A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Criteria for the Inclusion of Studies
2.3. Study Selection and Data Extraction
2.4. Adherence Analysis
2.5. Outcomes and Analysis
2.6. Ethical Consideration
3. Results
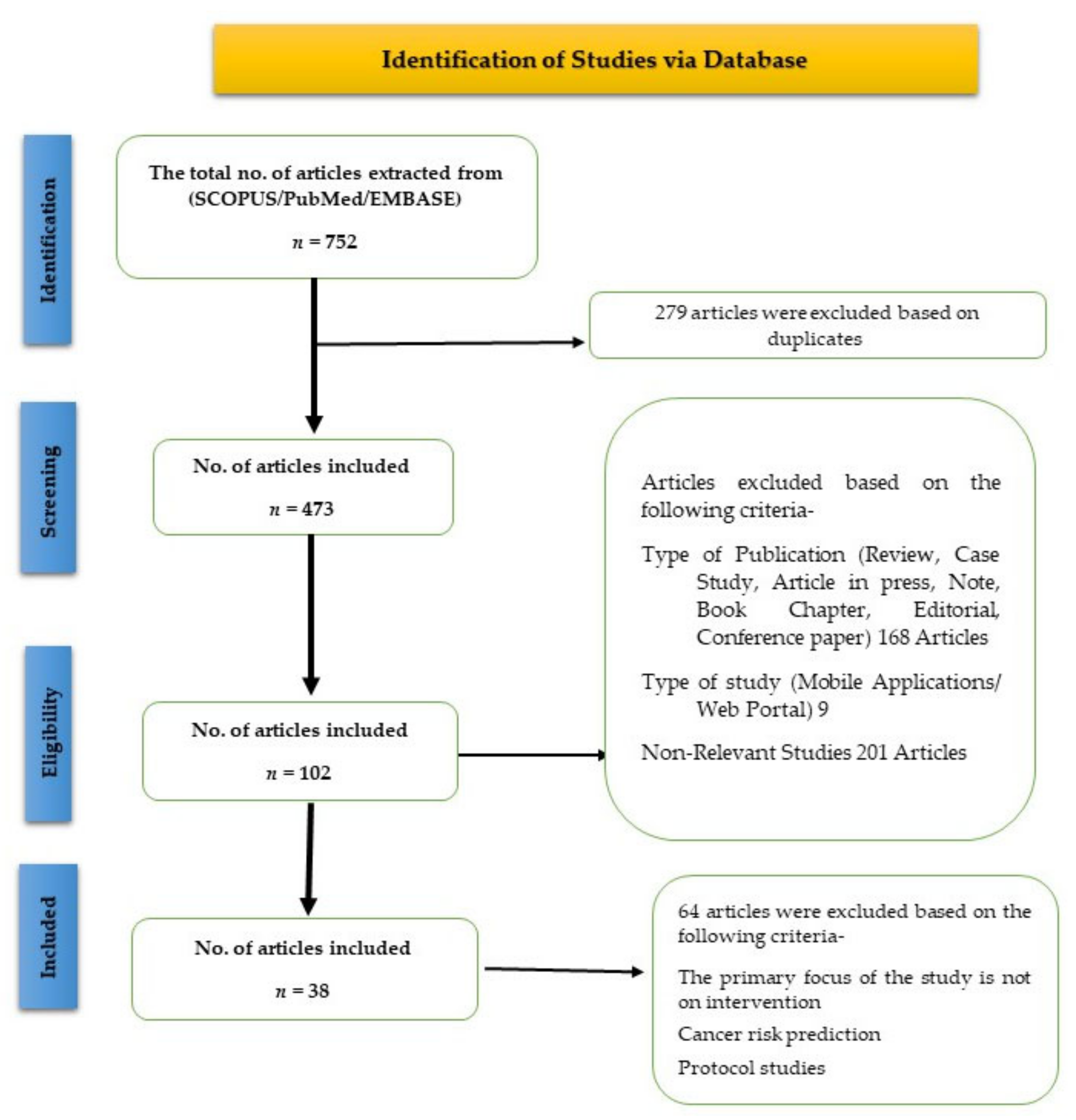
3.1. Study Selection
3.2. Study Characteristics
3.3. Characteristics of Research Participants
3.4. Measurement Tools
3.5. Major Study Focus
3.6. Intervention Methods
3.7. Interventional Outcomes
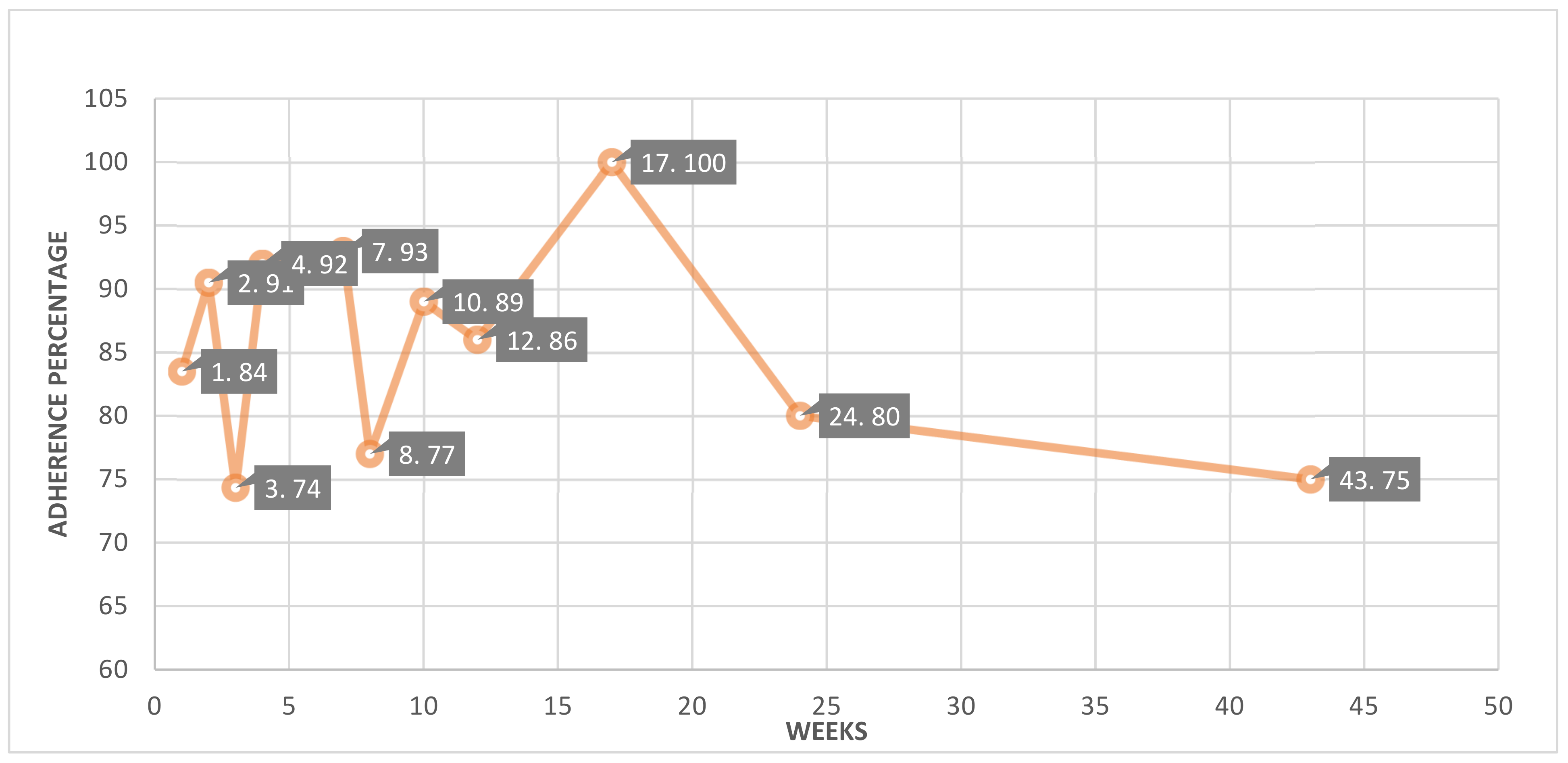
3.7.1. Adherence
3.7.2. Clinical Outcomes
4. Discussion
4.1. Summary and Findings
4.2. eHealth Tools for Cancer Care
4.3. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, A.E.; Jensen, R.E.; Basch, E.M. Leveraging Emerging Technologies and the “Internet of Things” to Improve the Quality of Cancer Care. J. Oncol. Pract. 2016, 12, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, A.; De Maria, C.; Di Pietro, L.; Sternini, F.; Audenino, A.L.; Bignardi, C. Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing. Front. Bioeng. Biotechnol. 2019, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Loucks, J. Wearable Technology in Health Care: Getting Better All the Time. TMT Predictions 2022; Deloitte: New York, NY, USA, 2021. [Google Scholar]
- Sabry, F.; Eltaras, T.; Labda, W.; Alzoubi, K.; Malluhi, Q. Machine Learning for Healthcare Wearable Devices: The Big Picture. J. Healthc. Eng. 2022, 2022, 4653923. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Wearable Technology Applications in Healthcare: A Literature Review. Online J. Nurs. Inform. 2019, 26, 23. [Google Scholar]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Per. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- Lazazzera, R.; Belhaj, Y.; Carrault, G. A New Wearable Device for Blood Pressure Estimation Using Photoplethysmogram. Sensors 2019, 19, 2557. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Aceto, G.; Persico, V.; Pescapé, A. Industry 4.0 and Health: Internet of Things, Big Data, and Cloud Computing for Healthcare 4.0. J. Ind. Inf. Integr. 2020, 18, 100129. [Google Scholar] [CrossRef]
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices-Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef]
- Park, J.; Look, K.A. Relationship Between Objective Financial Burden and the Health-Related Quality of Life and Mental Health of Patients With Cancer. J. Oncol. Pract. 2018, 14, e113–e121. [Google Scholar] [CrossRef]
- Chowthi-Williams, A.; Davis, G. Successful Change Management in Health Care: Being Emotionally and Cognitively Ready; Routledge: London, UK, 2022. [Google Scholar]
- Farahani, B.; Firouzi, F.; Chang, V.; Badaroglu, M.; Constant, N.; Mankodiya, K. Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare. Future Gener. Comput. Syst. 2018, 78, 659–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Reilly, C.M.; Bruner, D.W.; Mitchell, S.A.; Minasian, L.M.; Basch, E.; Dueck, A.C.; Cella, D.; Reeve, B.B. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 2013, 21, 1525–1550. [Google Scholar] [CrossRef]
- A Low, C.; Dey, A.K.; Ferreira, D.; Kamarck, T.; Sun, W.; Bae, S.; Doryab, A. Estimation of Symptom Severity During Chemotherapy From Passively Sensed Data: Exploratory Study. J. Med. Internet Res. 2017, 19, e420. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S.; Caplan, L.S.; Stone, R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: A review of health intervention studies. J. Cancer Surviv. 2020, 14, 386–392. [Google Scholar] [CrossRef]
- Herrington, W.G.; Goldsack, J.C.; Landray, M.J. Increasing the use of mobile technology-derived endpoints in clinical trials. Clin. Trials 2018, 15, 313–315. [Google Scholar] [CrossRef]
- Byrom, B.; Watson, C.; Doll, H.; Coons, S.J.; Eremenco, S.; Ballinger, R.; McCarthy, M.; Crescioni, M.; O’Donohoe, P.; Howry, C.; et al. Selection of and Evidentiary Considerations for Wearable Devices and Their Measurements for Use in Regulatory Decision Making: Recommendations from the ePRO Consortium. Value Health 2018, 21, 631–639. [Google Scholar] [CrossRef]
- Beauchamp, U.L.; Pappot, H.; Holländer-Mieritz, C. The Use of Wearables in Clinical Trials During Cancer Treatment: Systematic Review. JMIR mHealth and uHealth 2020, 8, e22006. [Google Scholar] [CrossRef]
- Ballinger, T.J.; Althouse, S.K.; Olsen, T.P.; Miller, K.D.; Sledge, J.S. A Personalized, Dynamic Physical Activity Intervention Is Feasible and Improves Energetic Capacity, Energy Expenditure, and Quality of Life in Breast Cancer Survivors. Front. Oncol. 2021, 11, 626180. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Vallance, J.K.; Buman, M.P.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Milton, S.; Friedenreich, C.M.; English, D.R.; et al. Effects of a wearable technology-based physical activity intervention on sleep quality in breast cancer survivors: The ACTIVATE Trial. J. Cancer Surviv. 2021, 15, 273–280. [Google Scholar] [CrossRef]
- Ferrante, J.M.; Lulla, A.; Williamson, J.D.; Devine, K.A.; Ohman-Strickland, P.; Bandera, E.V. Patterns of Fitbit Use and Activity Levels Among African American Breast Cancer Survivors During an eHealth Weight Loss Randomized Controlled Trial. Am. J. Health Promot. 2022, 36, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.M.; Nguyen, N.H.; Moore, M.M.; Reeves, M.M.; Rosenberg, D.E.; Boyle, T.; Vallance, J.K.; Milton, S.; Friedenreich, C.M.; English, D.R. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer 2019, 125, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Pope, Z.C.; Zeng, N.; Zhang, R.; Lee, H.Y.; Gao, Z. Effectiveness of Combined Smartwatch and Social Media Intervention on Breast Cancer Survivor Health Outcomes: A 10-Week Pilot Randomized Trial. J. Clin. Med. 2018, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Mcneil, J.; Brenner, D.R.; Stone, C.R.; O’Reilly, R.; Ruan, Y.; Vallance, J.K.; Courneya, K.S.; Thorpe, K.; Klein, D.J.; Friedenreich, C.M. Activity Tracker to Prescribe Various Exercise Intensities in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2019, 51, 930–940. [Google Scholar] [CrossRef]
- Wu, H.S.; Gal, R.; Van Sleeuwen, N.C.; Brombacher, A.C.; Ijsselsteijn, W.A.; May, A.M.; Monninkhof, E.M.; Jerome, G.; Arnoldussen, B.; Lynch, B. Breast Cancer Survivors’ Experiences With an Activity Tracker Integrated Into a Supervised Exercise Program: Qualitative Study. JMIR mHealth and uHealth 2019, 7, e10820. [Google Scholar] [CrossRef] [PubMed]
- Che Bakri, N.A.; Kwasnicki, R.M.; Dhillon, K.; Khan, N.; Ghandour, O.; Cairns, A.; Darzi, A.; Leff, D.R. Objective Assessment of Postoperative Morbidity After Breast Cancer Treatments with Wearable Activity Monitors: The “BRACELET” Study. Ann. Surg. Oncol. 2021, 28, 5597–5609. [Google Scholar] [CrossRef]
- Gandhi, A.; Samuel, S.R.; Kumar, K.V.; Saxena, P.P.; Mithra, P. Effect of a Pedometer-based Exercise Program on Cancer Related Fatigue and Quality of Life amongst Patients with Breast Cancer Receiving Chemotherapy. Asian Pac. J. Cancer Prev. 2020, 21, 1813–1818. [Google Scholar] [CrossRef]
- Nelson, S.H.; Weiner, L.S.; Natarajan, L.; Parker, B.A.; Patterson, R.E.; Hartman, S.J. Continuous, objective measurement of physical activity during chemotherapy for breast cancer: The Activity in Treatment pilot study. Transl. Behav. Med. 2020, 10, 1031–1038. [Google Scholar] [CrossRef]
- Champ, C.E.; Ohri, N.; Klement, R.J.; Cantor, M.; Beriwal, S.; Glaser, S.M.; Smith, R.P. Assessing Changes in the Activity Levels of Breast Cancer Patients During Radiation Therapy. Clin. Breast Cancer 2018, 18, e1–e6. [Google Scholar] [CrossRef]
- Helbrich, H.; Braun, M.; Hanusch, C.; Mueller, G.; Falk, H.; Flondor, R.; Harbeck, N.; Hermelink, K.; Wuerstlein, R.; Keim, S.; et al. Congruence and trajectories of device-measured and self-reported physical activity during therapy for early breast cancer. Breast Cancer Res. Treat. 2021, 188, 351–359. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Sun, Q.; Xiao, P.; Duan, Y.; Liu, X.; Zhou, J.; Xie, J.; Cheng, A.S. Effect of Two Interventions on Sleep Quality for Adolescent and Young Adult Cancer Survivors: A Pilot Randomized Controlled Trial. Cancer Nurs. 2022, 45, E560–E572. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, Z.; Nilanon, T.; Li, M.; Mejia, A.; Kolatkar, A.; Nocera, L.; Shahabi, C.; Philips, F.A.C.; Lee, J.S.; Hanlon, S.E.; et al. Quantified Kinematics to Evaluate Patient Chemotherapy Risks in Clinic. JCO Clin. Cancer Inform. 2020, 4, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Nilanon, T.; Nocera, L.P.; Martin, A.S.; Kolatkar, A.; May, M.; Hasnain, Z.; Ueno, N.T.; Yennu, S.; Alexander, A.; Mejia, A.E.; et al. Use of Wearable Activity Tracker in Patients With Cancer Undergoing Chemotherapy: Toward Evaluating Risk of Unplanned Health Care Encounters. JCO Clin. Cancer Inform. 2020, 4, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Yurkiewicz, I.R.; Simon, P.; Liedtke, M.; Dahl, G.; Dunn, T. Effect of Fitbit and iPad Wearable Technology in Health-Related Quality of Life in Adolescent and Young Adult Cancer Patients. J. Adolesc. Young Adult Oncol. 2018, 7, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, T.; Kawaguchi, T.; Azuma, K.; Suzuki, S.; Sano, Y.; Akatsu, M.; Torii, A.; Kamimura, T.; Ozawa, Y.; Tsuchida, A.; et al. Patient-generated health data collection using a wearable activity tracker in cancer patients-a feasibility study. Support. Care Cancer 2020, 28, 5953–5961. [Google Scholar] [CrossRef]
- Komarzynski, S.; Huang, Q.; Lévi, F.A.; Palesh, O.G.; Ulusakarya, A.; Bouchahda, M.; Haydar, M.; Wreglesworth, N.I.; Morère, J.-F.; Adam, R.; et al. The day after: Correlates of patient-reported outcomes with actigraphy-assessed sleep in cancer patients at home (inCASA project). Sleep 2019, 42, zsz146. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Maxwell-Smith, C.; Hince, D.; Bulsara, M.K.; Boyle, T.; Tan, P.; Levitt, M.; Salama, P.; Mohan, G.R.K.A.; Salfinger, S.; et al. The wearable activity technology and action-planning trial in cancer survivors: Physical activity maintenance post-intervention. J. Sci. Med. Sport 2021, 24, 902–907. [Google Scholar] [CrossRef]
- Devine, K.A.; Viola, A.; Levonyan-Radloff, K.; Mackowski, N.; Bozzini, B.; Chandler, A.; Xu, B.; Ohman-Strickland, P.; Mayans, S.; Farrar-Anton, A.; et al. Feasibility of FitSurvivor: A technology-enhanced group-based fitness intervention for adolescent and young adult survivors of childhood cancer. Pediatr. Blood Cancer 2020, 67, e28530. [Google Scholar] [CrossRef]
- Pavic, M.; Klaas, V.; Theile, G.; Kraft, J.; Tröster, G.; Guckenberger, M. Feasibility and Usability Aspects of Continuous Remote Monitoring of Health Status in Palliative Cancer Patients Using Wearables. Oncology 2020, 98, 386–395. [Google Scholar] [CrossRef]
- Mendoza, J.A.; Baker, K.S.; Moreno, M.A.; Whitlock, K.; Abbey-Lambertz, M.; Waite, A.; Colburn, T.; Chow, E.J. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr. Blood Cancer 2017, 64, e26660. [Google Scholar] [CrossRef]
- Cadmus-Bertram, L.; Tevaarwerk, A.J.; Sesto, M.E.; Gangnon, R.; Van Remortel, B.; Date, P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: A randomized controlled pilot trial. J. Cancer Surviv. 2019, 13, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.J.; Jiménez-Castuera, R.; Maxwell-Smith, C.; Bulsara, M.K.; Hince, D. Fitbit wear-time and patterns of activity in cancer survivors throughout a physical activity intervention and follow-up: Exploratory analysis from a randomised controlled trial. PLoS ONE 2020, 15, e0240967. [Google Scholar] [CrossRef] [PubMed]
- Maxwell-Smith, C.; Hince, D.; Cohen, P.; Bulsara, M.K.; Boyle, T.; Platell, C.; Tan, P.; Levitt, M.; Salama, P.; Tan, J.; et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psychooncology 2019, 28, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Chan, H.; Van Loon, K.; Kenfield, S.A.; Chan, J.M.; Mitchell, E.; Zhang, L.; Paciorek, A.; Joseph, G.; Laffan, A.; et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): A pilot randomized controlled trial. BMC Cancer 2019, 19, 218. [Google Scholar] [CrossRef]
- Cheong, I.Y.; An, S.Y.; Cha, W.C.; Rha, M.Y.; Kim, S.T.; Chang, D.K.; Hwang, J.H. Efficacy of Mobile Health Care Application and Wearable Device in Improvement of Physical Performance in Colorectal Cancer Patients Undergoing Chemotherapy. Clin. Colorectal Cancer 2018, 17, e353–e362. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Dhruva, A.; Atreya, C.E.; Kenfield, S.A.; Chan, J.M.; Milloy, A.; Kim, I.; Steiding, P.; Laffan, A.; Zhang, L.; et al. Feasibility and Acceptability of a Physical Activity Tracker and Text Messages to Promote Physical Activity During Chemotherapy for Colorectal Cancer: Pilot Randomized Controlled Trial (Smart Pace II). JMIR Cancer 2022, 8, e31576. [Google Scholar] [CrossRef]
- Shih, C.A.-O.; Chou, P.A.-O.; Chou, T.A.-O.; Huang, T.A.-O. Measurement of Cancer-Related Fatigue Based on Heart Rate Variability: Observational Study. J. Med. Internet Res. 2021, 23, e25791. [Google Scholar] [CrossRef]
- Bade, B.C.; Brooks, M.C.; Nietert, S.B.; Ulmer, A.; Thomas, D.D.; Nietert, P.J.; Scott, J.B.; Silvestri, G.A. Assessing the Correlation Between Physical Activity and Quality of Life in Advanced Lung Cancer. Integr. Cancer Ther. 2018, 17, 73–79. [Google Scholar] [CrossRef]
- Finley, D.J.; Stevens, C.J.; Emond, J.A.; Batsis, J.A.; Fay, K.A.; Darabos, C.; Sacks, O.A.; Cook, S.B.; Lyons, K.D. Potential effectiveness of a surgeon-delivered exercise prescription and an activity tracker on pre-operative exercise adherence and aerobic capacity of lung cancer patients. Surg. Oncol. 2021, 37, 101525. [Google Scholar] [CrossRef]
- Kong, S.; Park, H.Y.; Kang, D.; Lee, J.K.; Lee, G.; Kwon, O.J.; Shim, Y.M.; Zo, J.I.; Cho, J. Seasonal Variation in Physical Activity among Preoperative Patients with Lung Cancer Determined Using a Wearable Device. J. Clin. Med. 2020, 9, 349. [Google Scholar] [CrossRef]
- Ghods, A.; Shahrokni, A.; Ghasemzadeh, H.; Cook, D. Remote Monitoring of the Performance Status and Burden of Symptoms of Patients With Gastrointestinal Cancer Via a Consumer-Based Activity Tracker: Quantitative Cohort Study. JMIR Cancer 2021, 7, e22931. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Ho, T.-W.; Chang, Y.-T.; Hsu, C.; Tsai, C.J.; Lai, F.; Lin, M.-T. Wearable-Based Mobile Health App in Gastric Cancer Patients for Postoperative Physical Activity Monitoring: Focus Group Study. JMIR mHealth and uHealth 2019, 7, e11989. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, J.; An, S.Y.; Sinn, D.H.; Hwang, J.H. Efficacy and Safety of an mHealth App and Wearable Device in Physical Performance for Patients With Hepatocellular Carcinoma: Development and Usability Study. JMIR mHealth and uHealth 2020, 8, e14435. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.J.; Aibel, K.; Meyerhoff, R.; Wang, F.; Harpole, D.; Abernethy, A.P.; Leblanc, T.W. Actigraphy assessment of sleep quality among patients with acute myeloid leukaemia during induction chemotherapy. BMJ Supportive Palliat. Care 2018, 8, 274–277. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Naiki, T.; Tasaki, Y.; Kataoka, T.; Mimura, Y.; Kondo, Y.; Etani, T.; Iida, K.; Nozaki, S.; Ando, R.; et al. Effectiveness of continuous monitoring by activity tracker of patients undergoing chemotherapy for urothelial carcinoma. Cancer Treat. Res. Commun. 2020, 25, 100245. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.K.; Durst, D.A.; Gray, E.; Kwasny, M.; Heo, S.Y.; Banks, A.; Rogers, J.A. Sun exposure reduction by melanoma survivors with wearable sensor providing real-time UV exposure and daily text messages with structured goal setting. Arch. Dermatol. Res. 2021, 313, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, K.; Abuzour, A.; Khaira, M.; Mathers, A.; Chan, A.; Bui, V.; Lok, A.; Thabane, L.; Dolovich, L. The Use and Effects of Electronic Health Tools for Patient Self-Monitoring and Reporting of Outcomes Following Medication Use: Systematic Review. J. Med. Internet Res. 2018, 20, e294. [Google Scholar] [CrossRef]
- Bakker, J.P.; Goldsack, J.C.; Clarke, M.; Coravos, A.; Geoghegan, C.; Godfrey, A.; Heasley, M.G.; Karlin, D.; Manta, C.; Peterson, B.; et al. A systematic review of feasibility studies promoting the use of mobile technologies in clinical research. NPJ Digit. Med. 2019, 2, 47. [Google Scholar] [CrossRef]
- Gresham, G.; Schrack, J.; Gresham, L.M.; Shinde, A.M.; Hendifar, A.E.; Tuli, R.; Rimel, B.; Figlin, R.; Meinert, C.L.; Piantadosi, S. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp. Clin. Trials 2018, 64, 13–21. [Google Scholar] [CrossRef]
- Kadhim, K.T.; Alsahlany, A.M.; Wadi, S.M.; Kadhum, H.T. An Overview of Patient’s Health Status Monitoring System Based on Internet of Things (IoT). Wirel. Pers. Commun. 2020, 114, 2235–2262. [Google Scholar] [CrossRef]
| Country of Study | Topic (Type of Cancer and Status) | Study Design | Tools Used | Participant (%) Gender | |
|---|---|---|---|---|---|
| The United States of America [21] | Breast Cancer (n = 57) | Survivors | Feasibility Study | Wearable Device and Questionnaire | 100% Women |
| Australia [22] | Breast Cancer (n = 80) | Survivors | RCT | Wearable Device + Text Messages and Personal Interviews + Mobile Application | 100% Women |
| The United States of America [23] | Breast Cancer (n = 34) | Survivors | RCT | Wearable Device and Questionnaire (Correlation) | 100% Women |
| Australia [24] | Breast Cancer (n = 80) | Survivors | RCT | Wearable Device and Questionnaire (Correlation) | 100% Women |
| The United States of America [25] | Breast Cancer (n = 20) | Survivors | RCT | Wearable Device + Group Sessions and Phone Calls | 100% Women |
| Canada [26] | Breast Cancer (n = 41) | Survivors | RCT | Wearable Device and Questionnaires (Correlation) | 100% Women |
| The Netherlands [27] | Breast Cancer (n = 8) | Survivors | Qualitative Study | Wearable Device and Questionnaires | 100% Women |
| United Kingdom [28] | Breast Cancer (n = 39) | Under Treatment | Non-RCT | Wearable Device, Questionnaire, and Behavioral Counseling Session | 100% Women |
| India [29] | Breast Cancer (n = 44) | Under Treatment | Non-RCT | Wearable Device + General group session + Questionnaire + Mobile Application | 95.4% Women |
| The United States of America [30] | Breast Cancer (n = 32) | Under Treatment | Pilot Study | Wearable Device + Mobile application + Text Messages | 100% Women |
| The United States of America [31] | Breast Cancer (n = 10) | Under Treatment | RCT | Wearable Device and Questionnaire | 100% Women |
| Germany [32] | Breast Cancer (n = 99) | Under Treatment | Feasibility Study | Wearable Device and Questionnaire | 100% Women |
| Central China [33] | Mixed Cancer (n = 112) | Under Treatment | RCT | Wearable Device | 76.2% Women |
| The United States of America [34] | Mixed Cancer (n = 38) | Under Treatment | Utility Study/Predictive Study | Wearable Device + Mobile application and Interview | 52% Women |
| The United States of America [35] | Mixed Cancer (n = 41) | Under Treatment | Observational Study | Wearable Device + Mobile application + Questionnaire | 56% Women |
| The United States of America [36] | Mixed Cancer (n = 33) | Under Treatment | Prospective cohort Study | Wearable Devices and Spirometer | 57.5% Women |
| Japan [37] | Mixed Cancer (n = 30) | Under Treatment | Feasibility Study | Wearable Device | 70% Men |
| France [38] | Mixed Cancer (n = 31) | Under Treatment | Pilot Study | Wearable Device + Mobile Application + Questionnaire | 55% Men |
| Ireland [39] | Mixed Cancer (n = 61) | Survivors | RCT | Wearable Devices + Goal-setting session + Telephone-delivered health-coaching sessions | 50% Men |
| The United States of America [40] | Mixed Cancer (n = 32) | Survivors | Feasibility Study | Wearable Device + Two group sessions + support phone call | 51% Men |
| Switzerland [41] | Mixed Cancer (n = 30) | Survivors | Feasibility Study | Fitbit + iPad (preloaded apps) + Questionnaires | 70% Men |
| The United States of America [42] | Mixed Cancer (n = 59) | Survivors | Pilot Study | Wearable Device and Questionnaire | 59.3% Women |
| The United States of America [43] | Mixed Cancer (n = 47) | Survivors | RCT | Wearable Device + Questionnaire + Social Media Intervention (Health Education) | 96% Women |
| Australia [44] | Colorectal and Endometrial cancer (n = 29) | Survivors | RCT | Mobile application (in-app chat service) + Wearable device + Questionnaires | 58% Women |
| Western Australia [45] | Colorectal Cancer (n = 61) | Survivors | RCT | Wearable Device + mHealth app + Peer-based virtual support group + Qualitative Interviews | 50% Women |
| The United States of America [46] | Colorectal Cancer (n = 39) | Survivors | RCT | Wearable Device and Questionnaire-based study | 58% Women |
| South Korea [47] | Colorectal Cancer (n = 75) | Under Treatment | Feasibility Study | Wearable device + Questionnaires + e-Patient Diary | 58.7% Men |
| The United States of America [48] | Colorectal Cancer (n = 40) | Under Treatment | Pilot Study | Wearable Device and Questionnaire-based study | 56.8% Women |
| Taiwan [49] | Lung Cancer (n = 12) | Under Treatment | Observational Study | Wearable Device and Questionnaire-based study | 58.33% Men |
| The United States of America [50] | Lung Cancer (n = 30) | Under Treatment | Observational Study | Wearable Device + Questionnaire + Educational handbook + Social support + Email-based coaching | 67% Men |
| The United States of America [51] | Lung Cancer (n = 18) | Under Treatment | Observational Study | Wearable Device and Questionnaire (Correlation) | 44% Women |
| South Korea [52] | Lung Cancer (n = 555) | Under Treatment | Usability Study | Wearable Devices + Questionnaire+ Educational handbook+ Social support + Email-based coaching | 61% Men |
| The United States of America [53] | Gastric cancer (n = 27) | Under Treatment | Cohort Study | Wearable Device + Mobile Application | 62.96% Men |
| Taiwan [54] | Gastric Cancer (n = 43) | Under Treatment | Group Study | Wearable Devices + Questionnaires | 51% Men |
| South Korea [55] | Liver Cancer (n = 31) | Under Treatment | Usability Study | Wearable Device + Daily text messages+ Questionnaire | 84% Men |
| The United States of America [56] | Blood Cancer (n = 11) | Under Treatment | Feasibility Study | Diary + Accelerometer | 66.6% Men |
| Japan [57] | Urothelial Carcinoma (n = 21) | Under Treatment | Cohort Study | Wearable Device | 84% Men |
| The United States of America [58] | Skin Cancer (n = 60) | Survivor | Observational Study | Wearable Devices + Questionnaire + Interviews | 60% Women |
| Country of Study | Total Study Duration (in Weeks) | Intervention Duration (in Weeks) | Patients Recruited | Criteria for Evaluation | Adherence (in Percentage) |
|---|---|---|---|---|---|
| The United States of America [21] | 24 | 12 | 60 | Percentage of enrolled patients who completed all assessments (10 h per day for 4 days in a week) | 95 |
| Australia [22] | 24 | 12 | 83 | Based on the given assessment completion | 94 |
| The United States of America [23] | 52 | 24 | 44 | Collection of data (days with less than 1000 steps considered as non-adherent) | 65 |
| Australia [24] | 12 | 12 | 83 | Completeness of data collection | 96 |
| The United States of America [25] | 10 | 10 | 30 | Completeness of data collection | 67 |
| Canada [26] | 24 | 12 | 45 | Completeness of data collection | 88 |
| The Netherlands [27] | 12 | 12 | 10 | Based on data collection and total wearing days | 80 |
| United Kingdom [28] | 2 | 2 | 56 | Collected data on the different days (39 patients*14 days) | 89 |
| India [29] | 7 | 7 | 44 | Users’ tolerance ability to the intensity of the program that was set using the rate of perceived exertion (RPE) | 93 |
| The United States of America [30] | 17 | 17 | 32 | Days were considered “valid” if there was any wear time recorded (5 min threshold) | 100 |
| The United States of America [31] | 10 | 10 | 10 | Completeness of data collection | 100 |
| Germany [32] | 24 | 24 | 112 | Completeness of data collection | 95 |
| Central China [33] | 8 | 8 | 143 | Completeness of data collection | 78 |
| The United States of America [34] | 8 | 8 | 45 | Completeness of data collection | 84 |
| The United States of America [35] | 20 | 8 | 34 | Completeness of data collection | 68 |
| The United States of America [36] | 43 | 43 | 44 | Completeness of data collection | 75 |
| Japan [37] | 4 | 4 | 30 | Completeness of data collection | 90 |
| France [38] | 4 | 4 | 30 | Completeness of data collection | 86 |
| Ireland [39] | 24 | 12 | 68 | Completeness of data collection | 89 |
| The United States of America [40] | 52 | 12 | 49 | Completeness of data collection | 65 |
| Switzerland [41] | 12 | 12 | 30 | Completeness of data collection and qualitative analysis of interviews | 83 |
| The United States of America [42] | 10 | 10 | 59 | Completeness of data collection | 100 |
| The United States of America [43] | 12 | 12 | 50 | Completeness of data collection | 94 |
| Australia [44] | 24 | 12 | 34 | Based on participants who completed the study criterion, which is a minimum of 1000 steps or more denoted per day. | 82 |
| Western Australia [45] | 12 | 12 | 68 | Completeness of data collection | 94 |
| The United States of America [46] | 12 | 12 | 41 | Completeness of data collection | 81 |
| South Korea [47] | 12 | 12 | 102 | Completeness of data collection | 74 |
| The United States of America [48] | 12 | 12 | 44 | Completeness of data collection | 88 |
| Taiwan [49] | 1 | 1 | 12 | Completeness of data collection | 100 |
| The United States of America [50] | 1 | 1 | 39 | Completeness of data collection | 67 |
| The United States of America [51] | 3 | 3 | 30 | Completeness of data collection | 60 |
| South Korea [52] | 52 | 52 | 555 | Completeness of data collection | 100 |
| The United States of America [53] | 3 | 3 | 41 | Based on the rate of data collected during chemotherapy | 63 |
| Taiwan [54] | 4 | 4 | 43 | Completeness of data collection | 100 |
| South Korea [55] | 12 | 12 | 37 | Equivalent to the completion of the exercise program | 84 |
| The United States of America [56] | 2 | 2 | 12 | Completeness of data collection | 92 |
| Japan [57] | 12 | 12 | 28 | Completeness of data collection | 75 |
| The United States of America [58] | 3 | 3 | 60 | In-person interviews to examine the acceptability of the device and analysis of qualitative data | 100 |
| Country of Study | Cancer Type | Purpose | Reported Clinical Outcomes |
|---|---|---|---|
| The United States of America [21] | Breast Cancer | Behavioral health management (PA/QoL and fatigue) | High engagement among hospitalized patients and increased energy expenditure among cancer survivors. Outcomes depend on numerous factors related to users and their needs. |
| Australia [22] | Breast Cancer | Behavioral health management (sleep quality) | Changes in actigraphy (sleep efficiency) and PSQI global and subscales favored the intervention arm. Findings were not significant or clinically meaningful. |
| The United States of America [23] | Breast Cancer | Behavioral health management (physical activity/BMI/QoL/fatigue/ fitness/self-regulation and self-efficacy related to PA) | Self-monitoring, goal setting, and self-efficacy were significantly correlated with activity levels. Increased improvement in health was noted with an increase in PA. |
| Australia [24] | Breast Cancer | Behavioral health management (MVPA/Sedentary Behavior) | The intervention resulted in increases in MVPA and MVPA accrued in bouts of at least 10 consecutive min while reducing total and prolonged sitting times. A significant difference in MVPA was noted between groups at T2, favoring the intervention arm. |
| The United States of America [25] | Breast Cancer | Behavioral health management (PA- MVPA, Sedentary/physiological/ psychosocial/QoL variables) | No significant group differences were observed for changes over time for any variable. Both groups showed increased mean daily MVPA, light PA, energy expenditure, and steps/day. |
| Canada [26] | Breast Cancer | Behavioral health management (PA-MVPA, LIPA, Sedentary Behavior/Sleep quality/health-related Fitness Markers) | Increases in moderate-to-vigorous intensity PA and decreases in sedentary time were significantly greater in the lower-intensity PA group versus the control group at 12 weeks. Increases in V˙O2 max at 12 weeks in both intervention groups were significantly greater than the changes in the control group. Changes in PA and V˙O2 max remained at 24 weeks but differences between the intervention and control groups were not significant. |
| The Netherlands [27] | Breast Cancer | Behavioral health management (PA-Sedentary behavior) | The activity tracker motivated women to be physically active and increased their awareness of their sedentary lifestyle. Wearing an activity tracker raised lifestyle awareness in patients with breast cancer. |
| United Kingdom [28] | Breast Cancer | Behavioral health management (Upper Limb Function) | WAM improved on the surgical side of the upper limb with an increment in PA for the first week and showed a good correlation with DASH (0.0506) |
| India [29] | Breast Cancer | Behavioral health management (Fatigue/QoL//Functional Capacity/PA/Body Composition) | At the end of the 7-week intervention, functional capacity, quality of life, and skeletal mass were significantly improved, whereas fatigue and changes in total fat improved nonsignificantly. |
| The United States of America [30] | Breast Cancer | Behavioral health management (PA/MVPA/SB/Cognitive functions) | Participants decreased their activity from pre- to post-chemotherapy by 1 h/week in MVPA and 8 h/week in TPA during the decline. This is useful for determining the stage of chemotherapy in which PA starts to decline and patients need extra support for their care. |
| The United States of America [31] | Breast Cancer | Behavioral health management (PA/Sleep Metrics) | Overall step count decreased by an average of 54 steps per day from baseline during treatment. Although differences in step count, calories expended, and miles walked throughout the RT were minimal, they were significant because of the substantial number of events |
| Germany [32] | Breast Cancer | Behavioral health management (PA) | Coherence between self-reported and device data was strong (r = 0.566). Neither treatment nor week nor their interaction had effects on step counts. Self-reported activity time was lower for patients receiving chemotherapy than for those not receiving chemotherapy and lower in the 18th week than in the 3rd week |
| Central China [33] | Mixed Cancer | Behavioral health management (Asleep + QoL) | The baseline measurement was not significantly different among the three groups. However, after the intervention, a significant difference between the experimental and control groups was noted. Sleep quality and PA improved significantly but not the secondary outcomes. |
| The United States of America [34] | Mixed Cancer | Behavioral health management (Unplanned Healthcare Encounter/PA) | Kinematic features associated with physical activity showed a positive correlation. Chair-to-table kinematics are good predictors of unexpected hospitalization. Get- up-and-walk kinematics are good predictors of low physical activity |
| The United States of America [35] | Mixed Cancer | Behavioral health management (Unplanned Healthcare Encounter/PA) | This study demonstrated the feasibility of an outpatient wearable activity tracker. The results revealed a 50% disagreement with no association of these disagreements with UHEs and no correlation between the UHEs and ECOG scores. A correlation between (1) average METs and UHEs and (2) no sedentary physical activity hours and UHEs was noted |
| The United States of America [36] | Mixed Cancer | Behavioral health management (PA/QoL) | Significant improvements across all eight dimensions of HRQOL; most patients (85%) reported that they enjoyed wearing the Fitbit. Most felt that the Fitbit helped them to be more active (79%), whereas a minority (18%) felt their activity level was the same, and none reported becoming less active. |
| Japan [37] | Mixed Cancer | Behavioral health management (PA/Symptom Burden Assessment/Sleep/Fatigue) | Use of a wearable activity tracker for collecting PGHD in real time according to the protocol was feasible. With respect to adherence, the result was significant. The correlation between the assessed data was not significant |
| France [38] | Mixed Cancer | Behavioral health management (PA/Sleep) | Results provide evidence for both the feasibility and relevance of the combined objective and subjective remote monitoring of sleep and other symptoms in patients with cancer with single-night precision. This dynamic approach can help the development of novel therapeutics whose testing is warranted in patients with cancer |
| Ireland [39] | Mixed Cancer | Behavioral health management (MVPA/Cardiovascular risk factors and sedentary behavior) | The estimated difference between groups at 24 weeks supported higher MVPA; no change in MVPA in the intervention group was observed during the 12-week follow-up period, indicating a positive correlation with the improvement in cardiovascular risk factors. |
| The United States of America [40] | Mixed Cancer | Behavioral health Management (MVPA/QoL/ Fatigue/Fitness/Sedentary Behavior) | Results of the studies revealed some promising improvements in muscular strength that aligned with the intervention’s focus on strength training. |
| Switzerland [41] | Mixed Cancer | Behavioral health management (Symptom Analysis) | Remote monitoring of healthcare status in patients receiving palliative care with a limited life expectancy is feasible, and patients can handle the smartphone and sensor-equipped bracelet. Feedback toward the use of this monitoring system was mostly positive. |
| The United States of America [42] | Mixed Cancer | Behavioral health management (PA-SB and MVPA/QoL) | Intervention participants had a lower-than-expected engagement in the Facebook group component, (passive instead of active engagement); MVPA and sedentary time showed no significant difference b/w gaps |
| The United States of America [43] | Mixed Cancer | Behavioral health management (PA-MVPA) | Increased physical activity among cancer survivors was noted: the intervention group increased their daily steps. Moderate-to-vigorous-intensity activity performed in 10 min bouts increased, but no significant group-by-time differences for either light- or vigorous-intensity activity were noted |
| Australia [44] | Colorectal and endometrial Cancer | Behavioral health management (PA -Steensma) | Fitbit wear time (percentage of valid wear days = adherence) was consistent with a median adherence score of 100%. Comparison and correlation with actigraphy (MVPA) show that both devices are not correlated and do not show any type of association. |
| Western Australia [45] | Colorectal Cancer | Behavioral health management (MVPA/Cardiovascular Risk) | Despite a significant increase in MVPA, the change in the proportion of participants meeting the guidelines in relation to MV10 did not significantly differ by group. Reduction in DBP among intervention participants that were hypertensive. Fitbit was promising for low-intensity interventions. |
| The United States of America [46] | Colorectal Cancer | Behavioral health management (PA-MVPA/ Adverse events) | Intervention arm increased its MVPA by 13 min per day more than the control arm. Larger studies should be conducted to determine whether the intervention increases physical activity. |
| South Korea [47] | Colorectal Cancer | Behavioral health management (PA/QoL/Nutritional Status/Physical Performance) | Lower-extremity strength and cardiorespiratory endurance were significantly improved. Fatigue and nausea/vomiting symptoms were significantly relieved after the program. Most of the functional scales showed improvements, although the changes were not significant. |
| The United States of America [48] | Colorectal Cancer | Behavioral health management (PA/) | Pilot data show a nonsignificant decrease in moderate activity accumulated in bouts of at least 10 min in both arms (16–21 min per week). |
| Taiwan [49] | Lung Cancer | Behavioral health management (CRF) | The LF to HF ratio is highly correlated with the subjective BFI, particularly when measured during sleep time. Analytical results revealed that this ratio can be used to evaluate cancer fatigue because of a 3% mapping error in the BFI |
| The United States of America [50] | Lung Cancer | Behavioral health management (Steps/Day and MVPA/Sedentary Behavior/Cardiorespiratory Fitness) | Participants who received surgery in the spring, summer, autumn, and winter seasons, respectively, had lower PA and CRF than those who received surgery in other seasons. These results were consistent among all study subgroups. |
| The United States of America [51] | Lung Cancer | Behavioral health management (PA-Steps/QoL/ Symptoms/Functional Status/Dyspepsia) | Improved PA was associated with the early discharge of patients with GC undergoing gastrectomy. This was because patients with improved PA had resumed physical function, which was the main factor evaluated if patients were qualified to be discharged. |
| South Korea [52] | Lung Cancer | Behavioral health management (MVPA/Aerobic Capacity) | Eight (47%) of the seventeen participants demonstrated a clinically significant improvement of 14 m or more. The average improvement in aerobic capacity (13.8 m) was close to the minimum threshold for a clinically meaningful improvement of 14 m |
| The United States of America [53] | Gastric cancer | Behavioral health management (PA and Symptom Burden) | This study’s results indicate significant correlations between the number of the step count and two common performance statuses, which is consistent with previous research findings. Questionnaire findings indicated that active patients have a lower burden of symptoms. |
| Taiwan [54] | Gastric Cancer | Behavioral health management (PA/Sleep Metrics) | Results provide evidence for both the feasibility and relevance of the combined objective and subjective remote monitoring of sleep and other symptoms in patients with cancer with single-night precision. This dynamic approach can guide the development of novel therapeutic concepts whose testing is warranted in patients with cancer |
| South Korea [55] | Liver Cancer | Behavioral health management (Exercise Capacity/PA/QoL/Body Composition and Biochemical) | Compared with baseline, significant improvements were found in physical fitness measures, body composition, self-reported amount of physical activity, and pain. All symptoms improved, as observed in the QoL scales (i.e., EORTC-QLQ C30). |
| The United States of America [56] | Blood Cancer | Behavioral health management (PA/Sleep) | This study demonstrates the feasibility of collecting sleep data through actigraphy among hospitalized adults. Actigraphy measures suggested poor sleep. |
| Japan [57] | Urothelial Carcinoma | Behavioral health management (PA/QoL/ Adverse Events) | Significant correlations were noted between measurements performed using an oscillometer and a Fitbit during chemotherapy for patients. The measurement of fatigue using Fitbit was effective |
| The United States of America [58] | Skin Cancer | Preventive care | No differences in baseline knowledge or attitudes regarding sun exposure or protection were noted between the two groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Upadhyay, U.; Dhar, E.; Kuo, L.-J.; Syed-Abdul, S. A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population. Cancers 2022, 14, 4437. https://doi.org/10.3390/cancers14184437
Huang Y, Upadhyay U, Dhar E, Kuo L-J, Syed-Abdul S. A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population. Cancers. 2022; 14(18):4437. https://doi.org/10.3390/cancers14184437
Chicago/Turabian StyleHuang, Yaoru, Umashankar Upadhyay, Eshita Dhar, Li-Jen Kuo, and Shabbir Syed-Abdul. 2022. "A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population" Cancers 14, no. 18: 4437. https://doi.org/10.3390/cancers14184437
APA StyleHuang, Y., Upadhyay, U., Dhar, E., Kuo, L.-J., & Syed-Abdul, S. (2022). A Scoping Review to Assess Adherence to and Clinical Outcomes of Wearable Devices in the Cancer Population. Cancers, 14(18), 4437. https://doi.org/10.3390/cancers14184437






