Simple Summary
With the increasing number of breast cancer survivors and with the progress in survival of patients with metastatic breast cancer, consideration of survivorship issues becomes vital. Cardiotoxicity has been an important aspect in the management of early or metastatic breast cancer, not least due to the wide use of anthracyclines and, in HER2-positive breast cancer, anti-HER2 agents. Current baseline assessment includes clinical, such as history of cardiovascular comorbidities and lifestyle factors, and biochemical markers. Further biomarkers for tailored risk assessment and management remain an unmet need. Pharmacogenomics, an emerging field investigating how individual genetic variations, alone or in combination with polygenic risk scores, can impact drug metabolism and efficacy, and could be a complement. We hereby present a comprehensive review of the literature and the current landscape on the role of pharmacogenomics in cardiotoxicity prediction and its potential to become an additional biomarker in personalized risk assessment algorithms.
Abstract
Pharmacogenomics is an emerging field in oncology, one that could provide valuable input on identifying patients with inherent risk of toxicity, thus allowing for treatment tailoring and personalization on the basis of the clinical and genetic characteristics of a patient. Cardiotoxicity is a well-known side effect of anthracyclines and anti-HER2 agents, although at a much lower incidence for the latter. Data on single-nucleotide polymorphisms related to cardiotoxicity are emerging but are still scarce, mostly being of retrospective character and heterogeneous. A literature review was performed, aiming to describe current knowledge in pharmacogenomics and prediction of cardiotoxicity related to breast cancer systemic therapies and radiotherapies. Most available data regard genes encoding various enzymes related to anthracycline metabolism and HER2 polymorphisms. The available data are presented, together with the challenges and open questions in the field.
1. Introduction
About one-third of cancer cases in women are attributed to breast cancer, although incidence can vary between geographic regions [1]. Breast cancer incidence is projected to rise in the coming decades because of ageing and the increase in the global population [2], and simultaneously, the number of breast cancer survivors is expected to rise as a result of the advances in understanding the biology and management of breast cancer. Multimodal care is today the standard in Western countries, including a plethora of combinations of surgery, conventional chemotherapy, targeted therapies (including antihormonal therapy and anti-human epidermal growth factor receptor 2 (HER2)-agents), and radiotherapy.
The development of cardiotoxicity, both acute and chronic, represents a substantial source of short- and long-term morbidity and may drastically impact quality of life in breast cancer survivors. Cardiotoxicity is a broad term that encompasses either direct cardiovascular adverse effects or progression of established cardiovascular disease [3]. A reduction of left ventricle ejection fraction (LVEF) by 10%–15% points and/or to a value below the lower normal limit by echocardiography or multigated acquisition scan (MUGA) is often used to diagnose cancer-therapy-related cardiac dysfunction [4,5,6].
Efforts to ameliorate cancer-therapy-related cardiac dysfunction is vital to decrease the risk for acute morbidity, chronic health conditions, and decreased quality of life in cancer survivors. Several strategies have been explored, for example, by means of early risk stratification or medical interventions with cardioprotective drugs.
Early risk stratification is key to identifying the optimal candidates for intensified screening programs, treatment modifications, or initiation of cardio-protective strategies. Traditional risk factors associated with risk for cardiotoxicity include increasing age; smoking; obesity; lack of physical activity; comorbidities such as diabetes mellitus; hypertension; hyperlipidemia and pre-existing or history of cardiac conditions; type of cancer therapy; chest irradiation; and, in the case of anthracyclines, cumulative dose and concomitant administration with trastuzumab [3,6]. The European Society for Medical Oncology (ESMO) recommends baseline screening with electrocardiogram and evaluation of LVEF for all patients planed for cancer therapy, especially if the planned treatment is correlated to increased risk for cardiotoxicity [6]. The ground for the use of biomarkers such as troponin and brain natriuretic peptide (BNP) or the N-terminal prohormone of BNP (NT-proBNP) is not equally robust and they are mostly recommended for patients with high baseline risk for cardiovascular side effects [6,7].
Existing evidence for cardiotoxicity prevention with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or beta blockers have not yet provided significant clinical benefit, and interpretation is hampered by heterogeneity of the study populations and their baseline cardiotoxicity risk level, as well as of the definition of study endpoints. This inconsistency of the results in various randomized trials, often stratified by common risk factors, highlights the need for additional, objective, personalized, and risk-predictive biomarkers [8]. Ideally, a patient with increased risk for toxicity should be identified prior the initiation of a potentially cardiotoxic cancer treatment, allowing for modification of the planned treatment; initiation of preventive strategies; and, if necessary, establishment of a tailored monitoring schedule.
In this context, pharmacogenomics, an emerging field in clinical practice as well as in research, could be of great value. Pharmacogenomics examines how various genetic variants impact pharmacokinetics, i.e., how different genetic variants may influence the expression and function of the drug-metabolizing enzymes, as well as pharmacodynamics, i.e., how variants can affect expression of the drug targets and thereof the effect of the drug in the body. In this way, pharmacogenomics can provide insight on insufficient drug efficacy, variations in drug-related side effects, and drug-to-drug interactions. For example, allelic variations leading to high enzymatic cytochrome P450 family 2 (CYP2C19) and low CYP2D6 activity have been related to poor bioactivation of tamoxifen and subsequently worse breast cancer outcomes [9,10]. Additionally, in breast cancer patients treated with docetaxel, those with genetic variants leading to poor drug metabolism have been shown to have increased incidence of grade 3/4 toxicity (71%), compared to 45% among intermediate metabolizers and similarly to extensive or rapid metabolizers [11]. Hence, there is a strong rationale that allelic variants, single-nucleotide polymorphisms (SNPs), or substitution variants in processes involved in the function or metabolization of a drug could predispose to cardiotoxicity and could therefore serve as biomarkers for the prediction of treatment-related cardiotoxicity.
The utility of pharmacogenomics in the field of cardio-oncology is unclear thus far and the data are scarce. To this aim, we conducted a comprehensive literature review and present the available data on the predictive role of genetic variants for the development of acute and chronic cardiotoxicity in breast cancer survivors.
2. Chemotherapy
Anthracyclines and taxanes constitute the most commonly used regimens in early and metastatic breast cancer. Although the position of anthracyclines in neoadjuvant management of HER2-positive breast cancer is currently debated [12], their position in breast cancer care remains important. Anthracycline-related cardiotoxicity is described as a dose-dependent and irreversible cardiomyopathy, progressively leading to heart failure, and it was described already in 1967 [13]. Still, the exact pathogenic mechanism is not entirely known. Generation of reactive oxygen species (ROSs) and changes in iron metabolism were initially suggested as potential pathways, but in recent years, inhibition of topoisomerase 2β and subsequent mitochondrial dysfunction and cell death has been the most popular hypothesis [14,15].
In preclinical models using patient-derived cardiomyocytes, a missense variant S427L (rs2229774) of the retinoid acid receptor gamma (RARG) gene increased susceptibility to doxorubicin and subsequent cardiotoxicity [16].
Cytosolic carbonyl reductases (CBR) are enzymes involved in the metabolism of doxorubicin, and it has been demonstrated that different genotypes can significantly impact cardiotoxicity incidence [17]. In 92 breast cancer patients genotyped for the CBR3 gene, those with the CBR3 V244M variant had a significant reduction of LVEF six months after the initiation of doxorubicin, although the numerical reduction of LVEF was small [17]. Risk for cardiotoxicity, defined as asymptomatic LVEF decline <55%, did not correlate to any CBR3 V244M genotype [17]. The same variant was also identified as a risk variant in a retrospective series of n = 166 patients with breast cancer treated with doxorubicin [18]. They also demonstrated that the variant rs1045642 of the ATP binding cassette subfamily B member 1 (ABCB1) gene, encoding for the P-glycoprotein that is involved in transportation of the drug outside the cell, was found to be related to lower risk for cardiotoxicity (odds ratio (OR) 0.48; 95% confidence interval (CI): 0.23–1.00; p = 0.049) [18]. Another variant, namely, ABCC1 rs246221, was also identified as being correlated with cardiotoxicity in a retrospective study of 877 women [19]. In fact, LVEF decline correlated to heterozygous carriers (OR 1.59; 95% CI 1.1–2.3) [19].
In a study of 427 Asian women, the uridine glucuronosyltransferase 2B7 (UGT2B7) codon 161 SNP (C > T) was correlated with lower incidence of cardiotoxicity, defined as LVEF reduction ≥10% from baseline to <53%, heart failure, acute coronary artery syndrome, or fatal arrhythmia, among patients that received anthracycline and taxane adjuvant chemotherapy [20]. The TT homozygous alleles had the lowest incidence of cardiotoxicity (1.1%), followed by the heterozygous (3.1%), and finally the CC homozygous alleles, which were associated with the highest incidence (7.8%) [20]. In a multivariate analysis, the presence of a T-allele was found to be predictive of cardiotoxicity development, even when corrected for other known risk factors.
A meta-analysis, based on eight studies, investigated anthracycline-related cardiotoxicity (n = 84 genes related to n = 147 SNPs) and identified polymorphisms in three additional genes: (i) the ATP binding cassette subfamily C member 2 (ABCC2) gene, which encodes for the multidrug resistance protein 2 and is also involved in drug transport extracellularly (SNP rs8187710 OR 2.2; 95% CI 1.36–3.54); (ii) the cytochrome b-245 alpha chain (CYBA) gene that encodes a subunit of the NADPH oxidase enzyme complex (SNP rs4673 OR 1.55; 95% CI 1.05–2.30); and (iii) the Rac family small GTPase 2 (RAC2) gene that encodes for proteins that are involved in the generation of reactive oxygen species, among others (SNP rs13058338 OR 1.79; 95% CI 1.27–2.52) [21].
Another recent systematic review and meta-analysis on pharmacogenomic predictors for chemotherapy-induced cardiotoxicity included 41 studies, and all but three reported on anthracycline-containing regimens [22]. Among the 17 trials that were included in a meta-analysis, 14 SNPs were detected, of which 6 were found to be significantly associated with increased cardiotoxicity. Even here, some findings were consistent, demonstrating increased cardiotoxicity incidence in CYBA rs4673 polymorphism in ABCC2 rs8187710 and in RAC2 rs13058338. Additionally, the polymorphism rs776746 of the CYP3A5 gene and the ABCC1 rs45511401 polymorphism were also identified to be involved. SNPs reported to significantly affect risk for cardiotoxicity are summarized in Table 1.

Table 1.
Study characteristics, endpoint definition, and important findings in correlating genetic polymorphisms and risk for cardiotoxicity.
3. Targeted Therapies
3.1. Anti-HER2
Trastuzumab
Anti-HER2-therapy-induced cardiotoxicity is considered dose independent and largely reversible, without structural cardiomyocyte damage, and usually improves after treatment discontinuation and adequate cardiological care [3]. Despite being considered reversible, the risk of anti-HER2-related cardiotoxicity increases with the administration of anthracyclines concomitantly or in sequence within a short interval [23]. Treatment with trastuzumab has been described as disrupting the recovery of the cardiomyocytes and can lead to accentuated remodeling of the myocardium and fibrosis. Both in the adjuvant and metastatic setting, pertuzumab added to trastuzumab did not have a negative impact on cardiac safety [24,25], and trastuzumab emtansine (T-DM1) has also shown low cardiotoxicity potential [26,27].
A meta-analysis of 35 published trials by Leong et al. [28] identified 74 SNPs suggested to be related to cancer-therapy-induced hypertension, decrease in LVEF, and venous thromboembolism, and one in particular (HER2 codon 655 allele rs1136201) to augmenting the risk of trastuzumab-induced cardiotoxicity, as also demonstrated by a previous meta-analysis by the same group [21,28].
In a prospective observational study, the same HER2 codon 655 A > G polymorphism (or rs1136201) was also related to increased cardiotoxicity in an Asian population (univariate analysis OR 3.1, p = 0.008) [29]. In total, 91 patients were included and were followed during adjuvant therapy consisting of an anthracycline regimen first and subsequent initiation of trastuzumab. Twenty-six patients developed cardiotoxicity defined as LVEF decline of 10% from baseline and below 53% or on the basis of clinical criteria, i.e., diagnosis of heart failure, acute coronary artery syndrome, or fatal arrhythmia [29]. Carriers of the HER2 codon 655 A > G were eight times more likely to develop cardiotoxicity according to a multivariate analysis (p = 0.007).
On the contrary, a series with 140 Caucasian patients reporting findings of HER2 polymorphisms did not find a significant correlation of the codon 655 polymorphism and cardiotoxicity. The analysis was based on 29 patients with cardiotoxicity during adjuvant trastuzumab, defined as symptomatic heart failure or LVEF decline of 15% or of 10% if LVEF was less than 55%, as well as 111 controls [30]. An age-, ethnicity-, and hypertension-status-adjusted multivariable analysis demonstrated a correlation between the proline 1170 polymorphism of HER2 and risk for cardiotoxicity (adjusted OR = 2.60; 95% CI = 1.02 to 6.62, p = 0.046) [30]. However, no data on chemotherapy, hormonal therapy, or radiotherapy administration are provided, impeding correlation with other known confounding factors.
Similar findings were reported in a cohort of 177 European patients, 78 of which had cardiotoxicity defined according to LVEF decline as above [31]. Although mean LVEF among patients with HER2 codon 655 GG homologous allele was lower during adjuvant trastuzumab, this observation was not statistically significant. Of note, the 99 control patients were selected by chance, resulting in baseline imbalances between the groups. Twice as many patients with LVEF decline had left-sided breast cancer (60% vs. 36%, p = 0.002), which per se increases cardiotoxicity risk [31]. Additionally, the incidence of family history of heart disease (40 vs. 20%, p = 0.004) and lymph node burden (11% vs. 1%) were significantly higher in this group. Although not statistically significant, more patients in the control group had received non-anthracycline-containing regimens (13% vs. 8%).
A meta-analysis of four series with a total of 344 patients that received trastuzumab reported more than a five times higher risk of cardiotoxicity (varying definitions per study) in patients with the HER2 codon 655 AG polymorphism compared to the AA homologous one (OR 5.35; 95% CI 2.55–11.73, p < 0.0001) [32]. The AG polymorphism was significantly related to increased cardiotoxicity incidence in three of the four included cohorts [32,33,34,35]. The series published by Beauclair et al. did not report odds ratios, and interestingly, it was the only cohort with patients receiving trastuzumab in the metastatic setting [33].
Of note, the above-mentioned HER2 codon 655 polymorphism (rs1136201) has also been investigated as a breast-cancer-susceptible SNP, but this hypothesis has not been confirmed. Two small case–control studies in Austrian and Brazilian populations did not demonstrate a significant increase in breast cancer risk in patients carrying the SNP [36,37]. Moreover, a systematic review and meta-analysis also reported lack of such a relationship [38], although studies in controlled conditions and large populations would probably be required to safely exclude such a relationship.
Recently, Peddi et al. reported genotyping results from probably the largest dataset thus far, with 666 of the 3222 patients with early HER2-positive breast cancer included in the Breast Cancer International Research Group (BCIRG)-006 trial [39]. Of them, 224 patients received anthracycline and trastuzumab therapy in sequence, 226 did not receive anthracyclines, and 216 did not receive trastuzumab. Cardiac dysfunction was defined as LVEF decline of at least 10%, but also a broader definition of symptomatic heart failure was also used in a secondary analysis. No significant difference was seen among the investigated polymorphisms, rs1136201 (HER2 codon 655) and rs7853758 (SLC28A3 gene), although the AG heterologous allele demonstrated a small but significantly increased probability of cardiotoxicity when the group treated with anthracyclines and trastuzumab in sequence was analyzed separately [39].
The antioxidative-enzyme-encoding genes paraoxonase 1 (PON1), glutathione-S-transferases (GSTs), and catalase (CAT) stood out in a retrospective series of 101 patients with HER2-positive breast cancer that received adjuvant trastuzumab [40]. The vast majority (98%) of the patients had received anthracycline-containing regimens prior to trastuzumab administration, and 84% of them also underwent adjuvant radiotherapy, half of which was towards the left chest. In total, 36 patients had NT-proBNP equal to or higher than 125 ng/L [40], and two alleles of the PON1-gene were identified with opposing findings. Patients with at least PON1 rs662 polymorphism were characterized by significantly increased (≥125 ng/L) NT-proBNP at completion of the adjuvant trastuzumab (age-adjusted OR 5.41; 95% CI 2.12–13.78; p < 0.001), whereas those with PON1 rs854560 had significantly lower median NT-proBNP level (age-adjusted OR = 0.35; 95% CI 0.15–0.83; p = 0.017) [40]. In this study, LVEF levels did not correlate to any particular SNPs, but it is worth noting that there were only nine patients with LVEF lower than 55%, impeding any conclusions. Finally, carriers of the allele CAT rs1001179 were more likely to have symptoms comprising New York Heart Association (NYHA) class 2 (adjusted OR = 4.14, 95% CI 1.22–14.09).
3.2. CDK-4/6 Inhibitors
The cycline-D kinase 4/6 inhibitors palbociclib, abemaciclib, and ribociclib have revolutionized the management of metastatic estrogen receptor (ER)-positive breast cancer and enabled chemotherapy-free early line treatment regimens for patients without significant organ impairments due to their metastases, with comparable disease-related outcomes [41,42].
In the MONALEESA-2 trial comparing ribociclib plus letrozole to letrozole alone as first-line therapy, ribociclib was linked to QTc prolongation in 2.7% of the patients and none in the placebo-treated group [43]. In general, 3.3% of the ribociclib-treated patients experienced QTc interval prolongation of more than 480 ms. This finding does not seem to be a class action since the rest of the CDK4/6 inhibitors have thus far not demonstrated similar properties. Moreover, CDK4/6 inhibitors have not demonstrated significant increase in other types of cardiovascular toxicity such as coronary artery disease, thrombo-embolic events, or cardiomyopathy [44,45].
To the best of our knowledge, the possible predictive role of specific genotypes for ribociclib-associated QTc-prolongation has not been investigated. However, genetic variations known to be associated with drug-induced QTc-prolongation could probably be used to identify persons of increased risk, as described for anti-psychotic and thiazide-induced QTc-prolongation [46,47]. Candidate genes in this regard could be those encoding drug-metabolizing cytochrome P450 enzymes, drug transporters, or genes associated with QT interval duration and those correlated with congenital long QT syndromes [48]. For example, CYP3A is a cytochrome P450 enzyme essential for the hepatic metabolism of ribociclib, as well as palbociclib, and polymorphisms of the CYP3A gene could directly affect risk of toxicity and thereof QTc prolongation [49,50]. Future studies will hopefully shed light on this issue and aid in identifying patients at specifically high or low risk for QTc prolongation related to ribociclib and the risk for severe ventricular arrythmias.
3.3. Phosphatidylinositol 4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha Isoform (PIK3CA) Inhibitors
The PIK3CA inhibitor alpelisib was approved by the FDA in 2019 for administration in the metastatic setting, on the basis of the findings from the phase 3 Solar-1 trial [51,52,53]. Preclinical studies have suggested that PIK3CA inhibitors may be related to arrythmias, in contrast to drugs that inhibit other PI3K isoforms, through the induction of late sodium current [54,55]. Notably, the rate of cardiovascular events in the Solar-1 trial was very low: any grade cardiac arrest (one in the intervention group), cardiac failure (two in the control group), myocardial infarction (one in the control group), and sinus tachycardia (one in the control group) [51]. However, hyperglycemia is a frequently observed side effect, also known to increase the risk of cardiovascular morbidity in the long term. We were not able to identify studies that have investigated the relationship between the risk of treatment-related toxicity and specific genotypes of PIK3CA inhibitors.
3.4. Mammalian Target of Rapamycin (mTOR) Inhibitors
The results of the Breast Cancer Trials of Oral Everolimus-2 (BOLERO-2), comparing exemestane with and without the mTOR inhibitor everolimus, led to the approval of the combination therapy for the treatment of metastatic ER-positive breast cancer [56]. Everolimus has not been described as directly inflicting cardiac toxicity but is associated with increased risk of hyperglycemia, hyperlipidemia, and hypertension—known risk factors for cardiovascular disease [57].
In a study of 90 patients with metastatic breast cancer treated with everolimus and the aromatase inhibitor exemestane, SNPs (n = 12) in genes involved in everolimus pharmacokinetics and pharmacodynamics were genotyped and correlated with drug levels in the plasma and toxicity [58]. In this cohort, the CYP3A4*22 allele influenced plasma concentration of everolimus, and other SNPs were associated with treatment-related toxicities. However, no relationship between these genotypes and occurrence of cardiovascular events were reported in this study, and other data on cardiotoxicity are also lacking. CYP3A4*22 polymorphisms could, however, be candidates for future pharmacogenomic investigations on mTOR inhibitors and cardiotoxicity risk prediction.
3.5. Immune Checkpoint Inhibitors
Myocarditis in patients treated with immune checkpoint inhibitors is rare, occurring in about one percent of the patients and having an early onset, but its high mortality rates (50%) call for attention [59,60,61]. Importantly, no cases of myocarditis were reported in patients with advanced triple-negative breast cancer treated with atezolizumab or pembrolizumab in the Impassion130 and Keynote-522 trials, respectively, nor with pembrolizumab in the neoadjuvant setting in Keynote-355 [62,63,64]. Cytotoxic-T-lymphocyte-associated antigen-4 (CTLA-4) polymorphisms rs4553808, rs11571317, and rs231775 have been associated with response to therapy with ipilimumab for metastatic melanoma, and the CTLA-4 rs4553808 has also been correlated with higher incidence of endocrine side effects [65,66]. It is not possible to extrapolate whether the same polymorphisms could be predictive of cardiotoxicity, and given the rarity of the adverse event, it will probably be burdensome to map it, albeit important. Hence, we would like to suggest investigating CTLA-4 polymorphisms as potential predictors of immune-checkpoint-inhibitor-related myocarditis and cardiovascular toxicity.
Moreover, taking into consideration the mechanism of action of the checkpoint inhibitors, SNPs in genes related to the susceptibility loci of the major histocompatibility complex (MHC) could be potential candidates in the context. Polymorphisms shown to be related to autoimmune diseases have been suggested as potential predictive biomarkers of immune-related cardiotoxicity and other toxicities, but data supporting this hypothesis are currently lacking [67].
4. Endocrine Therapy
In general, cardiovascular effects of antihormonal drugs such as aromatase inhibitors (AIs) and selective estrogen receptor degraders (SERDs) seem to be relatively modest. In a large meta-analysis that included 19 RCTs with N = 62,345 patients treated with antihormonal therapies in the adjuvant setting after breast cancer surgery, AIs had a 19% (relative risk (RR) 1.19, 95% CI 1.07–1.34) increased risk of cardiovascular events compared with tamoxifen [68]. However, in this analysis, AIs were not associated with an increased risk for cardiovascular events compared with placebo in the extended-adjuvant setting (RR 1.01, 95% CI 0.85–1.20) [68]. Interestingly, in the adjuvant setting, tamoxifen was associated with a 33% (RR 0.67, 95% CI 0.45–0.98) decreased risk for cardiovascular events compared with placebo or no treatment [68]. On the basis of these data, it has been concluded that tamoxifen probably has cardioprotective effects.
SNPs in, for example, the drug metabolic enzyme cytochrome P450 19A1 (CYP19A1) and the estrogen receptor 1 (ERS1) gene have been related to the risk for AI-related side effects, but mainly in relation to the development of arthralgias, menopausal symptoms, and osteoporosis [69,70,71]. No studies identified as having explored the risks for cardiovascular events according to genetic polymorphisms have been identified.
However, we did identify one study that investigated the relationship between specific genotypes and the risk of the development of venous thrombosis related to treatment with the tamoxifen. In a study of 220 patients treated with tamoxifen, women with the XbaI (rs9340799) genotype and ESR1 Xbal/PvuII diplotype (rs9340799 and rs2234693) had an increased risk of a hazard ratio of 3.47 for the developing tamoxifen-associated thromboembolic events TTE (HR 3.47, 95% CI 0.97–12.44), results that persisted even after correction for other risk factors for TTE [72].
5. Radiotherapy
Left-sided chest radiotherapy is a known risk factor for short- or long-term cardiotoxicity. Radiotherapy can impact all layers of the heart (myocardium, pericardium, and endothelium) and can also lead to coronary artery stenosis [73]. In 57 breast cancer patients that underwent short partial breast irradiation (total dose 18–21Gy), a variant of the glutathione S-transferase pi 1 (GSTP1) gene was related to increased probability of grade 2 or above fibrosis or fat necrosis [74]. Similar analyses for cardiotoxicity and coronary artery stenosis are not available, but they should be considered.
6. Polygenic Risk Scores
The above-mentioned studies mainly focused on a few genetic markers associated with different degrees of cardiotoxicity. Genetic risk, however, most commonly constitutes of a combination of intermediate- and low-risk variants rather than a few high-risk genetic alterations [75], and risk prediction scores based on genome-wide association studies have been suggested to provide valuable information at the individual level [76]. A polygenic risk score including 1,745,180 variants was associated with higher risk of coronary artery disease in women with breast cancer, without baseline risk factors, even after adjustment for sociodemographic and medical confounders, including type of oncological treatment (HR 1.33; 95% CI 1.20–1.47) [77]. Among the 12,413 women included in this analysis, 38% had received adjuvant chemotherapy and 71% had received adjuvant radiotherapy. Moreover, adding a polygenic risk score to validated clinical risk scores for coronary artery disease in a large population improved the accuracy of risk prediction [78]. Although no information is provided on the individual risk of chemotherapy- or radiotherapy-related coronary artery disease or other types of cancer-therapy-induced cardiovascular toxicity, these two examples provide evidence of the usefulness of polygenic risk scores in the tailoring of the baseline risk assessment of the individual patient.
In addition to assessing the combined genetic risk from the PRS, co-medication and the impact of the interaction between different drugs and genes (drug–drug–gene interaction) has to be considered and is by far, less understood [79]. Interactions between co-administered drugs, interactions between genes, and drug–drug–gene interactions would need to be assessed in an algorithm for an improved cardiotoxicity risk prediction.
7. Discussion
The development of early and late cardiotoxicity is a potentially serious complication of breast cancer therapies that can lead to morbidity and can profoundly affect quality of life for cancer patients and survivors. Following improvement of breast cancer survival, there is an increasing number of breast cancer survivors, and thus diminishing long-term cardiotoxicity is essential. Estimation of the risk of development of cardiac toxicities from different cancer therapies should preferably be conducted prior to the initiation of therapy, and in this regard, pharmacogenomics provides a promising strategy that enables an individualized approach in establishing the risk–benefit balance for patients. This approach is currently advocated for in example fluoropyrimidine treatments, where SNPs of the gene of the drug metabolizing enzyme dihydropyrimidine dehydrogenase (DPD) are routinely assessed in many countries and, accordingly, dose modifications are considered [80].
We reviewed the literature for studies investigating the role of pharmacogenomics in identifying the risk level of cancer-therapy-related cardiotoxicity in breast cancer patients. Most studies involved patients treated with anthracyclines and/or HER2-targeted therapies, and modest correlations were found for specific variants in genes involved in drug metabolism, transport, and reactive oxygen species (ROS). Variants in such genes can modify the risk for development of cardiotoxicity through different mechanisms, for example, by altering the pharmacokinetics and pharmacodynamics of drugs that in turn lead to higher or prolonged drug plasma concentrations. Differences in the formation of ROS, which are thought to be one of the pathogenic factors in the development of anthracycline-induced cardiotoxicity, may lead to higher levels of ROS and thereby modify the risk for developing the specific toxicity.
Moreover, the polymorphism of the HER2 655 codon from A to G results in a valine (Val) amino acid instead of isoleucine (Ile), leading to a decreased tyrosine activity and thereby increased cell sensitivity to trastuzumab [81]. This could in turn lead also to increased sensitivity to adverse effects of trastuzumab on cardiomyocytes. Various studies have investigated correlations between HER2 655 polymorphisms and the risk of cardiotoxicity in patients treated with trastuzumab, but the findings of small retrospective series were not confirmed in a larger prospective cohort [39].
On the basis of the available data, no practical recommendations can yet be made to implement the use of pharmacogenomic strategies to ameliorate the risk for cardiac toxicity of very frequently used systemic drugs such as anthracyclines and trastuzumab in breast cancer management. Apparent issues related to the lack of conclusive findings may relate, amongst others, to the lack of a uniform definition of cardiotoxicity between different studies, as well as to heterogeneity in given systemic therapies. In addition, other candidate SNPs might prove to be more relevant, e.g., variants related to the development of other cardiac diseases such as dilated cardiomyopathy [82].
Data on relations between specific genetic variations and the development of cardiotoxicity from new-generation targeted therapies available in the management of breast cancer are still scarce. In the long term, pharmacogenomics could become a compliment to known and established stratification factors, not only for cardiotoxicity risk assessment but also for other types of severe and dose-limiting toxicities. A possible integrative model that could consist of a baseline assessment including established clinical risk factors for cardiovascular disease, testing for selected nucleotide polymorphisms, or individualizing treatment strategies on the basis of polygenic risk score calculations, as well as taking into consideration the expected benefits and risks of the oncological treatment indicated for the individual patient is presented in Figure 1.
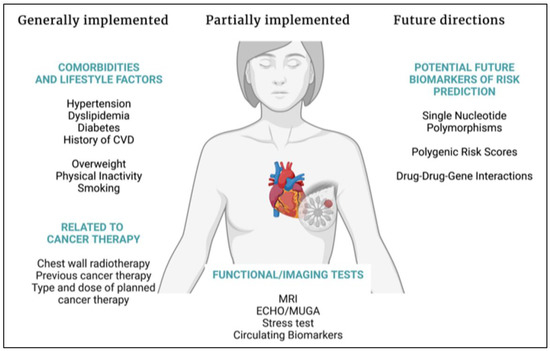
Figure 1.
Summary of factors to be assessed concordantly with oncological treatment planning. Abbreviations: CVD: cardiovascular disease, ECHO: echocardiogram, MRI: magnetic resonance imaging, MUGA: multigated acquisition scan. Created with BioRender.com.
This model could assist in the identification of the patients at most risk and inform us of the stratification for future studies investigating the utility of preventive strategies, as for example with beta-blocking agents or angiotensin receptor antagonists. This is an area that warrants further investigation since the exact impact of primary prevention for the prevention of cancer-treatment-related cardiotoxicity is still unclear [8].
Combining data from the available studies is cumbersome and should be performed with caution. Limitations in the comparison of the studies included in this review have been the heterogeneity in the cardiotoxicity definition, the methodology used to identify candidate genes and SNPs (wide versus directed analysis), the patient characteristics in each cohort and varying baseline risk for cardiotoxicity, and finally the heterogeneity in the assessed timepoints and length of follow-up. The advances in breast cancer management, as for example the shift to administrating anthracyclines and anti-HER2 agents in sequence and not concomitantly, as well as the increasing popularity of de-escalation efforts with anthracycline-free regimens in patients, will lower the risk for toxicity, and advances in radiotherapy techniques are expected to further decrease cardiotoxicity incidence. Thereof, small retrospective cohorts probably lack the power to identify genetic polymorphisms and undermine their significance. However, the use of pharmacogenomic parameters to predict and thereby prevent treatment-associated cardiotoxicity is tempting. There is an indisputably unmet need for prospective studies with well-defined patient populations, including a baseline cardiovascular phenotype, and outcome measures.
Suggestions for future studies include harmonization, including the definition of therapy-related cardiac dysfunction and the length of follow-up, and expansion of outcomes, including subclinical markers for cardiotoxicity. Additional candidate SNPs might be considered, e.g., genes known to predispose for other cardiac conditions, as well as enhanced/deepened explorations that take drug–drug–gene interactions into account [79]. The latter relates to potentially synergistically or antagonistically acting interactions that may further modify risks for treatment-related toxicities.
8. Conclusions
Moderate correlations with a number of candidate SNPs were found in relation to the development of cardiotoxicity caused by anthracyclines and/or HER2-targeted therapies, whereas data for other therapies is currently scarce, at best.
To facilitate clinical implementation of pharmacogenomics to predict cancer-treatment-related cardiotoxicity, future research efforts should aim to expand comparable approaches to other candidate SNPs as well as additional treatments such as checkpoint inhibitors and CDK4/6 inhibitors, and to include additional acute and chronic cardiotoxicity risk factors with the aim of creating a comprehensive baseline risk assessment. This will aid in further treatment tailoring and enhancing healthy cancer survivorship.
Author Contributions
Conceptualization, A.P.; writing—original draft preparation, R.A. and A.P.; writing—review and editing, R.A., S.B.-L. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from Percy Falks Stiftelse to A.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-an Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Sim, S.; Lövrot, J.; Lindh, J.D.; Bergh, J.; Xie, H. Effect of CYP2C19 and CYP2D6 genotype on tamoxifen treatment outcome indicates endogenous and exogenous interplay. Pharmacogenomics 2018, 19, 1027–1037. [Google Scholar] [CrossRef]
- Margolin, S.; Lindh, J.D.; Thorén, L.; Xie, H.; Koukel, L.; Dahl, M.-L.; Eliasson, E. CYP2D6 and adjuvant tamoxifen: Possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics 2013, 14, 613–622. [Google Scholar] [CrossRef]
- Sim, S.; Bergh, J.; Hellström, M.; Hatschek, T.; Xie, H. Pharmacogenetic impact of docetaxel on neoadjuvant treatment of breast cancer patients. Pharmacogenomics 2018, 19, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kimler, B.F.; O’Dea, A.P.; Nye, L.; Wang, Y.Y.; Yoder, R.; Staley, J.M.; Prochaska, L.; Wagner, J.; Amin, A.L.; et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin. Cancer Res. 2021, 27, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Tasaka, H.; Yu, K.-P.; Murphy, M.L.; Karnofsky, D.A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Huang, H.; Shafaattalab, S.; Maillet, A.; Lin, E.; Huang, K.; Laksman, Z.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 10363. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.K.; Karthikeyan, B.; Quiñones-Lombraña, A.; Blair, R.H.; Early, A.P.; Levine, E.G.; Sharma, U.C.; Blanco, J.G.; O’Connor, T. CBR3 V244M is associated with LVEF reduction in breast cancer patients treated with doxorubicin. Cardiooncology 2021, 7, 17. [Google Scholar] [CrossRef]
- Hertz, D.L.; Caram, M.V.; Kidwell, K.M.; Thibert, J.N.; Gersch, C.; Seewald, N.J.; Smerage, J.; Rubenfire, M.; Henry, N.L.; A Cooney, K.; et al. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics 2016, 17, 231–240. [Google Scholar] [CrossRef]
- Vulsteke, C.; Pfeil, A.M.; Maggen, C.; Schwenkglenks, M.; Pettengell, R.; Szucs, T.D.; Lambrechts, D.; Dieudonné, A.-S.; Hatse, S.; Neven, P.; et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 67–76. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Guo, Z.; Jiang, X.; Su, X.; Zhang, X. Correlation of UGT2B7 Polymorphism with Cardiotoxicity in Breast Cancer Patients Undergoing Epirubicin/Cyclophosphamide-Docetaxel Adjuvant Chemotherapy. Yonsei Med. J. 2019, 60, 30–37. [Google Scholar] [CrossRef]
- Leong, S.L.; Chaiyakunapruk, N.; Lee, S.W.H. Candidate Gene Association Studies of Anthracycline-induced Cardiotoxicity: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, G.; Guan, M.; Bapat, A.; Dai, Q.; Zhong, C.; Yang, T.; Luo, C.; An, N.; Liu, W.; et al. Potential Gene Association Studies of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 651269. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Trastuzumab Cardiotoxicity After Anthracycline Exposure Constitutes a Complex and Clinically Important Entity. JACC Heart Fail. 2019, 7, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Krop, I.E.; Suter, T.M.; Dang, C.T.; Dirix, L.; Romieu, G.; Zamagni, C.; Citron, M.L.; Campone, M.; Xu, N.; Smitt, M.; et al. Feasibility and Cardiac Safety of Trastuzumab Emtansine After Anthracycline-Based Chemotherapy As (neo) Adjuvant Therapy for Human Epidermal Growth Factor Receptor 2-Positive Early-Stage Breast Cancer. J. Clin. Oncol. 2015, 33, 1136–1142. [Google Scholar] [CrossRef]
- Pondé, N.; Ameye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; de Azambuja, E. Trastuzumab emtansine (T-DM1)-associated cardiotoxicity: Pooled analysis in advanced HER2-positive breast cancer. Eur. J. Cancer 2020, 126, 65–73. [Google Scholar] [CrossRef]
- Leong, S.L.; Chaiyakunapruk, N.; Tassaneeyakul, W.; Arunmanakul, P.; Nathisuwan, S.; Lee, S.W.H. Roles of pharmacogenomics in non-anthracycline antineoplastic-induced cardiovascular toxicities: A systematic review and meta-analysis of genotypes effect. Int. J. Cardiol. 2019, 280, 190–197. [Google Scholar] [CrossRef]
- Tan, L.; Su, X.; Li, X.; Li, H.; Hu, B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2020, 13, 286–294. [Google Scholar]
- Stanton, S.E.; Ward, M.M.; Christos, P.; Sanford, R.; Lam, C.; Cobham, M.V.; Donovan, D.; Scheff, R.J.; Cigler, T.; Moore, A.; et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer 2015, 15, 267. [Google Scholar] [CrossRef]
- Vazdar, L.; Gabrić, I.D.; Kruljac, I.; Pintarić, H.; Šeparović, R.; Biloš, L.S.K.; Pavlović, M.; Vuger, A.T.; Štefanović, M. Influence of Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in early-stage HER2 positive breast cancer. Sci. Rep. 2021, 11, 14395. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.G.; Dávila-Fajardo, C.L.; Martínez-González, L.J.; Carmona-Saez, P.; Pino, M.J.S.; Ramos, J.S.; Escobar, E.M.; Blancas, I.; Fernández, J.J.; Fernández, D.; et al. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: A meta-analysis. Pharm. Genom. 2015, 25, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Beauclair, S.; Formento, P.; Fischel, J.; Lescaut, W.; Largillier, R.; Chamorey, E.; Hofman, P.; Ferrero, J.; Pagès, G.; Milano, G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann. Oncol. 2007, 18, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.; Diorio, C.; Côté, M.-A.; Provencher, L.; Barabé, F.; Jacob, S.; St-Pierre, C.; Demers, E.; Tremblay-Lemay, R.; Nadeau-Larochelle, C.; et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013, 33, 2569–2576. [Google Scholar]
- Roca, L.; Diéras, V.; Roché, H.; Lappartient, E.; Kerbrat, P.; Cany, L.; Chieze, S.; Canon, J.-L.; Spielmann, M.; Penault-Llorca, F.; et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res. Treat. 2013, 139, 789–800. [Google Scholar] [CrossRef]
- Watrowski, R.; Castillo-Tong, D.C.; Wolf, A.; Schuster, E.; Fischer, M.B.; Speiser, P.; Zeillinger, R. HER2 Codon 655 (Ile/Val) Polymorphism and Breast Cancer in Austrian Women. Anticancer Res. 2015, 35, 5901–5904. [Google Scholar]
- Escórcio-Dourado, C.S.; Alves-Ribeiro, F.A.; Lima-Dourado, J.C.; dos Santos, A.R.; Pereira, R.D.O.; Tavares, C.B.; Silva, V.C.; Costa, P.V.L.; Soares-Júnior, J.M.; da Silva, B.B. Human Epidermal Growth Factor Receptor-2 gene polymorphism and breast cancer risk in women from the Northeastern region of Brazil. Clinics 2020, 75, e2360. [Google Scholar] [CrossRef]
- Dahabreh, I.J.; Murray, S. Lack of replication for the association between HER2 I655V polymorphism and breast cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2011, 35, 503–509. [Google Scholar] [CrossRef]
- Peddi, P.F.; Fasching, P.A.; Liu, D.; Quinaux, E.; Robert, N.J.; Valero, V.; Crown, J.; Falkson, C.; Brufsky, A.; Cunningham, J.M.; et al. Genetic Polymorphisms and Correlation with Treatment-Induced Cardiotoxicity and Prognosis in Patients with Breast Cancer. Clin. Cancer Res. 2022, 28, 1854–1862. [Google Scholar] [CrossRef]
- Marinko, T.; Konda, J.T.S.; Dolžan, V.; Goričar, K. Genetic Variability of Antioxidative Mechanisms and Cardiotoxicity after Adjuvant Radiotherapy in HER2-Positive Breast Cancer Patients. Dis. Markers 2020, 2020, 6645588. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial—PEARL☆. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Martel, S.; Maurer, C.; Lambertini, M.; Pondé, N.; De Azambuja, E. Breast cancer treatment-induced cardiotoxicity. Expert Opin. Drug Saf. 2017, 16, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Åberg, K.; Adkins, D.E.; Liu, Y.; McClay, J.L.; Bukszár, J.; Jia, P.; Zhao, Z.; Perkins, D.; Stroup, T.S.; Lieberman, J.A.; et al. Genome-wide association study of antipsychotic-induced QTc interval prolongation. Pharm. J. 2012, 12, 165–172. [Google Scholar] [CrossRef]
- Seyerle, A.A.; Sitlani, C.M.; Noordam, R.; Gogarten, S.M.; Li, J.; Li, X.; Evans, D.S.; Sun, F.; A Laaksonen, M.; Isaacs, A.; et al. Pharmacogenomics study of thiazide diuretics and QT interval in multi-ethnic populations: The cohorts for heart and aging research in genomic epidemiology. Pharm. J. 2018, 18, 215–226. [Google Scholar] [CrossRef]
- Niemeijer, M.N.; Berg, M.E.V.D.; Eijgelsheim, M.; Rijnbeek, P.R.; Stricker, B.H. Pharmacogenetics of Drug-Induced QT Interval Prolongation: An Update. Drug Saf. 2015, 38, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.E.; E R, N.; Danesi, R.; Girmenia, C.; Invernizzi, P.; Elvevi, A.; Uguccioni, M. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: A multidisciplinary approach is the key to success. Breast Cancer Res. Treat. 2019, 176, 483–494. [Google Scholar] [CrossRef]
- De Mattia, E.; Cecchin, E.; Guardascione, M.; Foltran, L.; Di Raimo, T.; Angelini, F.; D’Andrea, M.; Toffoli, G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3870–3896. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First PI3K Inhibitor for Breast Cancer. 2019. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-pi3k-inhibitor-breast-cancer (accessed on 12 April 2022).
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Meoli, D.F.; Moslehi, J.; Roden, D.M. Inhibition of the α-Subunit of Phosphoinositide 3-Kinase in Heart Increases Late Sodium Current and Is Arrhythmogenic. J. Pharmacol. Exp. Ther. 2018, 365, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ezeani, M.; Elom, S. Necessity to evaluate PI3K/Akt signalling pathway in proarrhythmia. Open Heart 2017, 4, e000596. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2011, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, G.; Roumpi, A.; Komporozos, C.; Syrigos, K. Everolimus as cancer therapy: Cardiotoxic or an unexpected antiatherogenic agent? A narrative review. Hell. J. Cardiol. 2018, 59, 196–200. [Google Scholar] [CrossRef]
- Pascual, T.; Apellaniz-Ruiz, M.; Pernaut, C.; Cueto-Felgueroso, C.; Villalba, P.; Álvarez, C.; Manso, L.; Inglada-Pérez, L.; Robledo, M.; Rodriguez-Antona, C.; et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS ONE 2017, 12, e0180192. [Google Scholar] [CrossRef]
- Castrillon, J.A.; Eng, C.; Cheng, F. Pharmacogenomics for immunotherapy and immune-related cardiotoxicity. Hum. Mol. Genet. 2020, 29, R186–R196. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.-E.; A Sosman, J.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Breunis, W.B.; Tarazona, E.; Chen, R.; Kiley, M.; Rosenberg, S.A.; Chanock, S.J. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J. Immunother. 2008, 31, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Ahlenstiel, G.; Piatkov, I. Pharmacogenetics of anticancer monoclonal antibodies. Cancer Drug Resist. 2019, 2, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Hoefsmit, E.P.; Rozeman, E.A.; Haanen, J.B.; Blank, C.U. Susceptible loci associated with autoimmune disease as potential biomarkers for checkpoint inhibitor-induced immune-related adverse events. ESMO Open 2019, 4, e000472. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.; Al-Qurashi, S.; Torabi, N.; Bouganim, N.; Suissa, S.; Azoulay, L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017, 28, 487–496. [Google Scholar] [CrossRef]
- Johansson, H.; The TEXT principal investigators; Gray, K.P.; Pagani, O.; Regan, M.M.; Viale, G.; Aristarco, V.; Macis, D.; Puccio, A.; Roux, S.; et al. Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Res. 2016, 18, 110. [Google Scholar] [CrossRef]
- Borrie, A.; Rose, F.A.; Choi, Y.-H.; Perera, F.E.; Read, N.; Sexton, T.; Lock, M.; Vandenberg, T.A.; Hahn, K.; Younus, J.; et al. Genetic and clinical predictors of arthralgia during letrozole or anastrozole therapy in breast cancer patients. Breast Cancer Res. Treat. 2020, 183, 365–372. [Google Scholar] [CrossRef]
- Baatjes, K.; Peeters, A.; McCaul, M.; Conradie, M.M.; Apffelstaedt, J.; Conradie, M.; Kotze, M.J. CYP19A1 rs10046 Pharmacogenetics in Postmenopausal Breast Cancer Patients Treated with Aromatase Inhibitors: One-year Follow-up. Curr. Pharm. Des. 2020, 26, 6007–6012. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Mccarty, C.A.; Wilke, R.A.; Glurich, I.; Engel, J.M.; Flockhart, D.A.; Nguyen, A.; Li, L.; Mi, D.; Skaar, T.C.; et al. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res. Treat. 2009, 115, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of coronary artery stenosis after radiation for breast cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Perentesis, J.P. Polymorphisms of drug metabolizing enzymes and markers of genotoxicity to identify patients with Hodgkin’s lymphoma at risk of treatment-related complications. Ann. Oncol. 2002, 13 (Suppl. 1), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.G. The common variants/multiple disease hypothesis of common complex genetic disorders. Med. Hypotheses 2004, 62, 309–317. [Google Scholar] [CrossRef]
- Sugrue, L.P.; Desikan, R.S. What Are Polygenic Scores and Why Are They Important? JAMA 2019, 321, 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.; Kaptoge, S.; Dennis, J.; Shah, M.; Tyrer, J.; Inouye, M.; Easton, D.F.; Pharoah, P.D.P. Genomic risk prediction of coronary artery disease in women with breast cancer: A prospective cohort study. Breast Cancer Res. 2021, 23, 94. [Google Scholar] [CrossRef]
- Elliott, J.; Bodinier, B.; Bond, T.A.; Chadeau-Hyam, M.; Evangelou, E.; Moons, K.G.M.; Dehghan, A.; Muller, D.; Elliott, P.; Tzoulaki, I. Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs. a Clinical Risk Score for Coronary Artery Disease. JAMA 2020, 323, 636–645. [Google Scholar] [CrossRef]
- Bruckmueller, H.; Cascorbi, I. Drug-Drug-Gene Interactions: A Call for Clinical Consideration. Clin. Pharmacol. Ther. 2021, 110, 549–551. [Google Scholar] [CrossRef]
- Meulendijks, D.; Henricks, L.; Sonke, G.; Deenen, M.J.; Froehlich, T.K.; Amstutz, U.; Largiader, C.; Jennings, B.; Marinaki, A.M.; Sanderson, J.D.; et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015, 16, 1639–1650. [Google Scholar] [CrossRef]
- Han, X.; Diao, L.; Xu, Y.; Xue, W.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. Association between the HER2 Ile655Val polymorphism and response to trastuzumab in women with operable primary breast cancer. Ann. Oncol. 2014, 25, 1158–1164. [Google Scholar] [CrossRef]
- Wasielewski, M.; van Spaendonck-Zwarts, K.Y.; Westerink, N.-D.L.; Jongbloed, J.D.H.; Postma, A.; A Gietema, J.; van Tintelen, J.P.; Berg, M.P.V.D. Potential genetic predisposition for anthracycline-associated cardiomyopathy in families with dilated cardiomyopathy. Open Heart 2014, 1, e000116. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).