Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture of 3 Meningioma Cell Lines
2.3. Primary Cell Culture of Freshly Excised Human Meningioma
2.4. Genomic Characterization
2.5. Cell Viability
2.6. BrdU (5-Bromo-2′-Deoxyuridine) Incorporation
2.7. Apoptosis Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Genomic Characterization of the Three Human Meningioma Cell Lines
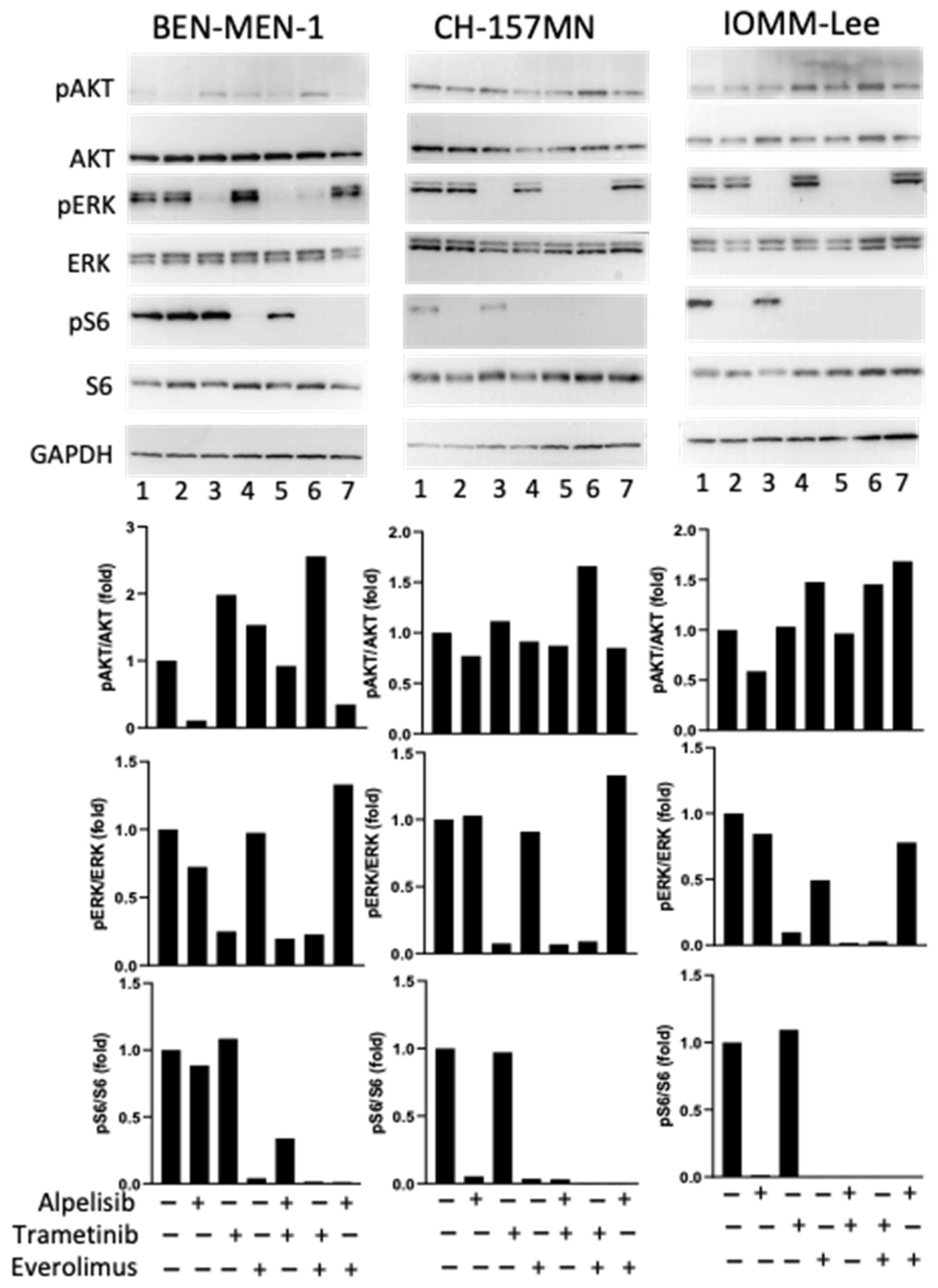
3.2. Drugs Effects on Signaling Pathways and Cell Viability on the 3 Meningioma Cell Lines
3.3. Clinical and Genomic Characterization of the Human Meningioma
3.4. Drug Effects on Signaling Pathways on Meningioma in Primary Culture
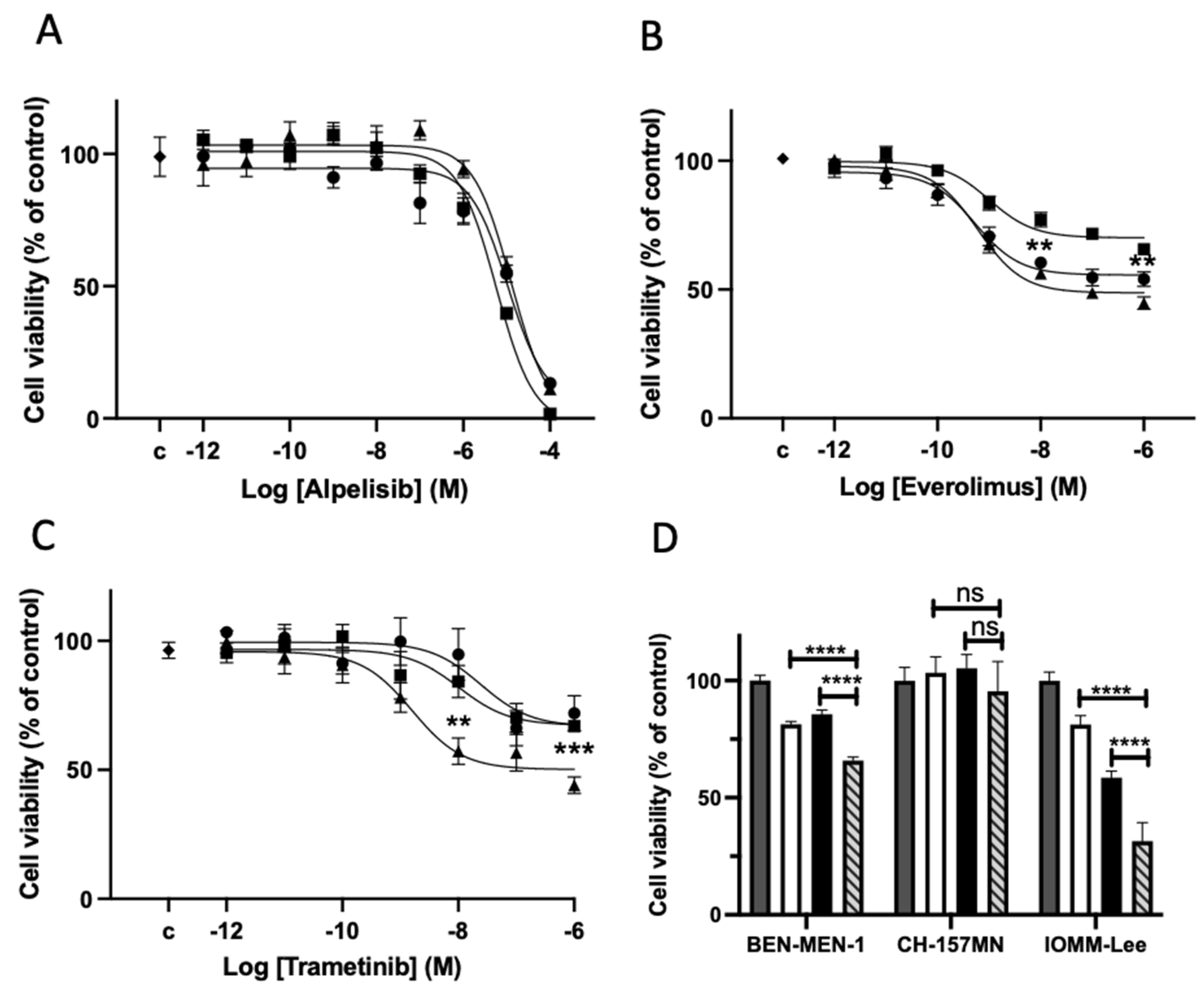
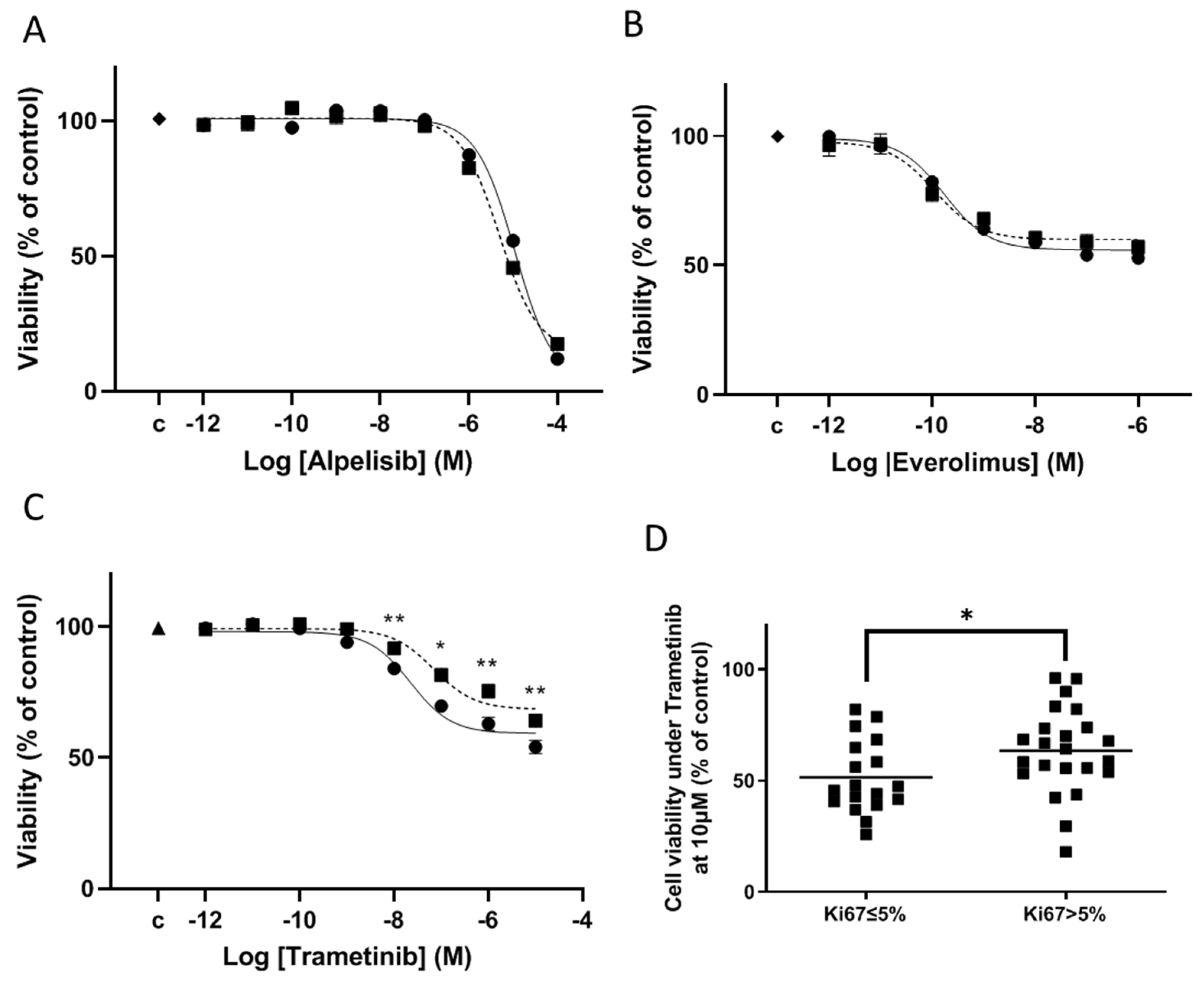
3.5. Drug Effects on Cell Viability on Meningioma in Primary Culture
3.6. Mechanism of Cell Viability Inhibition on Meningioma in Primary Culture
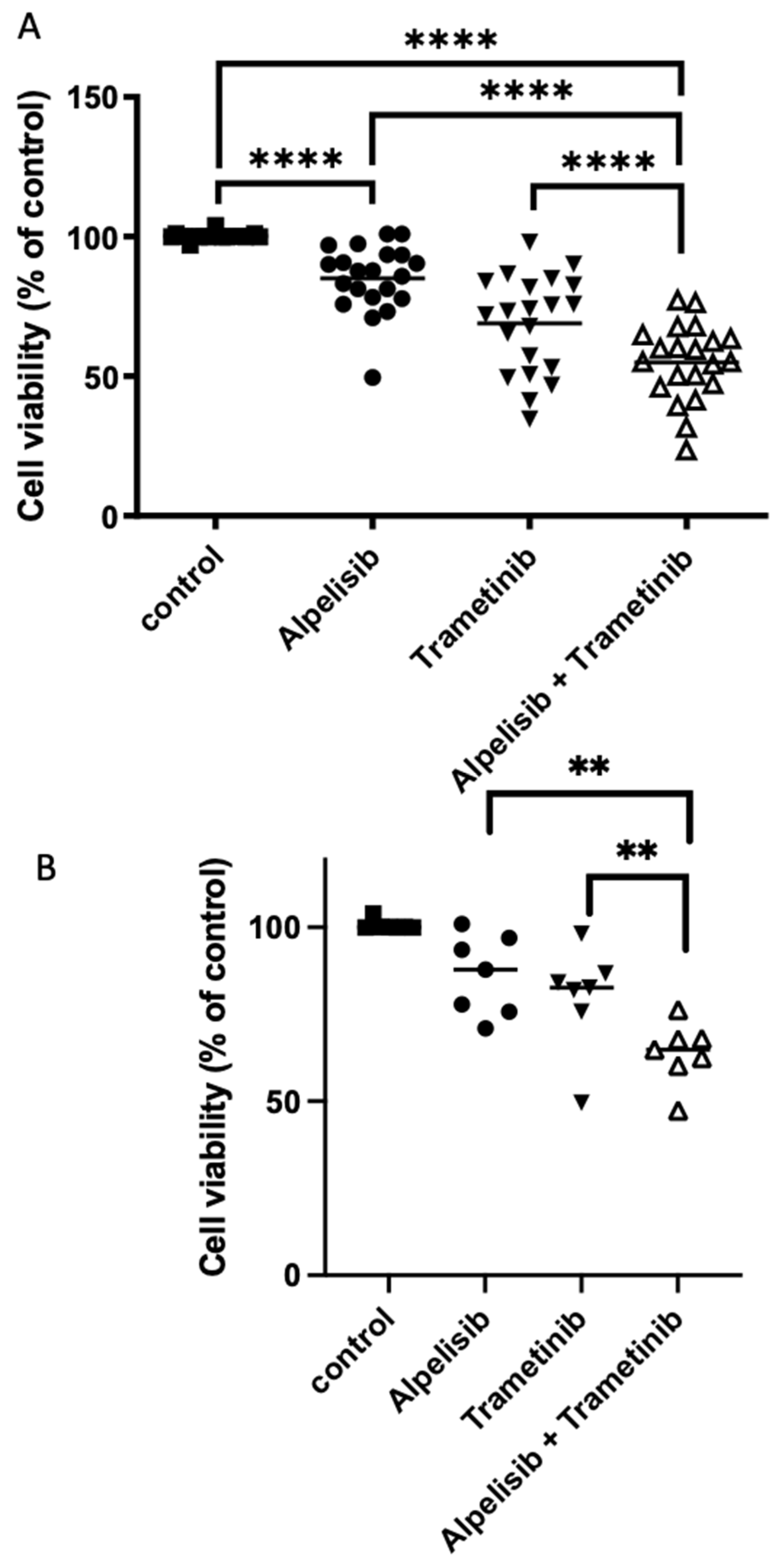
3.7. Effect of Combined Treatments Targeting Pi3K-Akt-mTOR and MAP Kinase Pathways on Meningioma in Primary Culture
4. Discussion
4.1. Pi3K-AKT-mTOR Pathway Targeting
4.2. MAP Kinase/ERK Pathway Targeting
4.3. Co-Targeting the Pi3K-AKT-mTOR and MAPkinase Pathways
4.4. Relevance and Limits of this In Vitro Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO Guidelines for the Diagnosis and Treatment of Meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical Benchmarks for Medical Therapy Trials in Surgery- and Radiation-Refractory Meningioma: A RANO Review. Neuro Oncol. 2014, 16, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Iwamoto, F.M.; Rudnick, J.D.; Norden, A.D.; Lee, E.Q.; Drappatz, J.; Omuro, A.; Kaley, T.J. Atypical and Anaplastic Meningiomas Treated with Bevacizumab. J. Neurooncol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II Trial of Sunitinib for Recurrent and Progressive Atypical and Anaplastic Meningioma. Neuro Oncol. 2015, 17, 116–121. [Google Scholar] [CrossRef]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Dias-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K Mutations Are as Common as AKT1 and SMO Mutations in Meningioma. Neuro Oncol. 2016, 18, 649–655. [Google Scholar] [CrossRef]
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/Merlin Is a Novel Negative Regulator of MTOR Complex 1, and Activation of MTORC1 Is Associated with Meningioma and Schwannoma Growth. Mol. Cell Biol. 2009, 29, 4250–4261. [Google Scholar] [CrossRef]
- Johnson, M.D.; Toms, S.A. Evidence for Phosphatidylinositol 3-Kinase–Akt–P70 S6K Pathway Activation and Transduction of Mitogenic Signals by Platelet-Derived Growth Factor in Meningioma Cells. J. Neurosurg. 2002, 97, 8. [Google Scholar] [CrossRef]
- Mawrin, C.; Sasse, T.; Kirches, E.; Kropf, S.; Schneider, T.; Grimm, C.; Pambor, C.; Vorwerk, C.K.; Firsching, R.; Lendeckel, U.; et al. Different Activation of Mitogen-Activated Protein Kinase and Akt Signaling Is Associated with Aggressive Phenotype of Human Meningiomas. Clin. Cancer Res. 2005, 11, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. MTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef]
- Pachow, D.; Wick, W.; Gutmann, D.H.; Mawrin, C. The MTOR Signaling Pathway as a Treatment Target for Intracranial Neoplasms. Neuro Oncol. 2015, 17, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Graillon, T.; Defilles, C.; Mohamed, A.; Lisbonis, C.; Germanetti, A.-L.; Chinot, O.; Figarella-Branger, D.; Roche, P.-H.; Adetchessi, T.; Fuentes, S.; et al. Combined Treatment by Octreotide and Everolimus: Octreotide Enhances Inhibitory Effect of Everolimus in Aggressive Meningiomas. J. Neurooncol. 2015, 124, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Romano, D.; Defilles, C.; Lisbonis, C.; Saveanu, A.; Figarella-Branger, D.; Roche, P.-H.; Fuentes, S.; Chinot, O.; Dufour, H.; et al. Pasireotide Is More Effective than Octreotide, Alone or Combined with Everolimus on Human Meningioma in Vitro. Oncotarget 2017, 8, 55361–55373. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clinical Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Ferrer, L.; Siffre, J.; Sanson, M.; Peyre, M.; Peyrière, H.; Mougel, G.; Autran, D.; Tabouret, E.; Figarella-Branger, D.; et al. Role of 3D Volume Growth Rate for Drug Activity Evaluation in Meningioma Clinical Trials: The Example of the CEVOREM Study. Neuro Oncol. 2021, 23, 1139–1147. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA -Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Xu, S.; Wang, S.; Tu, Y.; Xiong, Y.; Mei, J.; Wang, C. The Role of MAPK Signaling Pathway in the Her-2-Positive Meningiomas. Oncol. Rep. 2016, 36, 685–695. [Google Scholar] [CrossRef]
- Johnson, M.D.; O’Connell, M.; Vito, F.; Bakos, R.S. Increased STAT-3 and Synchronous Activation of Raf-1-MEK-1-MAPK, and Phosphatidylinositol 3-Kinase-Akt-MTOR Pathways in Atypical and Anaplastic Meningiomas. J. Neurooncol. 2009, 92, 129–136. [Google Scholar] [CrossRef]
- Kelly, R.J. Dabrafenib and Trametinib for the Treatment of Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2018, 18, 1063–1068. [Google Scholar] [CrossRef]
- Zeiser, R.; Andrlová, H.; Meiss, F. Trametinib (GSK1120212). In Small Molecules in Oncology; Martens, U.M., Ed.; Recent Results in Cancer Research; Springer International Publishing: Cham, Switzerland, 2018; Volume 211, pp. 91–100. ISBN 978-3-319-91441-1. [Google Scholar]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Özduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic Analysis of Non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Chteinberg, E.; Wetzels, S.; Gerritsen, W.; Temmerman, L.; van den Oord, J.; Biessen, E.; Kurz, A.K.; Winnepenninckx, V.; Zenke, M.; Speel, E.-J.; et al. Navitoclax Combined with Alpelisib Effectively Inhibits Merkel Cell Carcinoma Cell Growth in Vitro. Ther Adv. Med. Oncol. 2020, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.; Sendino Garví, E.; Stelloo, E.; Stangl, C.; Sereno, F.; Duran, K.; Groeneweg, J.; Paijens, S.; Nijman, H.; van Meurs, H.; et al. In Vitro Systematic Drug Testing Reveals Carboplatin, Paclitaxel, and Alpelisib as a Potential Novel Combination Treatment for Adult Granulosa Cell Tumors. Cancers 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Le Van, T.; Graillon, T.; Jacob, J.; Vauleon, E.; Feuvret, L.; Boch, A.-L.; Boetto, J.; Boone, M.; Bronnimann, C.; Caire, F.; et al. Multimodal Management of Surgery- and Radiation-Refractory Meningiomas: An Analysis of the French National Tumor Board Meeting on Meningiomas Cohort. J. Neurooncol. 2021, 153, 55–64. [Google Scholar] [CrossRef]
- Yesilöz, Ü.; Kirches, E.; Hartmann, C.; Scholz, J.; Kropf, S.; Sahm, F.; Nakamura, M.; Mawrin, C. Frequent AKT1E17K Mutations in Skull Base Meningiomas Are Associated with MTOR and ERK1/2 Activation and Reduced Time to Tumor Recurrence. Neuro Oncol. 2017, 19, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-Y.; Ma, W.-L.; Lu, Y.-S. Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives. TCRM 2021, 17, 193–207. [Google Scholar] [CrossRef]
- Mordechai, O.; Postovsky, S.; Vlodavsky, E.; Eran, A.; Constantini, S.; Dotan, E.; Cagnano, E.; Weyl-Ben-Arush, M. Metastatic Rhabdoid Meningioma with BRAF V600E Mutation and Good Response to Personalized Therapy: Case Report and Review of the Literature. Pediatric Hematol. Oncol. 2015, 32, 207–211. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsia, T.-C.; Yeh, M.-H.; Chen, T.-W.; Chen, Y.-J.; Chen, J.-T.; Wei, Y.-L.; Tu, C.-Y.; Huang, W.-C. MEK Inhibitors Induce Akt Activation and Drug Resistance by Suppressing Negative Feedback ERK-Mediated HER2 Phosphorylation at Thr701. Mol. Oncol. 2017, 11, 1273–1287. [Google Scholar] [CrossRef]
- Mudianto, T.; Campbell, K.M.; Webb, J.; Zolkind, P.; Skidmore, Z.L.; Riley, R.; Barnell, E.K.; Ozgenc, I.; Giri, T.; Dunn, G.P.; et al. Yap1 Mediates Trametinib Resistance in Head and Neck Squamous Cell Carcinomas. Clin. Cancer Res. 2021, 27, 2326–2339. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondielli, G.; Mougel, G.; Darriet, F.; Roche, C.; Querdray, A.; Lisbonis, C.; Appay, R.; Dufour, H.; Chinot, O.; Graillon, T.; et al. Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib. Cancers 2022, 14, 4448. https://doi.org/10.3390/cancers14184448
Mondielli G, Mougel G, Darriet F, Roche C, Querdray A, Lisbonis C, Appay R, Dufour H, Chinot O, Graillon T, et al. Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib. Cancers. 2022; 14(18):4448. https://doi.org/10.3390/cancers14184448
Chicago/Turabian StyleMondielli, Gregoire, Gregory Mougel, Florent Darriet, Catherine Roche, Adeline Querdray, Christophe Lisbonis, Romain Appay, Henry Dufour, Olivier Chinot, Thomas Graillon, and et al. 2022. "Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib" Cancers 14, no. 18: 4448. https://doi.org/10.3390/cancers14184448
APA StyleMondielli, G., Mougel, G., Darriet, F., Roche, C., Querdray, A., Lisbonis, C., Appay, R., Dufour, H., Chinot, O., Graillon, T., & Barlier, A. (2022). Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib. Cancers, 14(18), 4448. https://doi.org/10.3390/cancers14184448




