Serum FSH as a Useful Marker for the Differential Diagnosis of Ovarian Granulosa Cell Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum Sample and Clinical Data Collection
2.3. Statistical Analyses
3. Results
3.1. Clinical and Pathological Findings
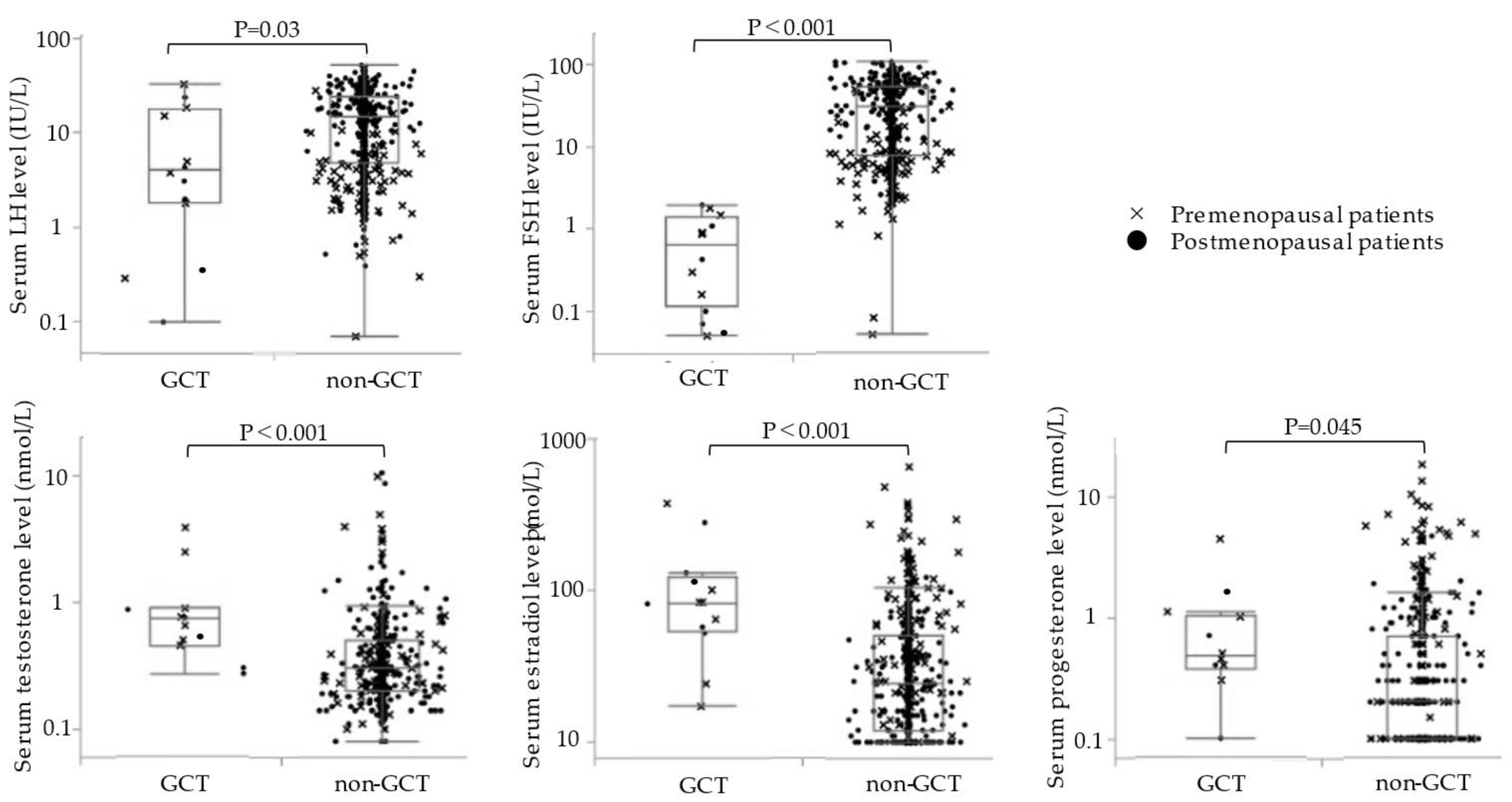
3.2. Serum Hormonal Levels in the GCT and Non-GCT Groups
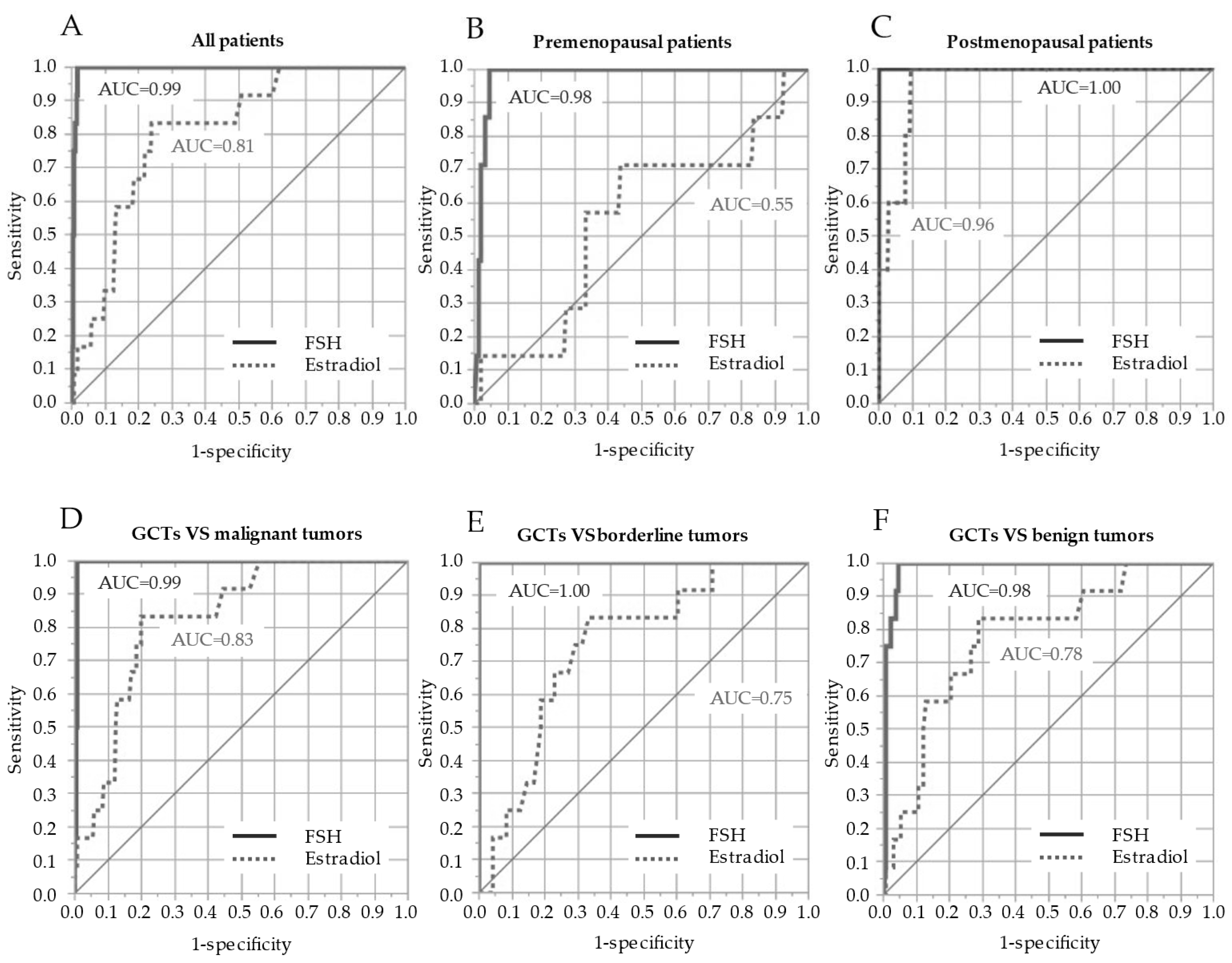
3.3. Accuracy of Serum FSH and Estradiol in GCT Diagnosis
4. Discussion
4.1. Key Findings of This Study
4.2. Relationship between the Serum Hormone Levels and GCTs
4.3. Diagnostic Value of the Serum FSH Level for GCTs
4.4. Role of FSH as a Tumor Marker to Follow up GCTs
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef]
- Young, R.H. Sex cord-stromal, steroid cell, and other ovarian tumors with endocrine, paraendocrine, and paraneoplastic manifestations. In Blaustein’s Pathology of the Female Genital Tract, 6th ed.; Kurman, R.J., Ellenson, L.H., Ronnett, B.M., Eds.; Springer: Boston, MA, USA, 2011; pp. 785–846. [Google Scholar]
- Pectasides, D.; Pectasides, E.; Psyrri, A. Granulosa cell tumor of the ovary. Cancer Treat. Rev. 2008, 34, 1–12. [Google Scholar] [CrossRef]
- Ottolina, J.; Ferrandina, G.; Gadducci, A.; Scollo, P.; Lorusso, D.; Giorda, G.; Breda, E.; Savarese, A.; Candiani, M.; Zullo, F.; et al. Is the endometrial evaluation routinely required in patients with adult granulosa cell tumors of the ovary? Gynecol. Oncol. 2015, 136, 230–234. [Google Scholar] [CrossRef]
- Kitamura, S.; Abiko, K.; Matsumura, N.; Nakai, H.; Akimoto, Y.; Tanimoto, H.; Konishi, I. Adult granulosa cell tumors of the ovary: A retrospective study of 30 cases with respect to the expression of steroid synthesis enzymes. J. Gynecol. Oncol. 2017, 28, e31. [Google Scholar] [CrossRef]
- Heinonen, P.K.; Koivula, T.; Rajaniemi, H.; Pystynen, P. Peripheral and ovarian venous concentrations of steroid and gonadotropin hormones in postmenopausal women with epithelial ovarian tumors. Gynecol. Oncol. 1986, 25, 1–10. [Google Scholar] [CrossRef]
- Matsuoka, A.; Tate, S.; Nishikimi, K.; Ishikawa, H.; Shozu, M. Elevated serum progesterone levels in postmenopausal women with mucinous ovarian tumors. Menopause 2016, 23, 544–549. [Google Scholar] [CrossRef]
- Colombo, N.; Parma, G.; Zanagnolo, V.; Insinga, A. Management of ovarian stromal cell tumors. J. Clin. Oncol. 2007, 25, 2944–2951. [Google Scholar] [CrossRef]
- Sun, H.D.; Lin, H.; Jao, M.S.; Wang, K.L.; Liou, W.S.; Hung, Y.C.; Chiang, Y.C.; Lu, C.H.; Lai, C.H.; Yu, M.H. A long-term follow-up study of 176 cases with adult-type ovarian granulosa cell tumors. Gynecol. Oncol. 2012, 124, 244–249. [Google Scholar] [CrossRef]
- NCCN. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 4 2022). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453 (accessed on 7 September 2022).
- Cheng, H.; Peng, J.; Yang, Z.; Zhang, G. Prognostic significance of lymphadenectomy in malignant ovarian sex cord stromal tumor: A retrospective cohort study and meta-analysis. Gynecol. Oncol. 2018, 148, 91–96. [Google Scholar] [CrossRef]
- Park, J.Y.; Jin, K.L.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, K.R.; Kim, Y.T.; Nam, J.H. Surgical staging and adjuvant chemotherapy in the management of patients with adult granulosa cell tumors of the ovary. Gynecol. Oncol. 2012, 125, 80–86. [Google Scholar] [CrossRef]
- Brown, J.; Sood, A.K.; Deavers, M.T.; Milojevic, L.; Gershenson, D.M. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecol. Oncol. 2009, 113, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.M.; Paley, P.; Garcia, R.; Goff, B.A. Patterns of spread and recurrence of sex cord-stromal tumors of the ovary. Gynecol. Oncol. 2011, 122, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.T., 3rd; Gaffey, T.A.; Malkasian, G.D., Jr.; Annegers, J.F. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet. Gynecol. 1980, 55, 231–238. [Google Scholar] [PubMed]
- Stenwig, J.T.; Hazekamp, J.T.; Beecham, J.B. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol. Oncol. 1979, 7, 136–152. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Gu, S.; Zhang, Y.; Liu, X.; Zhang, G. MR findings of primary ovarian granulosa cell tumor with focus on the differentiation with other ovarian sex cord-stromal tumors. J. Ovarian Res. 2018, 11, 46. [Google Scholar] [CrossRef]
- Elsherif, S.; Bourne, M.; Soule, E.; Lall, C.; Bhosale, P. Multimodality imaging and genomics of granulosa cell tumors. Abdom. Radiol. 2020, 45, 812–827. [Google Scholar] [CrossRef]
- Jung, S.E.; Rha, S.E.; Lee, J.M.; Park, S.Y.; Oh, S.N.; Cho, K.S.; Lee, E.J.; Byun, J.Y.; Hahn, S.T. CT and MRI findings of sex cord-stromal tumor of the ovary. Am. J. Roentgenol. 2005, 185, 207–215. [Google Scholar] [CrossRef]
- Stine, J.E.; Suri, A.; Gehrig, P.A.; Chiu, M.; Erickson, B.K.; Huh, W.K.; Soper, J.T.; Le, L.V.; Conner, M.G.; Kim, K.H. Pre-operative imaging with CA125 is a poor predictor for granulosa cell tumors. Gynecol. Oncol. 2013, 131, 59–62. [Google Scholar] [CrossRef]
- Robertson, D.M.; Lee, C.H.; Baerwald, A. Interactions between serum FSH, inhibin B and antral follicle count in the decline of serum AMH during the menstrual cycle in late reproductive age. Endocrinol. Diabetes Metab. 2020, 4, e00172. [Google Scholar] [CrossRef]
- Hildebrandt, R.H.; Rouse, R.V.; Longacre, T.A. Value of inhibin in the identification of granulosa cell tumors of the ovary. Hum. Pathol. 1997, 28, 1387–1395. [Google Scholar] [CrossRef]
- Mom, C.H.; Engelen, M.J.; Willemse, P.H.; Gietema, J.A.; ten Hoor, K.A.; de Vries, E.G.; van der Zee, A.G.J. Granulosa cell tumors of the ovary: The clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol. Oncol. 2007, 105, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.L.; Burger, H.G.; Mamers, P.; Jobling, T.; Bangah, M.; Quinn, M.; Grant, P.; Day, A.J.; Rome, R.; Campbell, J.J. Elevated serum inhibin concentrations in postmenopausal women with ovarian tumors. N. Engl. J. Med. 1993, 329, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Cahir, N.; Burger, H.G.; Mamers, P.; Groome, N. Inhibin forms in serum from postmenopausal women with ovarian cancers. Clin. Endocrinol. 1999, 50, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Murdock, C.; Allard, J.; Cisar, M.; Reid, E.; Nieman, L.; Segar, J. Pseudo-isolated FSH deficiency caused by an inhibin B-secreting granulosa cell tumour: Case report. Hum. Reprod. 2003, 18, 502–505. [Google Scholar] [CrossRef]
- Gica, C.; Cigaran, R.G.; Botezatu, R.; Panaitescu, A.M.; Cimpoca, B.; Peltecu, G.; Gica, N. Secondary amenorrhea and infertility due to an inhibin B producing granulosa cell tumor of the ovary. A rare case report and literature review. Medicina 2021, 57, 829. [Google Scholar] [CrossRef]
- Adefris, M.; Fekadu, E. Postmenopausal mild hirsutism and hyperandrogenemia due to granulosa cell tumor of the ovary: A case report. J. Med. Case Rep. 2017, 11, 242. [Google Scholar] [CrossRef]
- Cohen, I.; Nabriski, D.; Fishman, A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neoplasm in postmenopausal women. Gynecol. Oncol. Rep. 2015, 15, 12–15. [Google Scholar] [CrossRef][Green Version]
- Haltia, U.M.; Hallamaa, M.; Tapper, J.; Hynninen, J.; Alfthan, H.; Kalra, B.; Ritvos, O.; Heikinheimo, M.; Unkila-Kallio, L.; Perheentupa, A.; et al. Roles of human epididymis protein 4, carbohydrate antigen 125, inhibin B and anti-Mullerian hormone in the differential diagnosis and follow-up of ovarian granulosa cell tumors. Gynecol. Oncol. 2017, 144, 83–89. [Google Scholar] [CrossRef]
- Robertson, D.M.; Pruysers, E.; Jobling, T. Inhibin as a diagnostic marker for ovarian cancer. Cancer Lett. 2007, 249, 14–17. [Google Scholar] [CrossRef]
- Geerts, I.; Vergote, I.; Neven, P.; Billen, J. The role of inhibins B and antiMullerian hormone for diagnosis and follow-up of granulosa cell tumors. Int. J. Gynecol. Cancer 2009, 19, 847–855. [Google Scholar] [CrossRef]
- Jobling, T.; Mamers, P.; Healy, D.L.; MacLachlan, V.; Burger, H.G.; Quinn, M.; Rome, R.; Day, A.J. A prospective study of inhibin in granulosa cell tumors of the ovary. Gynecol. Oncol. 1994, 55, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Luisi, S.; Pautier, P.; Sabourin, J.C.; Rey, R.; Lhomme, C.; Bidart, J.M. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J. Clin. Endocrinol. Metab. 1998, 83, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.L.; Lee, M.M.; Scully, R.E.; Moncure, A.C.; Hirakawa, T.; Goodman, A.; Muntz, H.G.; Donahoe, P.K.; MacLaughlin, A.; Fuller, A.F., Jr. Mullerian inhibiting substance as a marker for ovarian sex-cord tumor. N. Engl. J. Med. 1992, 326, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Boggess, J.F.; Soules, M.R.; Goff, B.A.; Greer, B.E.; Cain, J.M.; Tamimi, H.K. Serum inhibin and disease status in women with ovarian granulosa cell tumors. Gynecol. Oncol. 1997, 64, 64–69. [Google Scholar] [CrossRef]
- Long, W.Q.; Ranchin, V.; Pautier, P.; Belville, C.; Denizot, P.; Cailla, H.; Lhomme, C.; Picard, J.Y.; Bidart, J.M.; Rey, R. Detection of minimal levels of serum anti-Mullerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J. Clin. Endocrinol. Metab. 2000, 85, 540–544. [Google Scholar] [CrossRef]
- Leung, D.T.H.; Fuller, P.J.; Chu, S. Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors. Int. J. Biochem. Cell Biol. 2016, 72, 51–54. [Google Scholar] [CrossRef]
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E.; et al. Mutation of FOXL2 in glanulosa cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef]
- Kim, M.S.; Hur, S.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of ovary and other human cancers. J. Pathol. 2010, 221, 147–152. [Google Scholar] [CrossRef]
- D’Angelo, E.; Mozos, A.; Nakayama, D.; Espinosa, I.; Catasus, L.; Munoz, J.; Prat, J. Prognostic significance of FOXL2 mutation and mRNA expression in adult and Juvenile granulosa cell tumors of the ovary. Mod. Pathol. 2011, 24, 1360–1367. [Google Scholar] [CrossRef]
- Rosario, R.; Wilson, M.; Cheng, W.T.; Payne, K.; Cohen, P.A.; Fong, P.; Shelling, A.N. Adult granulosa cell tumours (GCT): Clinicopathological outcomes including FOXL2 mutational status and expression. Gynecol. Oncol. 2013, 131, 325–329. [Google Scholar] [CrossRef]
- Oseto, K.; Suzumori, N.; Nishikawa, R.; Nishikawa, H.; Arakawa, A.; Ozaki, Y.; Asai, H.; Kawai, M.; Mizuno, K.; Takahashi, S.; et al. Mutational analysis of FOXL2 p.C134W and expression of bone morphogenetic protein 2 in Japanese patients with granulosa cell tumor of ovary. J. Obstet. Gynecol. Res. 2014, 40, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
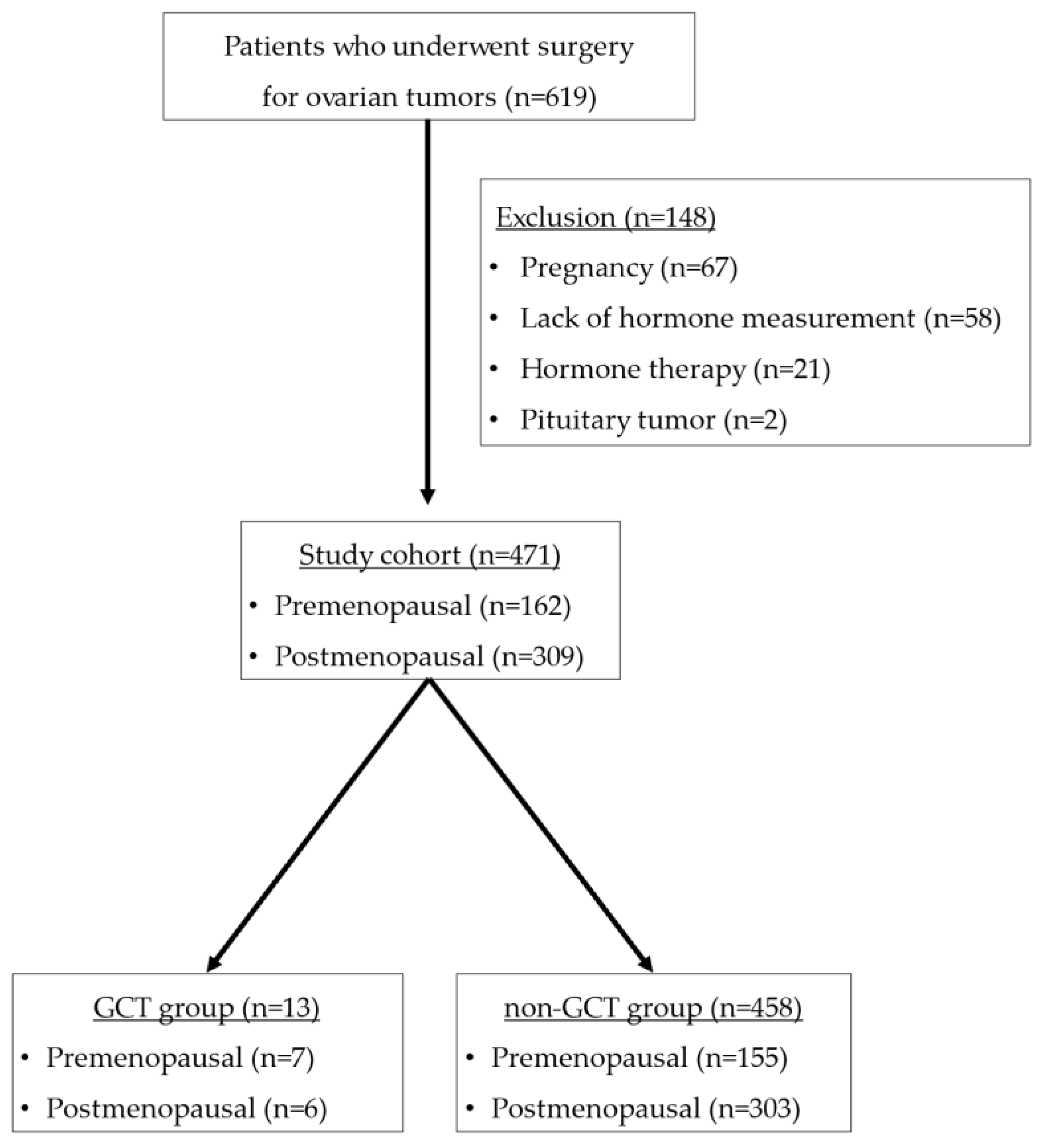
| Characteristic | All (n = 471) | GCT Group (n = 13) | Non-GCT Group (n = 458) | p-Value |
|---|---|---|---|---|
| Age (years) | 57 (8–92) | 44 (23–83) | 58 (8–92) | 0.08 |
| Menopausal status | ||||
| Premenopausal | 162 (34) | 7 (54) | 155 (34) | 0.1 |
| Postmenopausal | 309 (66) | 6 (46) | 303 (66) | |
| Menopause age (years) | 50 (40–60) | 51 (43–56) | 50 (40–60) | 0.6 |
| Time from menopause (years) | 14 (2–39) | 26 (3–33) | 14 (2–39) | 0.4 |
| Parity | ||||
| Nullipara | 152 (32) | 8 (62) | 144 (31) | 0.03 |
| Multipara | 319 (68) | 5 (38) | 314 (69) | |
| BMI (kg/m2) | 22 (14–42) | 21 (16–35) | 22 (14–42) | 0.1 |
| Preoperative CA125 (U/mL) | 83 (5–67,000) | 17 (6–240) | 90 (5–67,000) | 0.005 |
| Preoperative CA19-9 (U/mL) | 20 (0.1–75,000) | 10 (4–41) | 21 (0.1–75,000) | 0.03 |
| Features | Multivariate Logistic Regression Analysis | Receiver Operating Characteristic Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | AUC (95% CI) | Specificity | Sensitivity | |
| FSH | 0.046 | 0.0026–0.22 | 0.004 | 0.99 (0.981–0.997) | 100 | 98 |
| Testosterone | 0.44 | 0.12–1.4 | 0.2 | |||
| Estradiol | 0.98 | 0.96–0.998 | 0.046 | 0.81 (0.677–0.893) | 83 | 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, A.; Tate, S.; Nishikimi, K.; Kobayashi, T.; Otsuka, S.; Shozu, M. Serum FSH as a Useful Marker for the Differential Diagnosis of Ovarian Granulosa Cell Tumors. Cancers 2022, 14, 4480. https://doi.org/10.3390/cancers14184480
Matsuoka A, Tate S, Nishikimi K, Kobayashi T, Otsuka S, Shozu M. Serum FSH as a Useful Marker for the Differential Diagnosis of Ovarian Granulosa Cell Tumors. Cancers. 2022; 14(18):4480. https://doi.org/10.3390/cancers14184480
Chicago/Turabian StyleMatsuoka, Ayumu, Shinichi Tate, Kyoko Nishikimi, Tastuya Kobayashi, Satoyo Otsuka, and Makio Shozu. 2022. "Serum FSH as a Useful Marker for the Differential Diagnosis of Ovarian Granulosa Cell Tumors" Cancers 14, no. 18: 4480. https://doi.org/10.3390/cancers14184480
APA StyleMatsuoka, A., Tate, S., Nishikimi, K., Kobayashi, T., Otsuka, S., & Shozu, M. (2022). Serum FSH as a Useful Marker for the Differential Diagnosis of Ovarian Granulosa Cell Tumors. Cancers, 14(18), 4480. https://doi.org/10.3390/cancers14184480






