Node Metastasis. Technol. Cancer Res. Treat. 2021, 20, 15330338211035257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Liu, Z.; Hou, Y.; He, D.; Ge, X.; Bai, C.; Jiang, L.; Li, S. Primary salivary gland-type lung cancer: Clinicopathological analysis of 88 cases from China. J. Thorac. Oncol. 2013, 8, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A. Primary salivary gland-type tumors of the lung. Semin. Diagn. Pathol. 1995, 12, 106–122. [Google Scholar]
- Heitmiller, R.F.; Mathisen, D.J.; Ferry, J.A.; Mark, E.J.; Grillo, H.C. Mucoepidermoid lung tumors. Ann. Thorac. Surg. 1989, 47, 394–399. [Google Scholar] [CrossRef]
- Kang, D.Y.; Yoon, Y.S.; Kim, H.K.; Choi, Y.S.; Kim, K.; Shim, Y.M.; Kim, J. Primary salivary gland-type lung cancer: Surgical outcomes. Lung Cancer 2011, 72, 250–254. [Google Scholar] [CrossRef]
- Molina, J.R.; Aubry, M.C.; Lewis, J.E.; Wampfler, J.A.; Williams, B.A.; Midthun, D.E.; Yang, P.; Cassivi, S.D. Primary salivary gland-type lung cancer: Spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007, 110, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-L.; Li, C.-W.; Lin, W.-K.; Qiu, L.-H.; Xie, D. Incidence and survival analyses for occult lung cancer between 2004 and 2015: A population-based study. BMC Cancer 2021, 21, 1009. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Asamura, H.; Travis, W.D.; Rusch, V.W. Lung cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, P.; Xiao, Z.; Qiu, H.; Zhang, X.; Xiao, Y.; Zhang, B. Prognostic factors of primary pulmonary mucoepidermoid carcinoma: A clinical and pathological analysis of 34 cases. Int. J. Clin. Exp. Pathol. 2014, 7, 6792–6799. [Google Scholar] [PubMed]
- Lee, G.D.; Kang, D.K.; Kim, H.R.; Jang, S.J.; Kim, Y.-H.; Kim, D.K.; Park, S.-I. Surgical outcomes of pulmonary mucoepidermoid carcinoma: A review of 23 cases. Thorac. Cardiovasc. Surg. 2014, 62, 140–146. [Google Scholar] [PubMed]
- Hsieh, C.-C.; Sun, Y.-H.; Lin, S.-W.; Yeh, Y.-C.; Chan, M.-L. Surgical outcomes of pulmonary mucoepidermoid carcinoma: A review of 41 cases. PLoS ONE 2017, 12, e0176918. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xi, S.Y.; Hao, W.-W.; Yang, X.-H.; Deng, L.; Xu, Y.-X.; Wu, X.-Y.; Zeng, L.; Guo, K.-H.; Wang, H.-Y. Mutational landscape of primary pulmonary salivary gland-type tumors through targeted next-generation sequencing. Lung Cancer 2021, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Garcia, J.J.; Wehrs, R.N.; Colby, T.V.; Khoor, A.; Leslie, K.O.; Chen, L. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod. Pathol. 2014, 27, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, N.; Moran, C.A. Pulmonary mucoepidermoid carcinoma: Diagnosis and treatment. Expert Rev. Respir. Med. 2018, 12, 249–255. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, K.; Um, S.W.; Han, J.; Cho, J.H.; Kim, J.; Kim, H.; Jeong, B.H. Clinical outcomes and the role of bronchoscopic intervention in patients with primary pulmonary salivary gland-type tumors. Lung Cancer 2020, 146, 58–65. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, S.; Xue, Q.; Mu, J.; Gao, Y.; Tan, F.; Mao, Y.; Wang, D.; Zhao, J.; Gao, S.; et al. Treatment outcomes of patients with tracheobronchial mucoepidermoid carcinoma compared with those with adenoid cystic carcinoma. Eur. J. Surg. Oncol. 2020, 46 Pt A, 1888–1895. [Google Scholar] [CrossRef]
- Fosså, S.D.; Cvancarova, M.; Chen, L.; Allan, A.L.; Oldenburg, J.; Peterson, D.R.; Travis, L.B. Adverse prognostic factors for testicular cancer-specific survival: A population-based study of 27,948 patients. J. Clin. Oncol. 2011, 29, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Khan, S.A.; Chrischilles, E.A.; Schroeder, M.C. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988–2011. JAMA Surg. 2016, 151, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Wang, B.Y.; Huang, J.Y.; Chen, H.C.; Lin, C.H.; Lin, S.H.; Hung, W.H.; Cheng, Y.F. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J. Cancer Res. Clin. Oncol. 2020, 146, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, T.; Forjaz, G.; Antunes, L.; Bastos, J.; Mayer, A.; Aguiar, P.; Araújo, A.; Nunes, C. Lung cancer survival and sex-specific patterns in Portugal: A population-based analysis. Pulmonology 2021, 9, S2531-0437(21)00190-2. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).