Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Risk Factors Associated with the Onset of Cervical Cancer
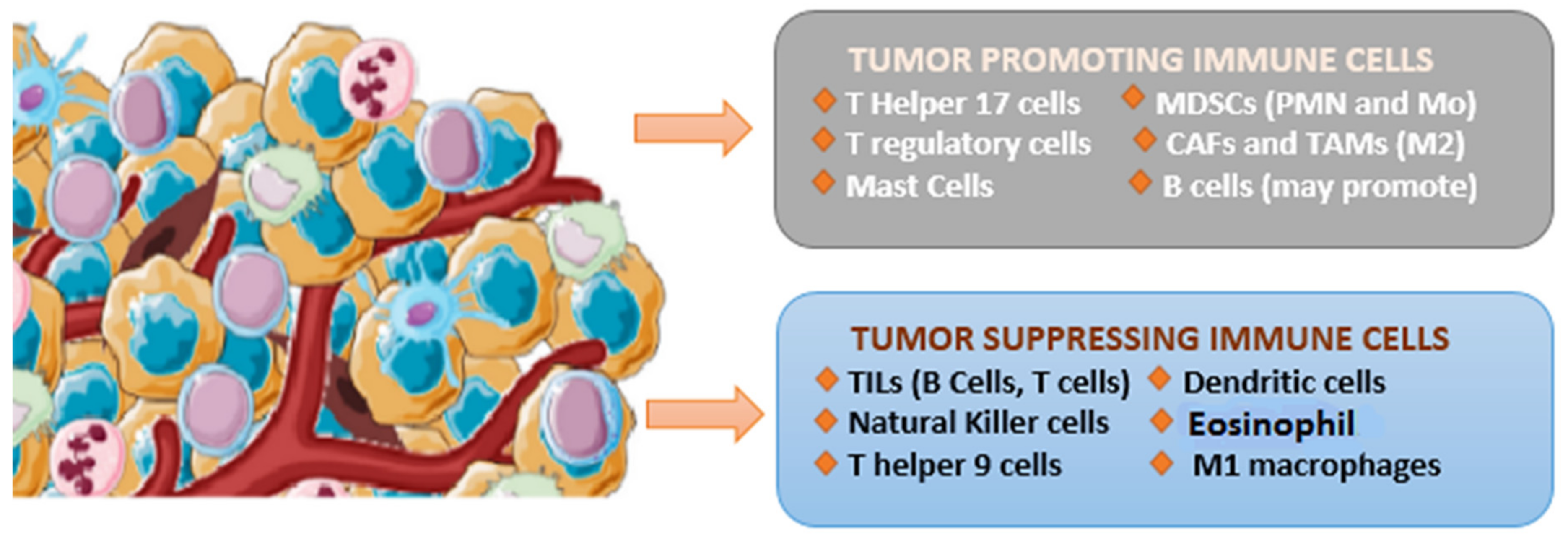
1.2. The Immunological Landscape of Cervical Cancer
1.3. Tumor-Infiltrating Lymphocyte (TIL)
1.4. Tumor-Associated Macrophages (TAMs)
1.5. Cancer-Associated Fibroblasts (CAFs)
1.6. Dendritic Cells (DCs)
1.7. B Cells
1.8. NK Cells
1.9. Myeloid-Derived Suppressor Cells (MDSCs)
1.10. Immunotherapy Approaches for the Treatment of Cervical Cancer
2. Immunotherapeutic Approaches for the Treatment of Cervical Cancer Include
- Immune checkpoint inhibitors.
- Adoptive cell therapies.
- Oncolytic virus therapy.
- Cancer vaccines.
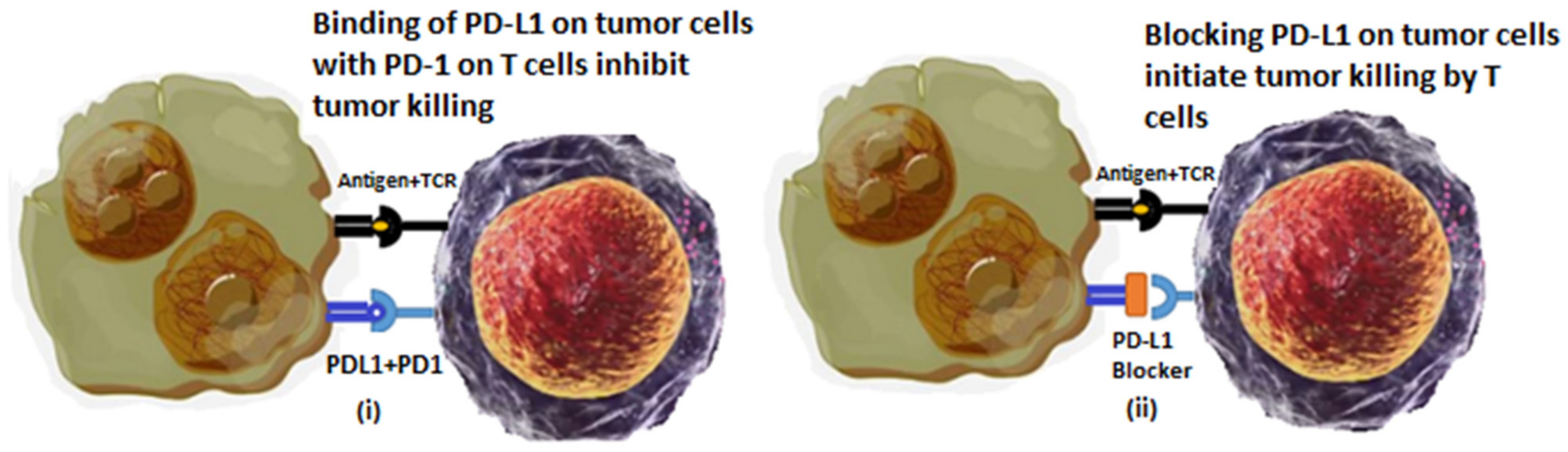
2.1. Immune Checkpoint Inhibitors
2.2. Adoptive Cell Therapies (ACTs)
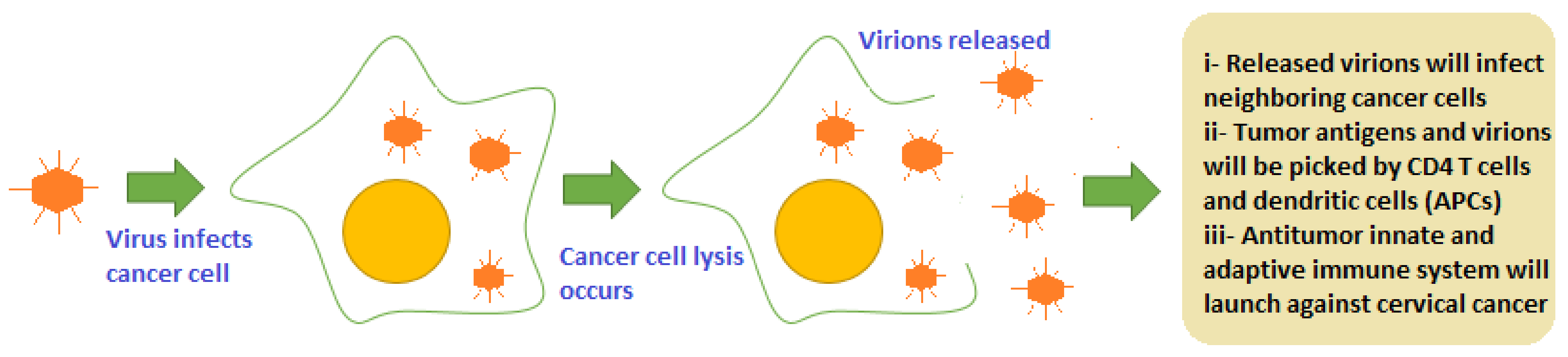
2.3. Oncolytic Viruses (OVs)
2.4. Cancer Vaccines
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Mao, Z.; Lai, Y.; Wan, T.; Zhang, K.; Zhou, B. A review of the research progress in T-lymphocyte immunity and cervical cancer. Transl. Cancer Res. 2020, 9, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Del Valle-Mendoza, J.; Becerra-Goicochea, L.; Aguilar-Luis, M.A.; Pinillos-Vilca, L.; Carrillo-Ng, H.; Silva-Caso, W.; Palomares-Reyes, C.; Taco-Masias, A.-A.; Aquino-Ortega, R.; Tinco-Valdez, C.; et al. Genotype-specific prevalence of human papillomavirus infection in asymptomatic Peruvian women: A community-based study. BMC Res. Notes 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Nadiya, K.; Sukhpal, K.; Sandhya, G. Risk Factors of Cervical Cancer: A Case-Control Study. Asia-Pac. J. Oncol. Nurs. 2019, 6, 308–314. [Google Scholar] [CrossRef]
- Luiza Genta, M. Human Papillomavirus 16 Is an Independent Predictor of Better Survival among Patients with Early Cervical Cancer; International Gynecologic Cancer Society: Louisville, KY, USA, 2016. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef] [PubMed]
- Jayshree, R.S. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions—Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 649815. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Zhu, L.; Hu, W.; Liu, B.; Xie, L. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer. Hum. Vaccines Immunother. 2022, 18, 2060019. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Dadaglio, G.; Oberkampf, M.; Di Carlo, S.; Peduto, L.; Laubreton, D.; Desrues, B.; Sun, C.-M.; Montagutelli, X.; Leclerc, C. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int. J. Cancer 2016, 139, 1358–1371. [Google Scholar] [CrossRef]
- Sammarco, M.L. Human Papillomavirus Infections, Cervical Cancer and Micrornas: An Overview and Implications for Public Health. MicroRNA 2020, 9, 174–186. [Google Scholar] [CrossRef]
- Chauhan, S.R.; Singhal, P.G.; Sharma, U.; Bandil, K.; Chakraborty, K.; Bharadwaj, M. Th9 cytokines curb cervical cancer progression and immune evasion. Hum. Immunol. 2019, 80, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8 + cytotoxic T lymphocytes in cancer immunotherapy: A Review. J. Cell. Physiol. 2018, 234, 8509–8521. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Bernsmeier, C.; van der Merwe, S.; Périanin, A. Innate immune cells in cirrhosis. J. Hepatol. 2020, 73, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Büttner, M.; Fabri, M.; Mougiakakos, D.; Bittenbring, J.T.; Hoffmann, M.H.; Beier, F.; Pasemann, S.; Jitschin, R.; Hofmann, A.D.; et al. Vitamin D–dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci. Transl. Med. 2015, 7, 282ra47. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage phenotypic mechanomodulation of enhancing bone regeneration by superparamagnetic scaffold upon magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Schaar, B.; Tallapragada, S.; Dorigo, O. Tumor associated macrophages in gynecologic cancers. Gynecol. Oncol. 2018, 149, 205–213. [Google Scholar] [CrossRef]
- Stone, S.C.; Rossetti, R.A.M.; Alvarez, K.L.F.; Carvalho, J.P.; Margarido, P.F.R.; Baracat, E.C.; Tacla, M.; Boccardo, E.; Yokochi, K.; Lorenzi, N.P.; et al. Lactate secreted by cervical cancer cells modulates macrophage phenotype. J. Leukoc. Biol. 2019, 105, 1041–1054. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef]
- Tran, L.; Theodorescu, D. Determinants of resistance to checkpoint inhibitors. Int. J. Mol. Sci. 2020, 21, 1594. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, L.; Moro, C.F.; Bankhead, P.; Kern, K.P.; Sadeak, I.; Meng, Q.; Rangelova, E.; Kaipe, H. Human pancreatic carcinoma-associated fibroblasts promote expression of co-inhibitory markers on CD4+ and CD8+ T-cells. Front. Immunol. 2019, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-J.; Yang, Y.; Wei, W.-F.; Wu, X.-G.; Yan, R.-M.; Zhou, C.-F.; Chen, X.-J.; Wu, S.; Wang, W.; Fan, L.-S. Tumor-secreted exosomal WNT2B activates fibroblasts to promote cervical cancer progression. Oncogenesis 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Cui, R.; Liu, C.; Zhang, G.; Zhang, Z. MTBHSP70-exfpr1-pulsed dendritic cells enhance the immune response against cervical cancer. J. Cancer 2019, 10, 6364–6373. [Google Scholar] [CrossRef] [PubMed]
- Strickler, H.D.; Martinson, J.; Desai, S.; Xie, X.; Burk, R.D.; Anastos, K.; Massad, L.S.; Minkoff, H.; Xue, X.; D’Souza, G.; et al. The relation of plasmacytoid dendritic cells (pdcs) and regulatory T-cells (Tregs) with HPV persistence in HIV-infected and HIV-uninfected women. Viral Immunol. 2014, 27, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, P.A.; Verhoeven, Y.; Jacobs, J.; Wouters, A.; Tjalma, W.; Lardon, F.; Van den Wyngaert, T.; Dewulf, J.; Smits, E.; Colpaert, C.; et al. Rank-rankl signaling in cancer of the uterine cervix: A Review. Int. J. Mol. Sci. 2019, 20, 2183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Y.; Du, R.; Pang, N.; Zhang, F.; Dong, D.; Ding, J.; Ding, Y. Role of regulatory B cells in the progression of cervical cancer. Mediat. Inflamm. 2019, 2019, 6519427. [Google Scholar] [CrossRef]
- Kim, S.S.; Shen, S.; Miyauchi, S.; Sanders, P.D.; Franiak-Pietryga, I.; Mell, L.; Gutkind, J.S.; Cohen, E.E.W.; Califano, J.A.; Sharabi, A.B. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res. 2020, 26, 3345–3359. [Google Scholar] [CrossRef]
- Lucena, A.A.S.; Guimarães, M.V.; Michelin, M.A.; Lodi, C.T.C.; Lima, M.I.; Murta, E.F.; Melo, V.H. Evaluation of T, B and natural killer lymphocyte in the cervical stroma of HIV-positive and negative patients with cervical intraepithelial neoplasia. Immunol. Lett. 2016, 169, 98–103. [Google Scholar] [CrossRef]
- Zhu, S.-Y.; Wu, Q.-Y.; Zhang, C.-X.; Wang, Q.; Ling, J.; Huang, X.-T.; Sun, X.; Yuan, M.; Wu, D.; Yin, H.-F. Mir-20A inhibits the killing effect of natural killer cells to cervical cancer cells by downregulating RUNX1. Biochem. Biophys. Res. Commun. 2018, 505, 309–316. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Nguyen, V.H.; Ichimura, H.; Pham, T.T.T.; Nguyen, C.H.; Pham, T.V.; Elbadry, M.I.; Yoshioka, K.; Tanaka, J.; Trung, L.Q.; et al. A functional polymorphism in the NKG2D gene modulates NK-cell cytotoxicity and is associated with susceptibility to human papilloma virus-related cancers. Sci. Rep. 2016, 6, 39231. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Li, C.-H.; Chu, L.-H.; Huang, P.-S.; Sheu, B.-C.; Huang, S.-C. Regulatory T cells suppress natural killer cell immunity in patients with human cervical carcinoma. Int. J. Gynecol. Cancer 2016, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, W.; Dahlan, E.G.; Saputra, K.; Sutrisno, T.C. Effect of electroacupuncture on natural-killer cells and tumor size in patients with cervical squamous-cell carcinoma: A randomized controlled trial. Med. Acupunct. 2019, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lü, B.; Zhao, P.; Lü, W. Increased circulating grmyeloid-derived suppressor cells correlated with tumor burden and survival in locally advanced cervical cancer patient. J. Cancer 2019, 10, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hua, K. Cervical cancer: Emerging immune landscape and treatment. OncoTargets Ther. 2020, 13, 8037–8047. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kwon, B.-E.; Hong, E.-H.; Shim, A.; Song, J.-H.; Kim, H.-M.; Chang, S.-Y.; Kim, Y.-J.; Kweon, M.-N.; Youn, J.-I.; et al. Interleukin-10 attenuates tumour growth by inhibiting interleukin-6/signal transducer and activator of transcription 3 signalling in myeloid-derived suppressor cells. Cancer Lett. 2016, 381, 156–164. [Google Scholar] [CrossRef]
- Galliverti, G.; Wullschleger, S.; Tichet, M.; Murugan, D.; Zangger, N.; Horton, W.; Korman, A.J.; Coussens, L.M.; Swartz, M.A.; Hanahan, D. Myeloid cells orchestrate systemic immunosuppression, impairing the efficacy of immunotherapy against HPV+ cancers. Cancer Immunol. Res. 2020, 8, 131–145. [Google Scholar] [CrossRef]
- Sherer, M.V.; Kotha, N.V.; Williamson, C.; Mayadev, J. Advances in immunotherapy for cervical cancer: Recent developments and Future Directions. Int. J. Gynecol. Cancer 2022, 32, 281–287. [Google Scholar] [CrossRef]
- Singh, V.; Sheikh, A.; Abourehab, M.A.; Kesharwani, P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors 2022, 12, 617. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Functions of immune checkpoint molecules beyond immune evasion. Adv. Exp. Med. Biol. 2020, 1248, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Mell, L.K.; Sharabi, A.B.; McHale, M.; Mayadev, J.S. Immunotherapy with radiotherapy and chemoradiotherapy for cervical cancer. Semin. Radiat. Oncol. 2020, 30, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. FDA Approves Pembrolizumab Combination for the First-Line Treatment of Cervical Cancer; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-combination-first-line-treatment-cervical-cancer#:~:text=On%20October%2013%2C2021%2C%20the,by%20an%20FDA%2Dapproved%20test (accessed on 7 July 2022).
- Anipindi, M.; Smith, R.J.; Gilani, M. Case report: Immune checkpoint inhibitors as a single agent in the treatment of metastatic cervical cancer. Front. Oncol. 2022, 12, 856944. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, C.; Zhu, J.; Sivik, J.; Drabick, J.J.; Mackley, H.B. Identifying the optimal fractionation schedules for improved response rates and survival in patients with metastatic melanoma treated with Ipilimumab and radiotherapy. Curr. Cancer Ther. Rev. 2020, 16, 78–85. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, H.; Fu, J. Combinations of radiotherapy with immunotherapy in cervical cancer. J. Cancer 2022, 13, 1480–1489. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.P.; Delord, J.P.; Evans, T.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Baldo, B.A. Immune-and non-immune-mediated adverse effects of monoclonal antibody therapy: A survey of 110 approved antibodies. Antibodies 2022, 11, 17. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J.B. Adoptive cellular therapies: The current landscape. Virchows Arch. 2018, 474, 449–461. [Google Scholar] [CrossRef]
- Simoni, Y.; Li, S.; Zhuang, S.; Heit, A.; Koo, S.-L.; Chow, I.-T.; Kwok, W.W.; Tan, I.B.; Tan, D.S.W.; Newell, E.W. Bystander CD4+ T cells infiltrate human tumors and are phenotypically distinct. OncoImmunology 2020, 11, 2012961. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus–targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, L. Faculty Opinions Recommendation of Efficacy of HPV-Based Screening for Prevention of Invasive Cervical Cancer: Follow-Up of Four European Randomised Controlled Trials; Faculty Opinions–Post-Publication Peer Review of the Biomedical Literature: London, UK, 2017. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus–associated epithelial cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Song, D.; Jia, X.; Liu, X.; Hu, L.; Lin, K.; Xiao, T.; Qiao, Y.; Zhang, J.; Dan, J.; Wong, C.; et al. Identification of the receptor of oncolytic virus M1 as a therapeutic predictor for multiple solid tumors. Signal Transduct. Target. Ther. 2022, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, E.V.; Yurchenko, K.S.; Shestopalov, A.M. The cytotoxic effect of the wild-type Newcastle disease virus strain on tumor cells in vitro. Cell Tissue Biol. 2020, 14, 243–249. [Google Scholar] [CrossRef]
- Keshavarz, M.; Nejad, A.S.M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle Disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020, 27, 47–52. [Google Scholar] [CrossRef]
- Duan, Y.; Bai, H.; Li, X.; Wang, D.; Wang, Y.; Cao, M.; Zhang, N.; Chen, H.; Wang, Y. Oncolytic adenovirus H101 synergizes with radiation in cervical cancer cells. Curr. Cancer Drug Targets 2021, 21, 619–630. [Google Scholar] [CrossRef]
- De Bakker, T.; Journe, F.; Descamps, G.; Saussez, S.; Dragan, T.; Ghanem, G.; Krayem, M.; Van Gestel, D. Restoring p53 function in head and neck squamous cell carcinoma to improve treatments. Front. Oncol. 2022, 11, 799993. [Google Scholar] [CrossRef]
- Burman, B.; Pesci, G.; Zamarin, D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers 2020, 12, 3552. [Google Scholar] [CrossRef]
- Ni, J.; Feng, H.; Xu, X.; Liu, T.; Ye, T.; Chen, K.; Li, G. Oncolytic vaccinia virus harboring aphrocallistes vastus lectin inhibits the growth of cervical cancer cells hela S3. Mar. Drugs 2021, 19, 532. [Google Scholar] [CrossRef]
- Kagabu, M.; Yoshino, N.; Saito, T.; Miura, Y.; Takeshita, R.; Murakami, K.; Kawamura, H.; Baba, T.; Sugiyama, T. The efficacy of a third-generation oncolytic Herpes Simplex Viral Therapy for an HPV-related uterine cervical cancer model. Int. J. Clin. Oncol. 2020, 26, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.-C.; Hung, C.-F. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Gardella, B.; Gritti, A.; Soleymaninejadian, E.; Pasquali, M.F.; Riemma, G.; La Verde, M.; Schettino, M.T.; Fortunato, N.; Torella, M.; Dominoni, M. New Perspectives in therapeutic vaccines for HPV: A critical review. Medicina 2022, 58, 860. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kawana, K.; Yokoyama, T.; Fujii, T.; Tomio, A.; Miura, S.; Tomio, K.; Kojima, S.; Oda, K.; Sewaki, T.; et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 2010, 28, 2810–2817. [Google Scholar] [CrossRef]
- Leitão, J.H. Listeria monocytogenes as a vector for cancer immunotherapy. Vaccines 2020, 8, 439. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2016, 27, 74–95. [Google Scholar] [CrossRef]
- Sioud, M. Releasing the Immune System Brakes Using siRNAs Enhances Cancer Immunotherapy. Cancers 2019, 11, 176. [Google Scholar] [CrossRef]
- Ferrall, L.; Lin, K.Y.; Roden, R.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4953–4973. [Google Scholar] [CrossRef]
- Rumfield, C.S.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic vaccines for HPV-associated malignancies. ImmunoTargets Ther. 2020, 9, 167–200. [Google Scholar] [CrossRef]
- Ta-Cin Vaccine with Anti-PD-1 Therapy in Recurrent HPV16-Associated Cancers—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT05132803 (accessed on 28 August 2022).
| Target | Agent | Advantages | Limitations |
|---|---|---|---|
| CTLA-4 | Ipilimumab | Used in solid and hematological malignancies Produces durable response even in advanced stage cancers Least side effects and patient compliance as compared to chemotherapy Have Biomarkers available to predict therapy response [50] | Therapeutic efficacy restricted to a limited number of patients and specific cancer in some cases Less effective in cancer with “cold” TME. Autoimmune-like toxicities: Nephritis Cytopenias Fatigue Myocarditis Hepatitis Hypophysitis Hypothyroidism Nephritis Uveitis Pneumonitis [51] |
| PD-1 | Cemiplimab | ||
| Pembrolizumab | |||
| Nivolumab | |||
| PD-L1 | Durvalumab | ||
| Atezolizumab | |||
| Avelumab |
| No. | NCT Number | Title | Phases | Enrollment | Combined Treatment | Status |
|---|---|---|---|---|---|---|
| 1 | NCT01585428 | Immunotherapy using tumor-infiltrating lymphocytes for patients with metastatic human papillomavirus-associated cancers | Phase 2 | 29 | - | Completed |
| 2 | NCT03108495 | Study of LN-145, autologous tumor-infiltrating lymphocytes in the treatment of patients with cervical carcinoma | Phase 2 | 138 | Pembrolizumab | Recruiting |
| 3 | NCT04674488 | TILs for treatment of metastatic or recurrent cervical cancer | Phase 1 | 15 | - | Recruiting |
| 4 | NCT05107739 | Study of DeTIL-0255 in adults with advanced malignancies | Phase 1 | - | Recruiting | |
| 5 | NCT04443296 | Study of tumor-infiltrating lymphocytes following CCRT in the treatment of patients with cervical carcinoma | Phase 1 | 10 | CCRT Concurrent chemoradiotherapy | Active, not recruiting |
| Virus | Description | Mechanism | Clinical Condition | Reference |
|---|---|---|---|---|
| Adenovirus | Non-enveloped virus with 90–100 nm size, have icosahedral nucleocapsid, which contain double-stranded DNA genome | Target tumor antigens specifically. Different Ad virus species bind with different receptors including CAR, αvβ5 integrin, HSPG, VCAM-1, MHC-Iα2 | ONYX-015 (FDA-approved in China) is used in synergy with standard chemotherapy agents 5-fluorouracil and cisplatin to treat head and neck squamous carcinoma. Another virus similar to Onyx-015 (E1B-55K/E3B-deleted), H101 is tested promising for use in combination with radiation therapy to treat metastatic cervical cancer. | [60] [61] |
| Newcastle Disease virus | Single-stranded, negative sense, enveloped RNA virus. Causes contagious bird disease | Targeted replication in interferon-defective cancer cells by binding with Sia receptors on tumor cells. Avoids problem of pre-existing immunity | NDV selectively kills cervical cancer cells by inducing ROS-mediated apoptosis. NDV triggers both innate and adaptive immune response in cervical cancer TME by causing inflammation and recruitment of CD4+ and CD8+ immune responses. | [62] |
| Vaccinia virus | Linear double-stranded DNA genome containing enveloped virus. Approx 360 × 270 × 250 nm in size | Can squeeze through leaky tumor vascular for targeted infection tumor cells. Binds with MARCO receptor (macrophage receptor with collagenous structure) | It has been reported that oncoVV (vaccinia varus) encoding AVL Aphrocallistes vastus lectin (AVL) genes enhanced the cytotoxic effect of oncolytic vaccinia virus (oncoVV) in cervical cancer both in vitro and in vivo. | [63] |
| Herpes simplex virus(HSV) | Linear, double stranded DNA genome virus with approximately 152 kbp length | Binds with at least 3 receptors which are over expressed/abnormally expressed on cancer cells. Receptors are HVEM, nectin-1 and 3-OS-HS. | As reported, triple-mutated, third-generation HSV therapy was targeted for HPV16- or HPV18-associated cervical carcinoma in which human Hela xenograft and TC-1 syngeneic models were studied. It was found that oncolytic HSV greatly inhibited cervical tumor growth, mediated apoptosis, and turned cervical cancer tumor “hot” for immune targeting. | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kousar, K.; Ahmad, T.; Naseer, F.; Kakar, S.; Anjum, S. Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma. Cancers 2022, 14, 4458. https://doi.org/10.3390/cancers14184458
Kousar K, Ahmad T, Naseer F, Kakar S, Anjum S. Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma. Cancers. 2022; 14(18):4458. https://doi.org/10.3390/cancers14184458
Chicago/Turabian StyleKousar, Kousain, Tahir Ahmad, Faiza Naseer, Salik Kakar, and Sadia Anjum. 2022. "Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma" Cancers 14, no. 18: 4458. https://doi.org/10.3390/cancers14184458
APA StyleKousar, K., Ahmad, T., Naseer, F., Kakar, S., & Anjum, S. (2022). Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma. Cancers, 14(18), 4458. https://doi.org/10.3390/cancers14184458






