Simple Summary
The incidence and mortality of hepatocellular carcinoma (HCC) in Asia are among the world leaders. By understanding the changes in prevalence and influencing factors of HCC, we can better understand the current situation in Asia and take measures to reduce the incidence.
Abstract
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, with high morbidity and mortality, and the incidence is on the rise. HCC imposes a heavy healthcare burden on Asian countries due to the presence of multiple HCC risk factors in this area. Chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, non-alcoholic liver disease (NAFLD), aflatoxin and alcohol intake are the causes of HCC that cannot be ignored. Compared with the pre-vaccination era, universal vaccination of newborns reduces the incidence of HCC. Anti-viral therapy with nucleos(t)ide analogues also causes a decline in HCC incidence. Early screening and direct-acting antiviral agent are beneficial to the prevention and treatment of HCV. For HCC caused by NAFLD and other reasons, lifestyle changes are imperative. This paper introduces the epidemiological trends of HCC in Asia and highlight future efforts. Focusing on prevention may be the most effective way to improve the prognosis of this hard-to-treat cancer.
1. Introduction
Liver cancer is one of the leading causes of cancer-related death in the world. The World Health Organization estimated that in 2020 there were about 905,677 new cases of liver cancer and about 830,180 people died of liver cancer in the world [1]. Hepatocellular carcinoma (HCC) is mostly mentioned when it comes to liver cancer and causes an important living and economic burden around the world, especially in Asia [2,3].
In Asia, liver cancer is the fifth most common cancer and the second most common cause of cancer-related death [1]. In 2020, there were about 609,596 new cases of liver cancer in Asia, accounting for about 72.5% of the total incidence of liver cancer in the world, with Age-Standardized Rate (ASR) per 100,000 of 11.6. As for mortality, liver cancer-related deaths in Asia were about 566,269, accounting for 72.4% of the total liver cancer deaths in the world, with ASR of 10.7 [1]. The incidence and mortality of liver cancer are higher in Asian men than in Asian women. Asian men have the fourth highest incidence of liver cancer and the second highest mortality. While for Asian women, the incidence of liver cancer ranks seventh and the mortality ranks sixth. In 2020, there were 471,999 cases of liver cancer reported in men and 184,993 in women in Asia. In terms of liver cancer deaths, 437,697 Asian men and 184,993 Asian women died of liver cancer [1].
The incidence of liver cancer in Asian populations shows different trends (Figure 1). East Asian regions (e.g., South Korea, Japan and China) and Southeast Asian regions (e.g., the Philippines) have significant declines of the incidence rates of liver cancer, as reflected by average annual percent change (AAPC) [4]. A study surveyed eight countries in Asia and discovered that six of them has a declined incidence rate since 1978, including China (AAPC = −1.6%), South Korea (AAPC = −2.2%) and the Philippines (AAPC = −1.7%), but rates has leveled off in South-Western Asia (e.g., Israel) [4]. Although most liver cancers turn out to be HCC, it is worth noting that HCC only takes up about 27% of liver cancers in Thailand. Although the incidence of primary liver cancer has grown in Thailand, the incidence of HCC has declined sharply since 2000 [4]. Moreover, GLOBOCAN 2020 shows a worrying trend that the incidence of liver cancer in many countries increasing significantly in recent years, including Iran, Afghanistan, Qatar, Azerbaijan, Iraq, and Nepal. Local governments should pay attention to this rising trend to avoid a large-scale epidemic of liver cancer [5].
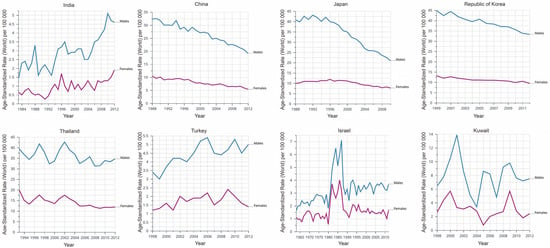
Figure 1.
Dynamic changes of the incidence of liver cancer in different Asian countries, based on CANCER OVER TIME|IARC (International Agency for Research on Cancer). Data are presented as incidence per 100,000.
2. Viral Hepatitis-Related HCC
2.1. Hepatitis B Virus (HBV)
HBV activates oncogenes and suppresses anti-oncogenes by integrating the genomes of oncogenic viral proteins into the host genome, leading to the occurrence of HCC [6]. Even for patients without cirrhosis, HCC occurs frequently in chronic HBV carriers, suggesting that HBV could be carcinogenic itself [7]. Hepatitis B virus infection is moderately to highly prevalent in the Asia-Pacific region, accounting for 75% of chronic hepatitis B positive patients globally [8]. We could find the highest incidence of liver cancer in East Asian countries, accompanied by a high rate of HBV [9]. The prevalence of HBV is almost 18% in China, 5% in India and 4% in Japan [10,11]. However, HBV vaccination in neonates has significantly affected the epidemiology of chronic HBV infection, which has declined in most parts of the world from 1990 to 2005, including Asian countries [12].
In Singapore, HBV infection is accounted for most liver disease and 66.6% of liver cancer-related deaths according to the GHE 2015 dataset [13]. Luckily, this situation has changed. The incidence of liver cancer in male decreased from 27.4 cases per 100,000 population in 1973–1977 to 17.2 cases in 2008–2012, while female incidence decreased from 6.9 cases per 100,000 population in 1973–1977 to 4.8, which may be attributed to the inclusion of HBV vaccination as part of Singapore’s national childhood immunization program in 1985 [14].
In 2001–2004, among those born in 1977–1980, the mortality rate due to HCC decreased significantly between the ages of 5 and 29 years (0·81 deaths per 100,000 for carcinoma to 0·05 per 100,000) in Taiwan, thanks to the immunization campaign in 1984 [15]. Of note, although the incidence of hepatocellular carcinoma has decreased since the 1984 immunization campaign, a community-based outreach screening in Taiwan estimated a total number of around 3 million HBsAg carriers from 1996 to 2005, indicating that the number of HBV carriers is still large [16].
The incidence of HCC in all age groups in Hong Kong has declined significantly over the past 25 years, partly due to a decline in HBV infection rates since the introduction of universal HBV vaccination in 1988. However, chronic HBV infection was still the main reason causing HCC in Hong Kong between 1992 and 2006, taking up 80% of all causes of HCC in 1992 and 78% in 2006 [17].
In addition to the promotion of vaccines, using nucleos(c)ide analogs (NAs) to inhibit the virus can improve liver inflammation and reverse liver fibrosis, thereby preventing chronic hepatitis from developing into cirrhosis and eventually developing into HCC [18]. Numerous studies have demonstrated the role of NAs in reducing HBV-related HCC. A Japanese study showed that the 5-year cumulative incidence of HCC in chronic hepatitis B (CHB) patients treated with entecavir was significantly lower than that in control groups [19]. In Hong Kong, entecavir-treated patients had a significantly lower risk of developing HCC compared with untreated cirrhosis patients [20]. A study in Taiwan also confirmed the role of NA in the development of HCC. CHB patients treated with NAs had significantly lower 7-year HCC incidence compared with untreated patients [21].
2.2. Hepatitis C Virus (HCV)
Chronic HCV infection is also an important cause of HCC. The prevalence of HCV is 10% in Mongolia, 6.7% in Pakistan, 2.7% in Thailand, 1.3% in China and 1% in India [22]. In 2012 HCV instead of HBV has become the most common cause of HCC in Pakistan and up to 58% of cases of HCC are attributable to HCV [23]. The main causes are the reuse of syringes and medical devices, contaminated blood or blood products, and the reuse of razors [24]. Notably, mass screening, treatment of 510,000 HCV cases per year in Pakistan, effective awareness campaigns and use of sterile equipment is estimated to significantly reduce HCV burden and prevent 116,000 new liver cancers over the next 15 years [25].
From 1958 to 1970, there was no significant change in hepatitis related HCC mortality in Japan. However, the incidence and death of HCC has increased exponentially since 1970 and peaked in 21st century, most likely due to the increase in HCV infections following World War II [26]. After a plateau in 2002–2004, the number of deaths due to HCC began to decline, reaching 28,889 in 2015 [27]. Between 1981 and 2003, the HCC incidence rate in Japanese men was higher than in women. Over 22 years, the incidence of male HCC increased from 29.2/100,000 cases in 1981 to 41.9/100,000 cases in 1987, and then fluctuated within the same range for eight years until a downward trend was observed in 1995. The incidence of liver cancer in women remained stable [28]. Despite the high incidence of HCC in Japan, the etiology is different from that in other Asian countries. In Japan, chronic HCV infection is more common than HBV infection, accounting for 79% of HCC and only 11% of HCC with HBV infection [29].
In the past twenty years, India’s HCC incidence increased, especially in Mumbai, Chennai and Bangalore. In the study cohort of 213 patients with HCC from 1999 to 2005, 83.1% were men, and the incidence of HCC was higher in men than in women [30]. In India, the main causes of HCC include HBV infection, HCV infection and alcohol consumption. HBV positive patients accounted for 70.42% [31]. Despite data showing that the incidence of HCV-related HCC in Asia has been decreasing since 2006, the potential risk of HCV infection cannot be ignored [5].
3. NAFLD and Obesity-Related HCC
NAFLD is defined as having more than 5% fat accumulation in liver cells, with the absence of HBV or HCV infection, and no excessive alcohol consumption [32]. NAFLD is gradually becoming the most common chronic liver disease in developed countries. However, due to the westernization of dietary habits in Asian countries, NAFLD may also be prevalent in the near future [33]. NAFLD is strongly associated with HCC [34]. Data on the impact of autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, and IGG4-related liver diseases on liver-related morbidity and mortality in the Asia-Pacific region are insufficient, so the following sections will be focused on NAFLD.
The etiology of HCC in Asia is undergoing a transition from viral factors to non-viral factors, including NAFLD. According to GHE2015 data, NAFLD and other causes account for 10.5% of all liver cancer deaths in mainland China [13]. Changing lifestyles and eating habits have led to an increase in the prevalence of NAFLD in mainland China [35]. The estimated total number of epidemic NAFLD cases in China in 2016 was 243.67 million and 7000 cases became HCC [36]. The prevalence of NAFLD-associated HCC cases is estimated to be increasing in all countries/regions studied, such as 47% in Japan (from 2200 cases in 2016 to 3240 cases by 2030). Among all years, China had the highest incidence of HCC, increasing from 14,090 cases (2016) to 26,240 cases (2030), an increase of 86%, whereas Japan saw the lowest increase (44%), from 1050 cases a year to 1520 [36]. In Korea, a study found that the proportion of HCC patients associated with NAFLD increased from 3.8% in 2001–2005 to 12.2% in 2006–2010 [37]. Although data for other countries are lacking, the incidence of NAFLD-related HCC is expected to increase rapidly in the future, considering that many Asian countries have developed NAFLD in the past two decades due to sedentary lifestyles and over-nutrition [38].
As a liver manifestation of metabolic syndrome, NAFLD is closely associated with obesity. Asia is the fastest growing country in terms of obesity, with China, for example, seeing a 414% rise in the prevalence of overweight and obesity between 1982 and 2002 [39]. Several population-based studies from Taiwan have shown that metabolic syndrome significantly increases the risk of HCC in patients with HBV or HCV. Being overweight leads to increased lipid peroxidation and oxidative stress and then makes it easier for chronic hepatitis B to become liver cancer [40]. A study in 2008 found that diabetes is also an HCC susceptibility prediction index, maybe associated with HBV and HCV infection status [41]. Diabetes contributes to a higher risk of HCC progression in HCV-infected patients and a higher risk of all-cause mortality in patients with or without HCC [42]. According to several national health surveys in Asia, the prevalence of overweight and obese people has increased [43]. Epidemiological studies have shown that type 2 diabetes mellitus (DM) is also a major risk factor for liver cancer and is commonly associated with NAFLD [44]. By 2030, it is expected that the largest group of DM patients will be found in India and China. The other four Asian countries including Indonesia, Pakistan, Bangladesh and the Philippines are among the top 10 countries with the highest prevalence of DM. It is not hard to see that obesity and DM may play a role in liver cancer in the future [45].
4. Aflatoxin-Related HCC
Aflatoxins are a family of carcinogens produced by fungal species such as Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius in warm, humid environments [7]. In Asia, these mycotoxins contaminate foods such as corn, peanuts and soybeans and cause liver damage [46]. Long-term low level dietary exposure to aflatoxin is a risk factor for HCC in Asia [47].
One reason for the decline in liver cancer incidence rates is the aflatoxin reduction program. Food processing plants of Philippines voluntarily began monitoring aflatoxin, leading to a significant reduction in its levels, thereby reducing the incidence of HCC [48]. Qidong once had the highest incidence of liver cancer in the world. Since 1985, policies allowing rice to be substituted for corn in the diet have resulted in a 40-fold reduction in aflatoxin-albumin adducts. This resulted in a significant decrease in the incidence of HCC in men (ASR = 89.9 from 1983 to 1987, ASR = 60.9 from 2008 to 2012, −32.3%) and a slight decrease in women (ASR = 24.5 from 1983 to 1987, ASR = 21.5 from 2008 to 2012, −12.2%) [48,49]. The proportion of aflatoxin-albumin adducts in randomly selected serum samples collected since 1980 was found to have decreased from 100% in 1982 to 23% in 2009 and 7% in 2012. It is considered that 65% of the decrease in primary liver cancer deaths in Asia is resulted from the reduction in aflatoxin exposure between 1982 and 2009 [49].
5. Alcoholic Liver Disease
Many studies have shown that heavy drinking (>50–70 g/d over several years) is associated with HCC [50]. A relatively low level of aldehyde dehydrogenase, mitochondria (ALDH2) among Asian populations may exacerbate liver damage caused by alcoholism [51]. Alcohol intake in Asia plays a smaller role in HCC than in the United States and Europe. In Middle East countries, alcohol consumption is relatively low [52]. Although research on alcoholic liver disease trends in Asia is limited, the incidence of alcoholic liver disease is also increasing annually among hospitalized patients [35].
The most recent data, from 2014, showed that Asia-Pacific countries’ drinking scoring patterns were divided into two main tiers: China, Japan and Singapore scored 2. In Japan, the ratio of HCC patients with non-viral causes continues to grow, and the lifelong alcohol consumption could be a potential cause [53]. The other tier includes other countries such as India, the Southern Republic, Malaysia, the Philippines, Thailand, Indonesia, Laos and Cambodia, with scores of 3 [54]. A high score is linked to a higher risk of drinking [55]. For the definition of drinking scoring patterns, please refer to Rehm et al. [56]. The increase in alcohol intake across the Asia-Pacific region between 2006 and 2016 May have contributed to an increase in age-standardized liver cancer rates [57]. The reason for the increase in alcohol consumption is not clear, but it could be due to lower taxes on alcohol [58].
6. Estimated Trend of Liver Cancer in Asia
We estimated the number of the future incidence rate of liver cancer by 2040 by CANCERTOMORROW|IARC. Overall, China may have the highest total number of liver cancer cases in Asia. Until 2040, the incidence of liver cancer in male is almost always higher than in female in Asian countries (Figure 2). We speculate that this trend is inevitably related to lifestyle. Countries where the incidence of liver cancer is expected to increase rapidly include the United Arab Emirates, Qatar, Kuwait and the Syrian Arab Republic. The rise of incidence rate in Japan, Georgia, Democratic People Republic of Korea Armenia and China is relatively flat (Table 1).
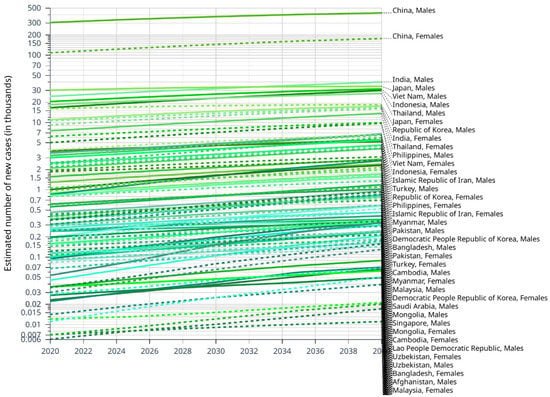
Figure 2.
Estimated number of new cases of liver cancer from 2020 to 2040, Males and Females, age [0–85+], based on CANCER TOMORROW|IARC.

Table 1.
Estimated number of new cases of liver cancer in Asia from 2020 to 2040, Incidence, both sexes, age [0–85+], based on CANCERTOMORROW|IARC.
7. Prevention Methods of HCC in Asia
7.1. Prevention of Viral Hepatitis-Related HCC
Most HBV infections are caused by vertical transmission, that is, mother-to-child transmission [59]. Preventing HBV infection is an important step to prevent HCC. Compared with the pre-vaccination era, universal vaccination of newborns can significantly reduce the incidence of HCC. WHO recommends that all newborns get HBV vaccine within 24 h [60]. Some countries recommend that hepatitis B immune globulin be administered at the same time as the vaccine to establish passive immunity, this combination is more effective [61]. In patients with chronic HBV carriers, certain factors can increase the risk of HCC, including advanced age, males, cirrhosis, drinking, chronic HCV or HIV infection, metabolic syndrome, and genetic polymorphisms [17,62]. Although many of these factors cannot be changed, antiviral drugs can be used to significantly control viral replication and reduce HBV DNA levels, such as interferon (IFN) and nucleoside analogs (NA) [59].
HCV-related liver cancer is another avoidable cause of primary cancer. Recent guidelines recommend screening infants and people at risk of HCV infection [59], even covering everyone born between 1945 and 1965 [63]. In addition, the AASLD/American Academy of Infectious Diseases guidelines recommend that all patients with chronic HCV infection should receive antiviral treatment [64]. For HCV, direct-acting antiviral (DAA) drugs could remarkably increase the cure rate. Therefore, all HCV patients should consider DAA treatment.
7.2. Maintain a Healthy Lifestyle
In a meta-analysis of 38,940 cancer cases, Esposito et al. [65] found an association between metabolic syndrome and HCC, and the association was stronger in Asians. The risk factors of NAFLD-related HCC include NAFLD, obesity, Patatin-like phospholipase-3 gene, diabetes, and metabolic syndrome [59]. Although the results of clinical trials have shown that potential treatments for NASH are inconsistent, diet and weight loss have brought positive results for NAFLD patients [66]. Weight loss of 7% to 10% is an intervention goal for most lifestyles and can improve liver enzymes and histology [59]. In addition, according to the AASLD guidelines, every NAFLD or liver cirrhosis patient should have a HCC screening every six months [67]. For patients with type 2 diabetes and metabolic syndrome, weight loss and regular aerobic exercise can reduce insulin resistance and improve inflammation [68]. In China, overweight and obesity increased by 414% between 1982 and 2002, which may contribute to NAFLD becoming an increasingly common cause of HCC in Asia [69]. Although lifestyle factors are strongly associated with NAFLD and an association with HCC has been demonstrated in animal studies, data on the relationship between humans and HCC are scarce. In animal models, the incidence of HCC in animals with exercise behavior is lower than in sedentary animals [70]. A systematic review and meta-analysis of 14 prospective studies confirmed an inverse association between physical activity and HCC risk [71]. However, due to the complex differences among individual lifestyle factors, it is difficult to accurately analyze their impact.
Several studies have shown that consuming vegetables reduces the risk of liver cancer. In the Shanghai Women’s and Men’s Health Study [72], which included 267 cases of liver cancer, vegetable-based dietary patterns were inversely associated with liver cancer, such as high intakes of celery, mushrooms, scallion vegetables, and beans/legume products. In Japan, vegetable consumption was also associated with a similar reduction in HCC risk [73]. Certain phytochemicals found in fruits, vegetables, herbs, and medicinal plants and dietary antioxidants, such as coenzyme Q, vitamin C, vitamin E and selenium, have been recommended to prevent HCC [74]. Furthermore, the Mediterranean diet also has a protective effect on HCC patients [75].
Reducing dietary aflatoxin exposure is a key step in reducing the prevalence in areas with high HCC epidemic burden [76]. Beginning in the 1980s, several agricultural policy reforms, such as improved storage of rice and maize, were implemented in highly aflatoxin-exposed areas such as eastern China, which resulted in a 65% reduction in HCC-related mortality between 1982 and 2009 [77].
A meta-analysis conducted by the World Cancer Research Foundation found that every 10 g of alcohol intake per day increases the statistical risk of HCC by 4%, avoiding heavy drinking can reduce HCC burden [78]. Increasing alcohol consumption among the Asian population plays an enormous role in the burden of HCC in this part of the world and is a matter of great concern [79].
In addition to diet and exercise factors, smoking is well known to be a risk factor for HCC, quitting smoking may of great value in preventing HCC and other malignant tumors.
7.3. Pharmacological Therapies
Sorafenib has been approved for the treatment of advanced HCC patients worldwide with Child-Pugh grade A liver function and produced similar survival benefits in this diverse patient population [80]. Patients with Child-Pugh grade B liver function do not have excessive risk with sorafenib, but they are more likely to develop liver decompensation. Therefore, sorafenib should be used with caution in patients with Child-Pugh score 7 and is not recommended in patients with Child-Pugh score > 7 or decompensated cirrhosis [80]. Regorafenib has stronger inhibitory activity on a variety of angiogenic pathways and carcinogenic pathways [81]. In addition to sorafenib and regorafenib, there are a variety of molecular targeted drugs, including sunitinib, brivanib, linifanib, ramucirumab, erlotinib and everolimus have also been investigated. However, they did not show a survival advantage in a phase III randomized controlled trial [80].
In recent years, significant breakthroughs have been made in agents targeting immune checkpoint proteins such as cytotoxic T-lymphocyte antigen-4 and programmed cell death-1 [82,83]. Their cancer-killing effects are achieved by maintaining T cell activation. Clinical trials of immune checkpoint inhibitors alone or in combination with immunotherapy are ongoing [80].
8. Conclusions
East Asia remains one of the regions with the highest burden of hepatocellular carcinoma worldwide [84]. We review that the following factors are contributing to the changing trends in liver cancer in the Asia-Pacific region: the development of country-specific prevention strategies, such as the promotion of healthier lifestyles, the reduce of smoking and alcohol use and community-based health promotion programs; environmental interventions, such as improved grain storage and crop substitution to prevent aflatoxin contamination; better medical interventions, such as new antiviral treatments for patients with HBV or HCV infection (Table 2 and Figure 3) [85].

Table 2.
The changing epidemiology of HCC in Asia.
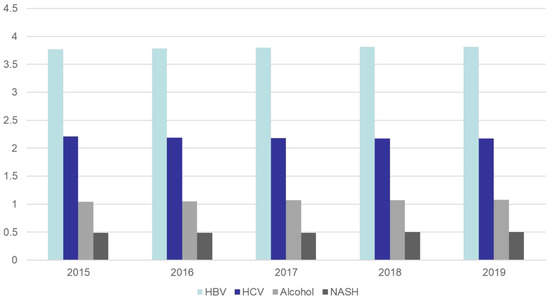
Figure 3.
According to the Global Burden of Disease Database, changes in the incidence of liver cancer by different causes in Asia from 2015 to 2019, expressed as rate per 100,000 [5].
With the effective implementation of HBV and treatment regimens for HCV, the epidemiological attribution of HCC is shifting from viral hepatitis to NAFLD [84]. Here, we reviewed the epidemiological trends of HCC in Asia and highlight future efforts, such as improving the lifestyle of patients (Figure 4). Focusing on prevention may be the most effective way to improve the prognosis of this hard-to-treat cancer.
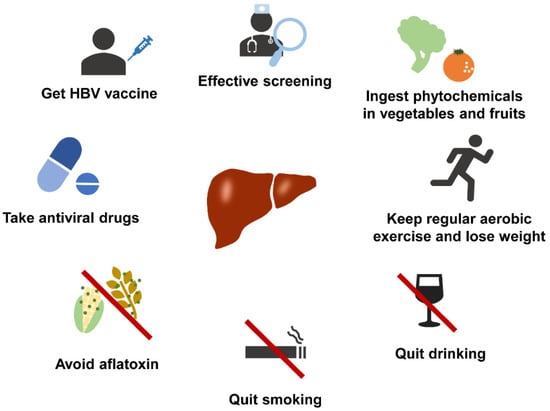
Figure 4.
Methods to reduce the incidence of HCC.
The current effective measures for the prevention and treatment of HCC include vaccination, effective screening, application of antiviral drugs, intake of fresh fruits and vegetables, regular aerobic exercise, weight loss, alcohol prohibition, smoking ban, and avoidance of aflatoxin intake.
Author Contributions
Y.L., Writing—Original Draft; L.L., Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Key R & D Program of China (Grant No. 2019YFA0709300), National Natural Science Foundation of China (Grant No. U19A2008, No. 81972307, and No. 82102705), China Postdoctoral Science Foundation (Grant No. 2020M671911, No. 2020TQ0313), Natural Science Foundation of Anhui Province (Grant No. 2008085QH376).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAPC | average annual percent change |
| ASR | Age-Standardized Rate |
| CHB | chronic hepatitis B |
| DAA | direct-acting antiviral |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| IFN | interferon |
| NAFLD | non-alcoholic liver disease |
| NAs | nucleos(c)ide analogs |
References
- WHO. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. 2020. Available online: https://gco.iarc.fr/today/ (accessed on 1 September 2022).
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Zhang, C.H.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e1224. [Google Scholar] [CrossRef]
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. [Google Scholar] [CrossRef]
- Liaw, Y.F. Antiviral therapy of chronic hepatitis B: Opportunities and challenges in Asia. J. Hepatol. 2009, 51, 403–410. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, L.Y.; Yu, M.W. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J. Gastroenterol. Hepatol. 2000, 15, E3–E6. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P. Epidemiology of Viral Hepatitis and Liver Diseases in India. Euroasian J. Hepatogastroenterol. 2015, 5, 34–36. [Google Scholar] [CrossRef]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009, 27, 6550–6557. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. 2016. Available online: https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000_2015/en/ (accessed on 10 June 2017).
- Ang, S.F.; Ng, E.S.; Li, H.; Ong, Y.H.; Choo, S.P.; Ngeow, J.; Toh, H.C.; Lim, K.H.; Yap, H.Y.; Tan, C.K.; et al. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS ONE 2015, 10, e0118658. [Google Scholar] [CrossRef]
- Chiang, C.J.; Yang, Y.W.; You, S.L.; Lai, M.S.; Chen, C.J. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013, 310, 974–976. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, P.M.; Huang, G.T.; Lee, H.S.; Sung, J.L.; Sheu, J.C. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J. Formos Med. Assoc. 2007, 106, 148–155. [Google Scholar] [CrossRef]
- Yuen, M.F.; Cheng, C.C.; Lauder, I.J.; Lam, S.K.; Ooi, C.G.; Lai, C.L. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000, 31, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Yuen, M.F. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology 2013, 57, 399–408. [Google Scholar] [CrossRef]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Mak, C.W.; Lee, S.K.; Ip, Z.M.; Lam, A.T.; Iu, H.W.; Leung, J.M.; Lai, J.W.; Lo, A.O.; et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013, 58, 1537–1547. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, J.T.; Ho, H.J.; Su, C.W.; Lee, T.Y.; Wang, S.Y.; Wu, C.; Wu, J.C. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: A nationwide cohort study. Gastroenterology 2014, 147, 143–151.e5. [Google Scholar] [CrossRef]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61 (Suppl. 1), S45–S57. [Google Scholar] [CrossRef] [Green Version]
- Butt, A.S.; Abbas, Z.; Jafri, W. Hepatocellular carcinoma in pakistan: Where do we stand? Hepat. Mon. 2012, 12, e6023. [Google Scholar] [CrossRef]
- Ahmed, B.; Ali, T.; Qureshi, H.; Hamid, S. Population-attributable estimates for risk factors associated with hepatitis B and C: Policy implications for Pakistan and other South Asian countries. Hepatol. Int. 2013, 7, 500–507. [Google Scholar] [CrossRef]
- Bosan, A.; Qureshi, H.; Bile, K.M.; Ahmad, I.; Hafiz, R. A review of hepatitis viral infections in Pakistan. J. Pak. Med. Assoc. 2010, 60, 1045–1058. [Google Scholar]
- Okuda, K.; Ohtsuki, T.; Obata, H.; Tomimatsu, M.; Okazaki, N.; Hasegawa, H.; Nakajima, Y.; Ohnishi, K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985, 56, 918–928. [Google Scholar] [CrossRef]
- Kudo, M. Management of Hepatocellular Carcinoma in Japan as a World-Leading Model. Liver Cancer 2018, 7, 134–147. [Google Scholar] [CrossRef]
- Tanaka, H.; Imai, Y.; Hiramatsu, N.; Ito, Y.; Imanaka, K.; Oshita, M.; Hijioka, T.; Katayama, K.; Yabuuchi, I.; Yoshihara, H.; et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann. Intern Med. 2008, 148, 820–826. [Google Scholar] [CrossRef]
- Umemura, T.; Ichijo, T.; Yoshizawa, K.; Tanaka, E.; Kiyosawa, K. Epidemiology of hepatocellular carcinoma in Japan. J. Gastroenterol. 2009, 44 (Suppl. 19), 102–107. [Google Scholar] [CrossRef]
- Yeole, B.B. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac. J. Cancer Prev. 2008, 9, 97–100. [Google Scholar]
- Kumar, M.; Kumar, R.; Hissar, S.S.; Saraswat, M.K.; Sharma, B.C.; Sakhuja, P.; Sarin, S.K. Risk factors analysis for hepatocellular carcinoma in patients with and without cirrhosis: A case-control study of 213 hepatocellular carcinoma patients from India. J. Gastroenterol. Hepatol. 2007, 22, 1104–1111. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Wong, V.W.; Hui, A.Y.; Tsang, S.W.; Chan, J.L.; Wong, G.L.; Chan, A.W.; So, W.Y.; Cheng, A.Y.; Tong, P.C.; Chan, F.K.; et al. Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2006, 24, 1215–1222. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Fan, J.G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 1), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Kwack, M.S.; Jang, E.S.; You, S.J.; Lee, J.H.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion 2011, 84 (Suppl. 1), 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Asia Pacific Cohort Studies Collaboration. The burden of overweight and obesity in the Asia-Pacific region. Obes. Rev. 2007, 8, 191–196. [Google Scholar] [CrossRef]
- Yu, M.W.; Shih, W.L.; Lin, C.L.; Liu, C.J.; Jian, J.W.; Tsai, K.S.; Chen, C.J. Body-mass index and progression of hepatitis B: A population-based cohort study in men. J. Clin. Oncol. 2008, 26, 5576–5582. [Google Scholar] [CrossRef]
- Chen, C.L.; Yang, H.I.; Yang, W.S.; Liu, C.J.; Chen, P.J.; You, S.L.; Wang, L.Y.; Sun, C.A.; Lu, S.N.; Chen, D.S.; et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology 2008, 135, 111–121. [Google Scholar] [CrossRef]
- Huang, T.S.; Lin, C.L.; Lu, M.J.; Yeh, C.T.; Liang, K.H.; Sun, C.C.; Shyu, Y.C.; Chien, R.N. Diabetes, hepatocellular carcinoma, and mortality in hepatitis C-infected patients: A population-based cohort study. J. Gastroenterol. Hepatol. 2017, 32, 1355–1362. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C. Rising burden of obesity in Asia. J. Obes. 2010, 2010, 868573. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Crespo, D.M.; Kang, H.S.; Al-Osaimi, A.M. Obesity and hepatocellular carcinoma. Gastroenterology 2004, 127 (Suppl. 1), S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, J.H.; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Zimmet, P.; Son, H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Magnussen, A.; Parsi, M.A. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, T.; Thorgeirsson, S.S.; Zhan, Q.; Chen, J.; Park, J.H.; Lu, P.; Hsia, C.C.; Wang, N.; Xu, L.; et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013, 34, 1800–1805. [Google Scholar] [CrossRef]
- Chen, J.G.; Egner, P.A.; Ng, D.; Jacobson, L.P.; Munoz, A.; Zhu, Y.R.; Qian, G.S.; Wu, F.; Yuan, J.M.; Groopman, J.D.; et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. 2013, 6, 1038–1045. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hwang, L.Y.; Hatten, C.J.; Swaim, M.; Li, D.; Abbruzzese, J.L.; Beasley, P.; Patt, Y.Z. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002, 36, 1206–1213. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef]
- Poustchi, H.; Sepanlou, S.; Esmaili, S.; Mehrabi, N.; Ansarymoghadam, A. Hepatocellular carcinoma in the world and the middle East. Middle East J. Dig. Dis. 2010, 2, 31–41. [Google Scholar]
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef]
- Observation, G.H. Patterns of Drinking Score-By Country. 2014. Available online: http://apps.who.int/gho/data/node.main.A1048?lang=en?showonly=GISAH (accessed on 1 September 2022).
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Rehn, N.; Room, R.; Monteiro, M.; Gmel, G.; Jernigan, D.; Frick, U. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur. Addict. Res. 2003, 9, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Obsearvatory, G.H. Estimate of 5-Year Change in Recorded Alcohol per Capita (15+ Years) Consumption, 2006–2010. 2014. Available online: http://www.who.int/gho/alcohol/consumption_levels/change_adult_percapita/en/ (accessed on 1 September 2022).
- Wong, M.C.S.; Huang, J.L.W.; George, J.; Huang, J.; Leung, C.; Eslam, M.; Chan, H.L.Y.; Ng, S.C. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 57–73. [Google Scholar] [CrossRef]
- Mak, L.Y.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Torres, H.A.; LoConte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 262–279. [Google Scholar] [CrossRef]
- WHO. Immunization, Vaccines and Biologicals: Hepatitis B. 2017. Available online: www.who.int/immunization/diseases/hepatitisB/en/ (accessed on 1 September 2022).
- Lee, C.; Gong, Y.; Brok, J.; Boxall, E.H.; Gluud, C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: Systematic review and meta-analysis. BMJ 2006, 332, 328–336. [Google Scholar] [CrossRef]
- Varbobitis, I.; Papatheodoridis, G.V. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin. Mol. Hepatol. 2016, 22, 319–326. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm. Rep. 1998, 47, 1–39. [Google Scholar]
- AASLD-IDSA. When and in Whom to Initiate HCV Therapy. 2017. Available online: http://www.hcvguidelines.org/evaluate/when-whom (accessed on 1 September 2022).
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Degasperi, E.; Colombo, M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2016, 1, 156–164. [Google Scholar] [CrossRef]
- Konyn, P.; Ahmed, A.; Kim, D. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1295–1307. [Google Scholar] [CrossRef]
- Piguet, A.C.; Saran, U.; Simillion, C.; Keller, I.; Terracciano, L.; Reeves, H.L.; Dufour, J.F. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J. Hepatol. 2015, 62, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, S.E.; Leitzmann, M.F.; Linseisen, J.; Schlesinger, S. Physical Activity and the Risk of Liver Cancer: A Systematic Review and Meta-Analysis of Prospective Studies and a Bias Analysis. J. Natl. Cancer Inst. 2019, 111, 1142–1151. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Y.B.; Li, H.L.; Yang, G.; Cai, H.; Ji, B.T.; Gao, Y.T.; Zheng, W.; Shu, X.O. Vegetable-based dietary pattern and liver cancer risk: Results from the Shanghai women’s and men’s health studies. Cancer Sci. 2013, 104, 1353–1361. [Google Scholar] [CrossRef]
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Tanaka, Y.; Mizokami, M.; Tsugane, S.; Group, J.S. Vegetable, fruit and antioxidant nutrient consumption and subsequent risk of hepatocellular carcinoma: A prospective cohort study in Japan. Br. J. Cancer 2009, 100, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Montella, M.; Crispo, A.; Giudice, A. HCC, diet and metabolic factors: Diet and HCC. Hepat. Mon. 2011, 11, 159–162. [Google Scholar]
- Turati, F.; Trichopoulos, D.; Polesel, J.; Bravi, F.; Rossi, M.; Talamini, R.; Franceschi, S.; Montella, M.; Trichopoulou, A.; La Vecchia, C.; et al. Mediterranean diet and hepatocellular carcinoma. J. Hepatol. 2014, 60, 606–611. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Goh, G.B.; Chang, P.E.; Tan, C.K. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 919–928. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Liver Cancer. 2015. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/liver-cancer-report.pdf (accessed on 1 September 2022).
- Jafri, W.; Kamran, M. Hepatocellular Carcinoma in Asia: A Challenging Situation. Euroasian J. Hepatogastroenterol. 2019, 9, 27–33. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schutz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Turati, F.; La Vecchia, C. Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).