Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Lines and Cell Culture
2.3. Construction of Plasmid
2.4. PCR Assay
2.5. Western Blotting
2.6. Animal Models and Vaccine Immunization
2.7. Preparation of Single-Cell Suspension
2.8. Flow Cytometry Analysis
2.9. Determination of CD8+ T Lymphocyte Proliferation
2.10. Cytotoxic T Lymphocyte (CTL)-Mediated Tumor Cell Killing Assay
2.11. ELISPOT Assay
2.12. In Vivo Depletion of CD8+ T Cell
2.13. IHC Staining
2.14. Statistical Analyses
3. Results
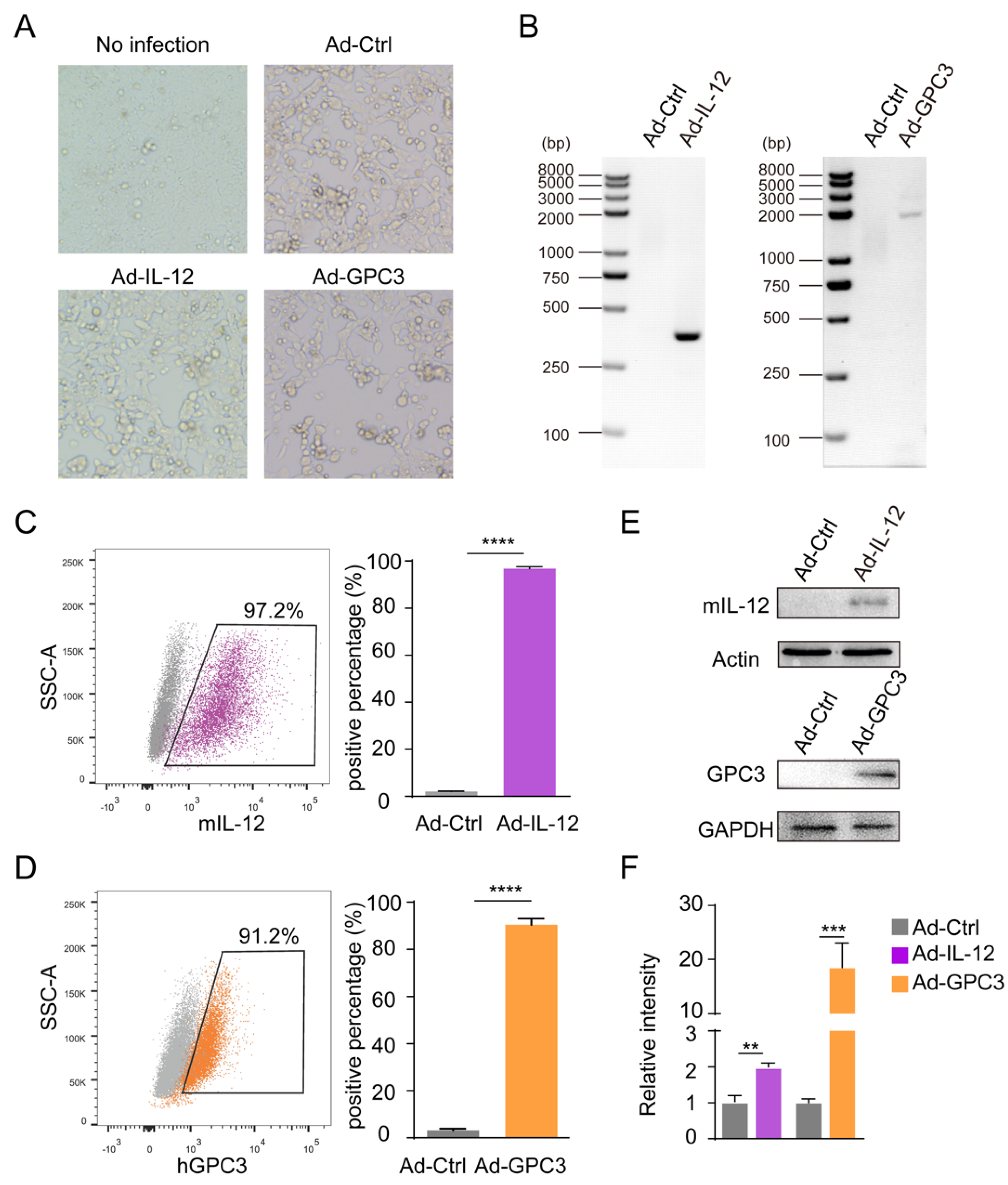
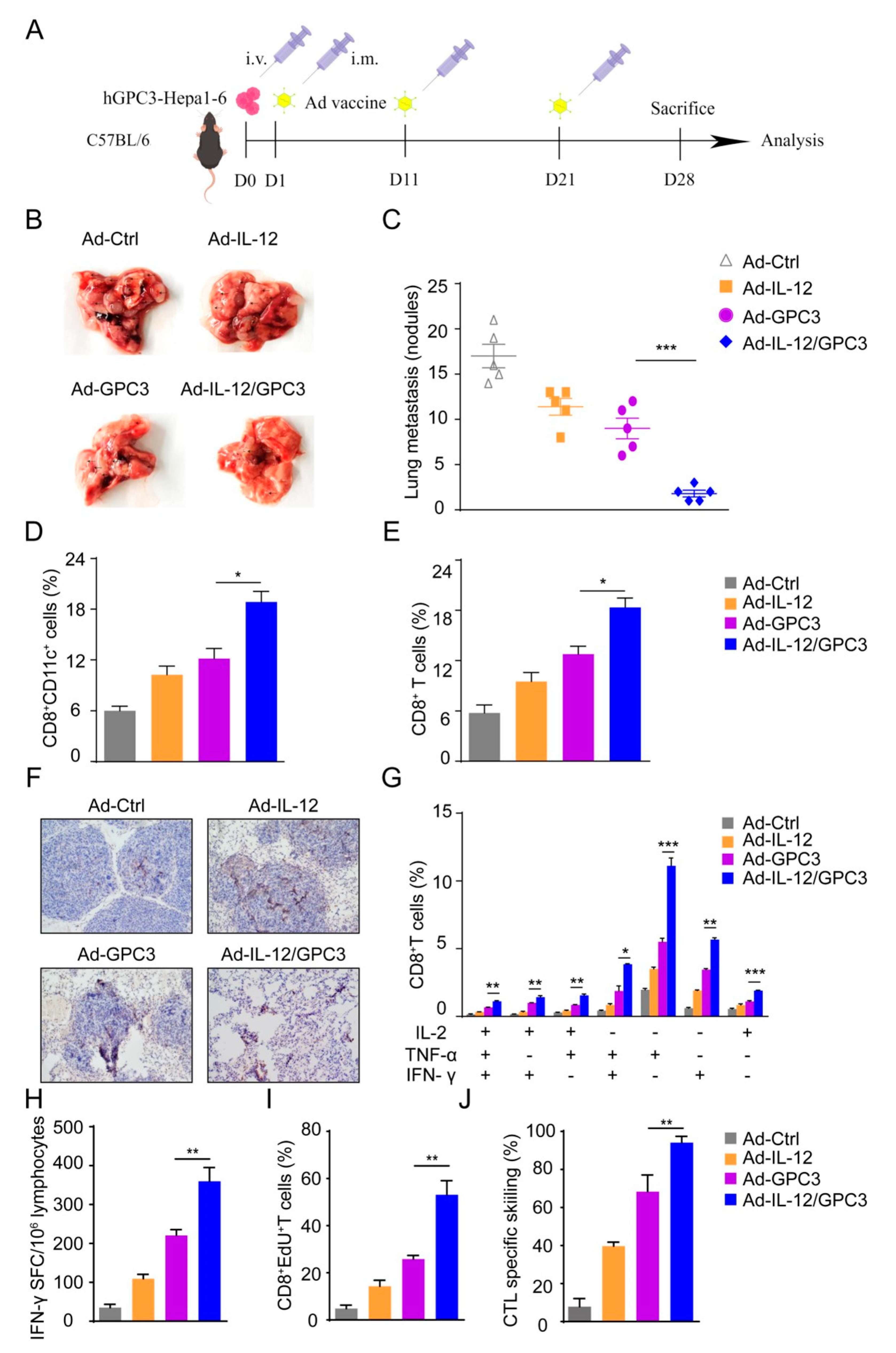
3.1. Preparation and Identification of Ad-IL-12/GPC3 Vaccine
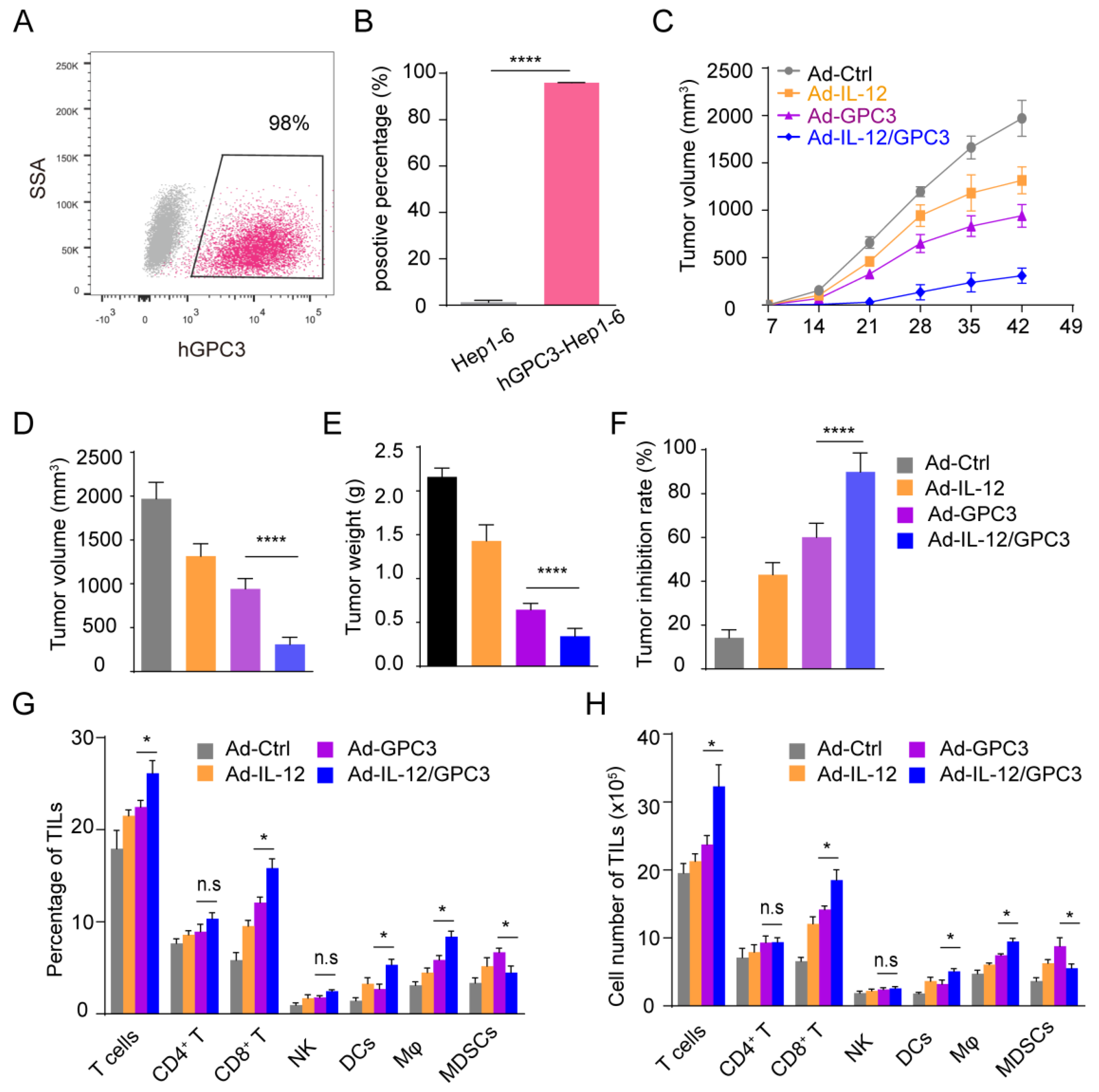
3.2. Ad-IL12/GPC3 Vaccine Inhibits Tumor Growth and Stimulates Cytotoxic T Cells
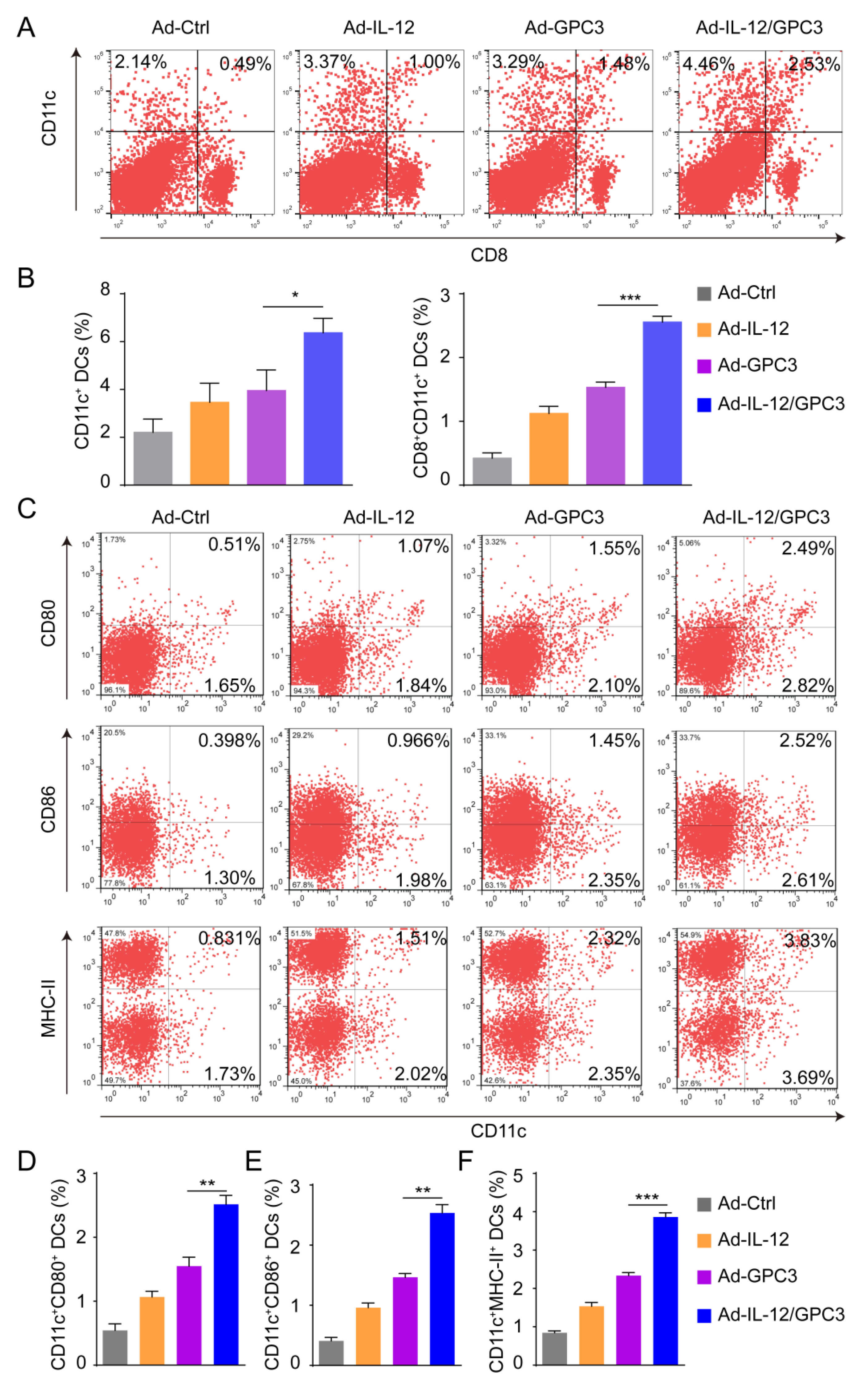
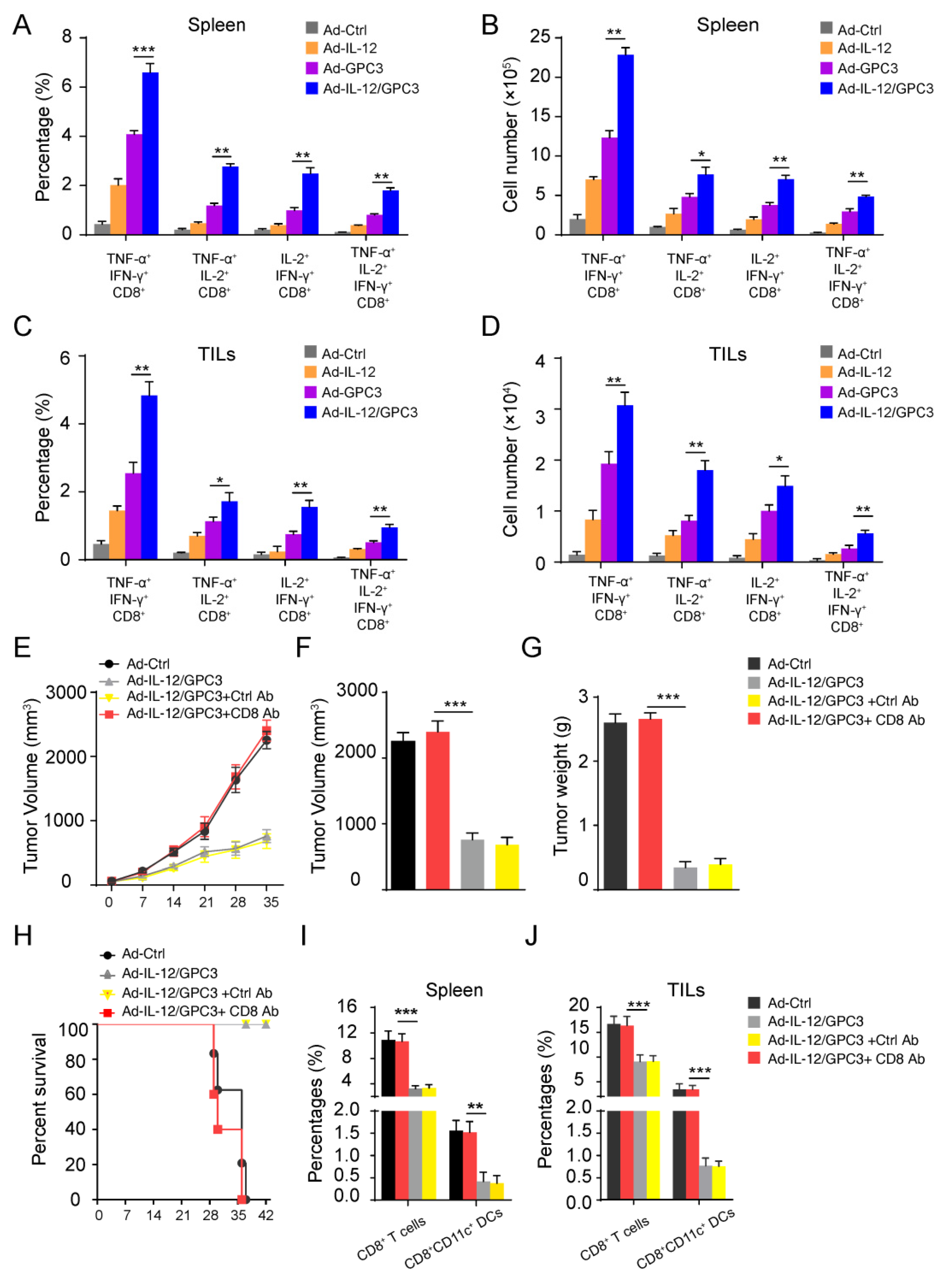
3.3. Co-Immunization with Ad-IL-12 Promotes the Increase and Maturation of CD11c+ and CD8+CD11c+ DC Subsets and Induces Strong Immune Responses of CD8 T Cells
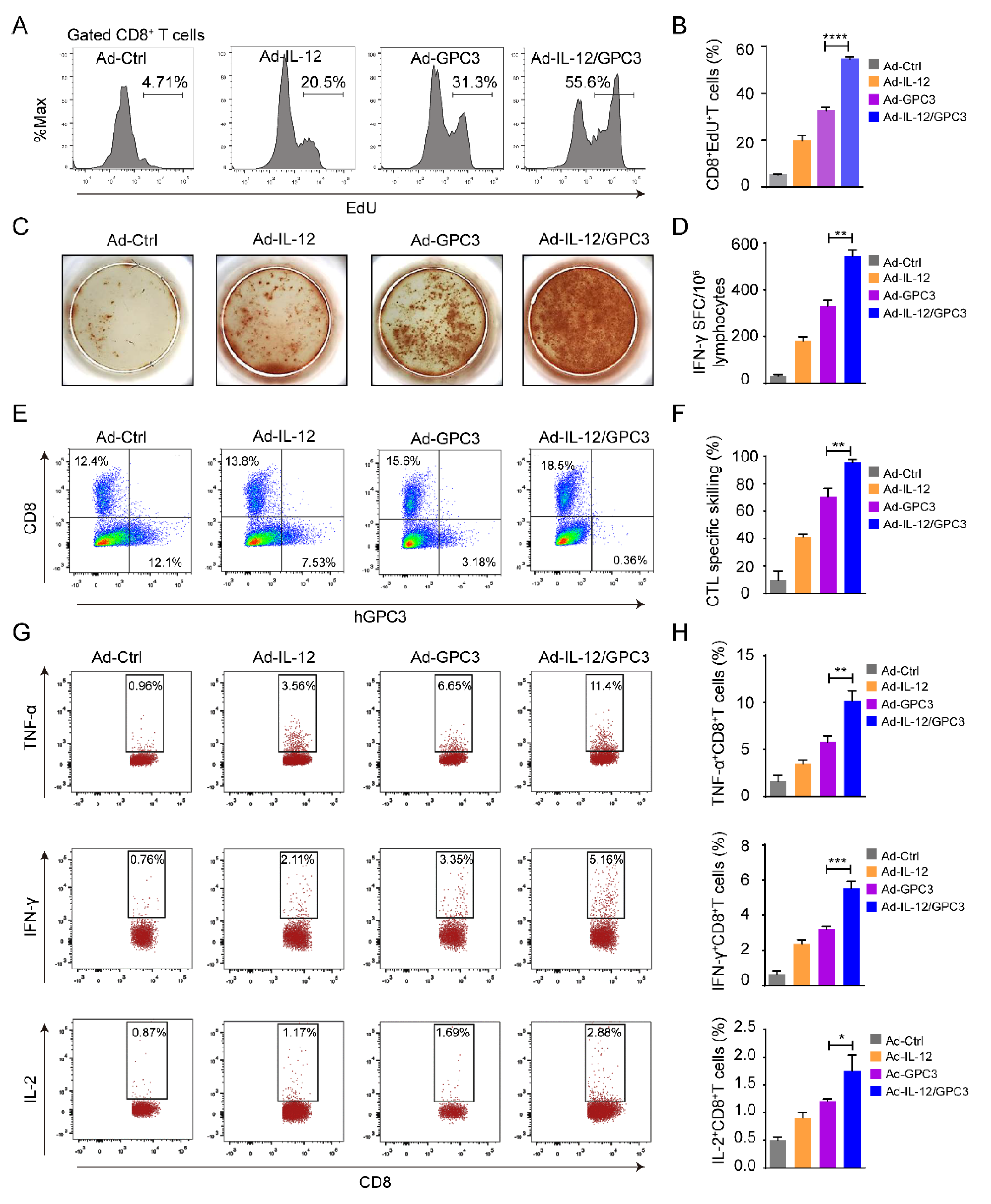
3.4. The Multifunctional CD8+ T Cell Immune Responses Are Necessary for the Anti-Tumor Effect
3.5. Ad-IL-12/hGPC3 Vaccine Inhibits Tumor Lung Metastasis by Promoting Multifunctional CD8+ T-Cell Responses in the Lung Metastasis Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Clavien, P.-A.; Petrowsky, H.; DeOliveira, M.L.; Graf, R. Strategies for safer liver surgery and partial liver transplantation. New Engl. J. Med. 2007, 356, 1545–1559. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World. J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Sherman, M. Hepatocellular carcinoma: Epidemiology, risk factors, and screening. Semin. Liver Dis. 2005, 25, 143–154. [Google Scholar] [CrossRef]
- Tatsumi, T.; Takehara, T.; Katayama, K.; Mochizuki, K.; Yamamoto, M.; Kanto, T.; Sasaki, Y.; Kasahara, A.; Hayashi, N. Expression of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on human hepatocellular carcinoma. Hepatology 1997, 25, 1108–1114. [Google Scholar] [CrossRef]
- Pillarisetty, V.G.; Shah, A.B.; Miller, G.; Bleier, J.I.; DeMatteo, R.P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 2004, 172, 1009–1017. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Nakatsura, T.; Yoshitake, Y.; Senju, S.; Monji, M.; Komori, H.; Motomura, Y.; Hosaka, S.; Beppu, T.; Ishiko, T.; Kamohara, H.; et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun. 2003, 306, 16–25. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, H.; Zheng, J.; Liu, Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J. Cancer 2020, 11, 2008–2021. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Yoshikawa, T.; Fujinami, N.; Saito, K.; Mizuno, S.; Sawada, Y.; Endo, I.; Nakatsura, T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology 2017, 6, e1346764. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichon, T.; Kulach, N.; Czapla, J.; Matuszczak, S.; Szala, S. Combined Tumor Cell-Based Vaccination and Interleukin-12 Gene Therapy Polarizes the Tumor Microenvironment in Mice. Arch. Immunol. Ther. Exp. 2015, 63, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Lasek, W.; Zagozdzon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef]
- Stern, A.S.; Podlaski, F.J.; Hulmes, J.D.; Pan, Y.C.; Quinn, P.M.; Wolitzky, A.G.; Familletti, P.C.; Stremlo, D.L.; Truitt, T.; Chizzonite, R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 1990, 87, 6808–6812. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Weiss, J.M.; Subleski, J.J.; Wigginton, J.M.; Wiltrout, R.H. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin. Biol. Ther. 2007, 7, 1705–1721. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Herndon, J.M.; Goedegebuure, P.; Belt, B.A.; Satpathy, A.T.; Fleming, T.P.; Hansen, T.H.; Murphy, K.M.; Gillanders, W.E. Cross-dressed CD8α+/CD103+ dendritic cells prime CD8+ T cells following vaccination. Proc. Natl. Acad. Sci. USA 2012, 109, 12716–12721. [Google Scholar] [CrossRef]
- Neves, H.; Kwok, H.F. Recent advances in the field of anti-cancer immunotherapy. BBA Clin. 2015, 3, 280–288. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.S. Rapid Advances in Immunotherapy to Treat Cancer. Clin. Pharmacol. Ther. 2018, 103, 540–544. [Google Scholar] [CrossRef]
- Sun, W. Recent advances in cancer immunotherapy. J. Hematol. Oncol. 2017, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Zhai, Y.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Topalian, S.L.; Restifo, N.P.; Seipp, C.A.; Einhorn, J.H.; et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl. Cancer Inst. 1998, 90, 1894–1900. [Google Scholar] [CrossRef]
- Bolhassani, A.; Safaiyan, S.; Rafati, S. Improvement of different vaccine delivery systems for cancer therapy. Mol. Cancer 2011, 10, 3. [Google Scholar] [CrossRef]
- Top, F.H., Jr. Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J. Biol. Med. 1975, 48, 185–195. [Google Scholar]
- Chaloner-Larsson, G.; Contreras, G.; Furesz, J.; Boucher, D.W.; Krepps, D.; Humphreys, G.R.; Mohanna, S.M. Immunization of Canadian Armed Forces personnel with live types 4 and 7 adenovirus vaccines. Can. J. Public Health. 1986, 77, 367–370. [Google Scholar]
- Stewart, A.K.; Lassam, N.J.; Quirt, I.C.; Bailey, D.J.; Rotstein, L.E.; Krajden, M.; Dessureault, S.; Gallinger, S.; Cappe, D.; Wan, Y.; et al. Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: Results of a phase 1 clinical trial. Gene. Ther. 1999, 6, 350–363. [Google Scholar] [CrossRef][Green Version]
- Kusumoto, M.; Umeda, S.; Ikubo, A.; Aoki, Y.; Tawfik, O.; Oben, R.; Williamson, S.; Jewell, W.; Suzuki, T. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol. Immunother. 2001, 50, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Gao, W.; Wang, R.; Chen, W.; Man, Y.-G.; Figg, W.D.; Wang, X.W.; Dimitrov, D.S.; Ho, M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2013, 110, E1083–E1091. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Puig, O.; Daniele, B.; Kudo, M.; Merle, P.; Park, J.-W.; Ross, P.; Peron, J.-M.; Ebert, O.; Chan, S.; et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2016, 65, 289–295. [Google Scholar] [CrossRef]
- Schmidt, N.; Neumann-Haefelin, C.; Thimme, R. Cellular immune responses to hepatocellular carcinoma: Lessons for immunotherapy. Dig. Dis. 2012, 30, 483–491. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Lin, C.C.; Chou, C.W.; Shiau, A.L.; Tu, C.F.; Ko, T.M.; Chen, Y.L.; Yang, B.C.; Tao, M.H.; Lai, M.D. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Lambricht, L.; Vanvarenberg, K.; de Beuckelaer, A.; van Hoecke, L.; Grooten, J.; Ucakar, B.; Lipnik, P.; Sanders, N.N.; Lienenklaus, S.; Preat, V.; et al. Coadministration of a Plasmid Encoding HIV-1 Gag Enhances the Efficacy of Cancer DNA Vaccines. Mol. Ther. 2016, 24, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, H.; Shi, B.; Di, S.; Sun, R.; Su, J.; Liu, Y.; Li, H.; Jiang, H.; Li, Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Li, Y.; Liu, J.; Chen, W.; Wei, Z.; Luo, Y.; Liu, H.; Qi, Y.; Wang, F.; Sui, J. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol. Ther. 2021, 29, 1572–1584. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, L.; Luo, Y.; Cai, Q.; Jiang, X.; Liao, C.; Wei, H. Optimization of GPC3-specific chimeric antigen receptor structure and its effect on killing hepatocellular carcinoma cells. Bioengineered 2021, 12, 3674–3683. [Google Scholar] [CrossRef]
- del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological properties and clinical application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef]
- Hu, J.; Sun, C.; Bernatchez, C.; Xia, X.; Hwu, P.; Dotti, G.; Li, S. T-cell Homing Therapy for Reducing Regulatory T Cells and Preserving Effector T-cell Function in Large Solid Tumors. Clin. Cancer Res. 2018, 24, 2920–2934. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, J.; Yang, T.; Li, Y.; Zhu, R.; Hou, Y.; Liu, Y. Co-delivery of IL-12 cytokine gene and cisplatin prodrug by a polymetformin-conjugated nanosystem for lung cancer chemo-gene treatment through chemotherapy sensitization and tumor microenvironment modulation. Acta. Biomater. 2021, 128, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.Z.; Ramos, H.J.; Davis, L.S.; Forman, J.; Farrar, J.D. IL-12 selectively programs effector pathways that are stably expressed in human CD8+ effector memory T cells in vivo. Blood 2011, 118, 3890–3900. [Google Scholar] [CrossRef]
- Halwani, R.; Boyer, J.D.; Yassine-Diab, B.; Haddad, E.K.; Robinson, T.M.; Kumar, S.; Parkinson, R.; Wu, L.; Sidhu, M.K.; Phillipson-Weiner, R.; et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J. Immunol. 2008, 180, 7969–7979. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Lu, Z.; Ding, J.; Jiang, N.; Wang, J.; Yang, J.; Song, J.; Chen, H.; Fang, L.; Li, H.; et al. Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma. Cancers 2022, 14, 4512. https://doi.org/10.3390/cancers14184512
Zheng Y, Lu Z, Ding J, Jiang N, Wang J, Yang J, Song J, Chen H, Fang L, Li H, et al. Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma. Cancers. 2022; 14(18):4512. https://doi.org/10.3390/cancers14184512
Chicago/Turabian StyleZheng, Yanyan, Zheng Lu, Jiage Ding, Nan Jiang, Jiawei Wang, Jie Yang, Jingyuan Song, Hongliang Chen, Lin Fang, Huizhong Li, and et al. 2022. "Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma" Cancers 14, no. 18: 4512. https://doi.org/10.3390/cancers14184512
APA StyleZheng, Y., Lu, Z., Ding, J., Jiang, N., Wang, J., Yang, J., Song, J., Chen, H., Fang, L., Li, H., Tian, H., Wang, G., Zheng, J., & Chai, D. (2022). Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma. Cancers, 14(18), 4512. https://doi.org/10.3390/cancers14184512






