Surgical Resection Is Superior to TACE in the Treatment of HCC in a Well Selected Cohort of BCLC-B Elderly Patients—A Retrospective Observational Study
Abstract
Simple Summary
Abstract
1. Introduction
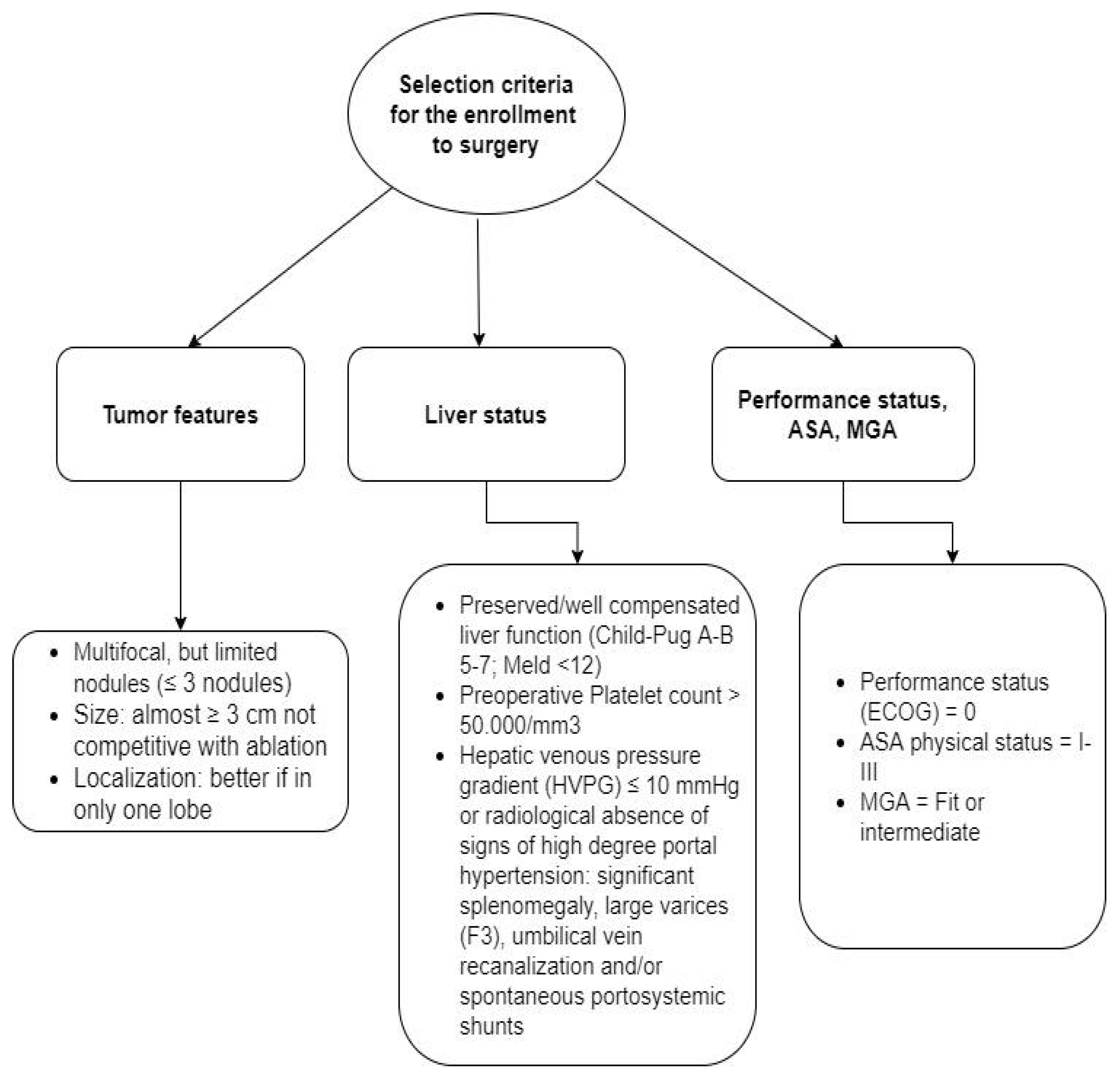
2. Materials and Methods
Statistical Analysis
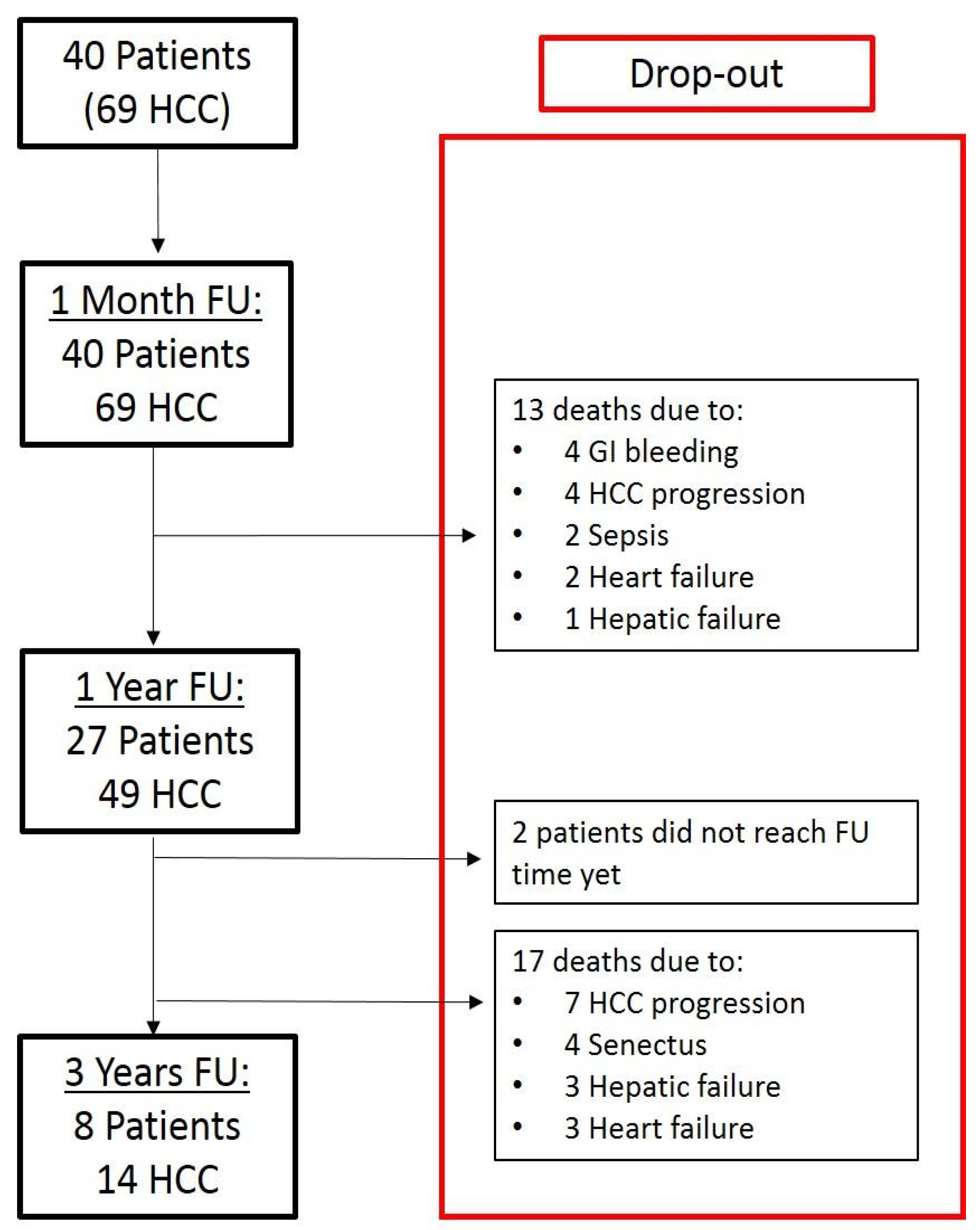
3. Results
3.1. Surgical Resection
3.1.1. Clinical-General Characteristics of Patients
3.1.2. Perioperative Morbidity and Mortality
3.1.3. Overall Survival
3.2. Follow-Up and Recurrence
3.3. Quality of Life Analysis
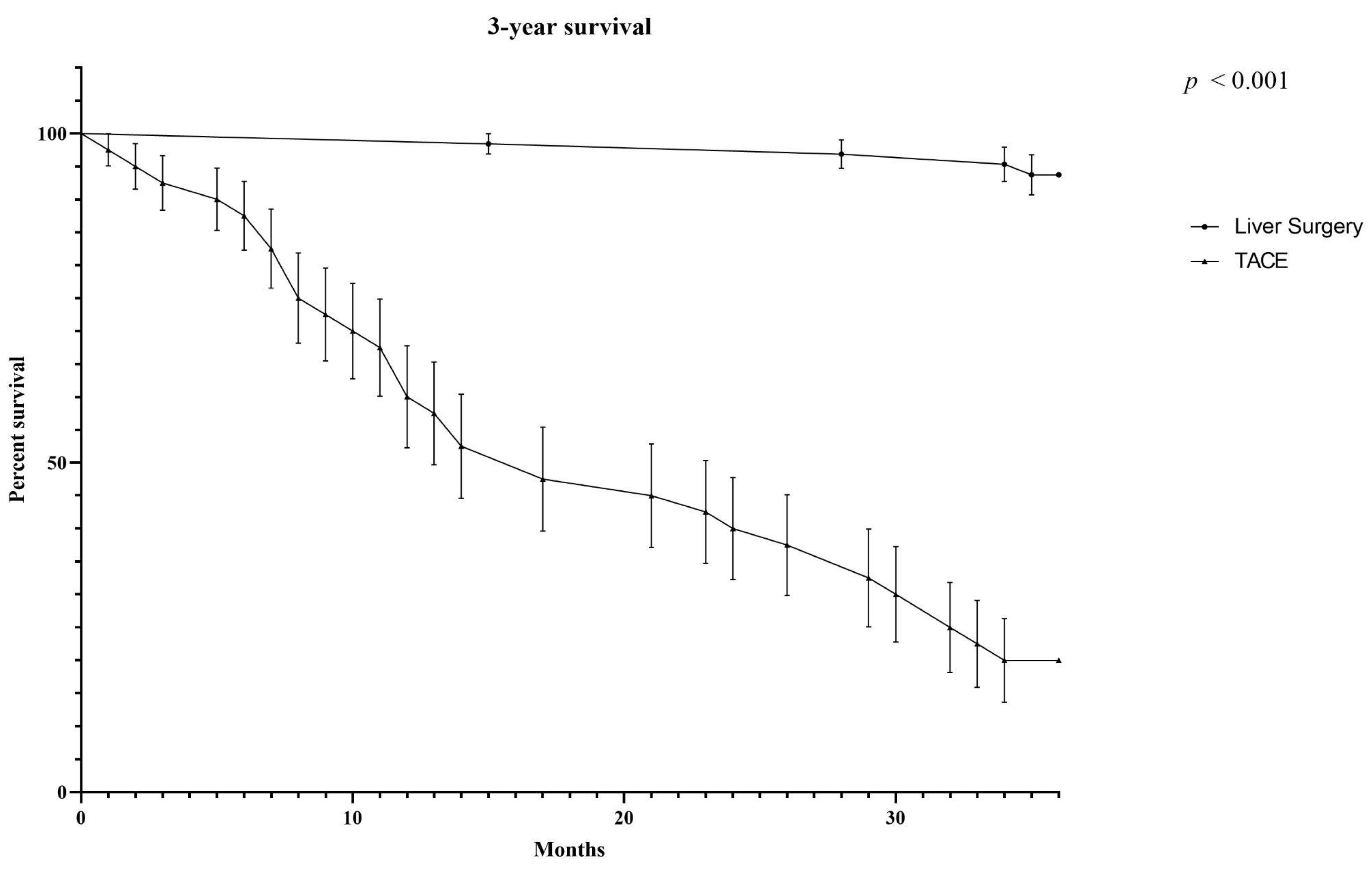
3.4. Comparison of Surgery and TACE in BCLC-B Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2020. Available online: http://globocan.iarc.fr (accessed on 5 June 2022).
- Brozzetti, S.; Bini, S.; Chiarella, L.; Fazzi, K.; Di Martino, M.; Bezzi, M. HCC in elderly patients. Curative intraoperative strategies and management in recurrences. In Liver Cancer; Tech Open: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Desai, A.; Sandhu, S.; Lai, J.-P.; Sandhu, D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Z. Survival improvement and prognosis for hepatocellular carcinoma: Analysis of the SEER database. BMC Cancer 2021, 21, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Basso, U.; Monfardini, S. Multidimensional geriatric evaluation in elderly cancer patients: A practical approach. Eur. J. Cancer Care (Engl.) 2004, 13, 424–433. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Raccomandazioni Multisocietarie Italiane (AISF, AIOM, IT-IHPBA, SIC, SIRM, SITO) Per la Gestione Clinica Integrata Del Paziente Con Epatocarcinoma. Available online: https://www.webaisf.org/aisf-guidelines-e-position-papers/page/3/ (accessed on 13 June 2022).
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Italian Liver Cancer (ITA.LI.CA) group. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: A multicentre study. J. Hepatol. 2015, 62, 617–624. [Google Scholar] [CrossRef]
- Lowe, M.C.; D’Angelica, M.I. Anatomy of Hepatic Resectional Surgery. Surg. Clin. N. Am. 2016, 96, 183–195. [Google Scholar] [CrossRef]
- Tellapuri, S.; Sutphin, P.D.; Beg, M.S.; Singal, A.G.; Kalva, S.P. Staging systems of hepatocellular carcinoma: A review. Indian J. Gastroenterol. 2018, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver; European Organization For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Wu, F.X.; Li, H. Hepatic resection associated with good survival for selected patients with multinodular hepatocellular carcinoma. Tumour Biol. 2014, 35, 8355–8358. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, S.; Kosaka, T.; Eguchi, S. Hepatic resection for hepatocellular carcinoma. Hepatoma Res. 2018, 4, 50. [Google Scholar] [CrossRef][Green Version]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Merath, K.; Paredes, A.Z.; Sahara, K.; Ratti, F.; Marques, H.P.; Soubrane, O.; Lam, V.; et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona clinic liver cancer guidelines: A multi-institutional analysis of 1010 patients. Surgery 2019, 166, 967–974. [Google Scholar] [CrossRef]
- Ng, K.K.; Vauthey, J.N.; Pawlik, T.M.; Lauwers, G.Y.; Regimbeau, J.M.; Belghiti, J.; Ikai, I.; Yamaoka, Y.; Curley, S.C.; Nagorney, D.M.; et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? results from a multi-institutional database. Ann. Surg. Oncol. 2005, 12, 364–373. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Poon, R.T.; Abdalla, E.K.; Zorzi, D.; Ikai, I.; Curley, S.A.; Nagorney, D.M.; Belghiti, J.; Ng, I.O.; Yamaoka, Y.; et al. International Cooperative Study Group on Hepatocellular Carcinoma. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch. Surg. 2005, 140, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Lee, K.H.; Wai, C.T.; Wagholikar, G.; Tan, K.C. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann. Surg. Oncol. 2007, 14, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Bagante, F.; Sahara, K.; Moris, D.; Madison Hyer, J.; Wu, L.; Ratti, F.; Marques, H.P.; Soubrane, O.; Paredes, A.Z.; et al. Prognosis after resection of Barcelona clinic liver cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: A comprehensive assessment of the current BCLC classification. Ann. Surg. Oncol. 2019, 26, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Sankar, A.; Johnson, S.R.; Beattie, W.S.; Tait, G.; Wijeysundera, D.N. Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. Br. J. Anaesth. 2014, 113, 424–432. [Google Scholar] [CrossRef]
- Brozzetti, S.; Bezzi, M.; De Sanctis, G.M.; Andreoli, G.M.; De Angelis, M.; Miccini, M.; Galati, F.; Panetta, V.; Furlan, C.; De Santis, D.; et al. Elderly and very elderly patients with hepatocellular carcinoma. Strategy for a first line treatment. Ann. Ital. Chir. 2014, 85, 120–128. [Google Scholar]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994, 20, 15–20. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Hsieh, C.B.; Chen, C.J.; Chen, T.W.; Yu, J.C.; Shen, K.L.; Chang, T.M.; Liu, Y.C. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J. Gastroenterol. 2004, 10, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qua Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Argirò, R.; De Rubeis, G.; Rocco, B.; Corradini, S.G.; Corona, M.; Nardis, P.G.; Saba, L.; Mennini, G.; Fiorentino, F.; et al. Polyethylene Glycol Epirubicin-Loaded Transcatheter Arterial Chemoembolization Procedures Utilizing a Combined Approach with 100 and 200 μm Microspheres: A Promising Alternative to Current Standards. J. Vasc. Interv. Radiol. 2019, 30, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J.; et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Bruix, J.; Llovet, J.M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002, 35, 519–524. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Lin, C.T.; Hsu, K.F.; Chen, T.W.; Yu, J.C.; Chan, D.C.; Yu, C.Y.; Hsieh, T.Y.; Fan, H.L.; Kuo, S.M.; Chung, K.P.; et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: Change for treatment of choice? World J. Surg. 2010, 34, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Wang, M.; Tan, K.; Dou, W.; Fan, Q.; Li, H.; Du, X.; Liu, L. Identifying optimal candidates for liver resection or transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Brambilla, G.; Carnaghi, C.; Tommasini, M.A.; Torzilli, G. Is it time to reconsider the BCLC/AASLD therapeutic flow-chart? J. Surg. Oncol. 2010, 102, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Giuliante, F.; Ardito, F.; Pinna, A.D.; Sarno, G.; Giulini, S.M.; Ercolani, G.; Portolani, N.; Torzilli, G.; Donadon, M.; Aldrighetti, L.; et al. Liver resection for hepatocellular carcinoma ≤3 cm: Results of an Italian multicenter study on 588 patients. J. Am. Coll. Surg. 2012, 215, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Xiang, B.D.; Gong, W.F.; Ke, Y.; Mo, Q.G.; Ma, L.; Liu, X.; Li, L.Q. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS ONE 2013, 8, 68193. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.S.; Lo, G.H.; Hsu, Y.C.; Hsu, C.C.; Wu, T.C.; Yeh, J.H.; Hsiao, P.; Hsieh, P.M.; Lin, H.Y.; et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2020, 20, 99. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsieh, P.M.; Lin, H.Y.; Hung, C.M.; Lo, G.H.; Hsu, Y.C.; Lu, I.C.; Lee, C.Y.; Wu, T.C.; Yeh, J.H.; et al. Surgical resection significantly promotes the overall survival of patients with hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2021, 21, 220–231. [Google Scholar] [CrossRef]
- Berardi, G.; Morise, Z.; Sposito, C.; Igarashi, K.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; Kubo, S.; Tanaka, S.; et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2020, 72, 75–84. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Xu, H.; Wei, Y.; Li, B. Is laparoscopic liver resection suitable for selected patients with BCLC stage B HCC? A propensity score-matched analysis. HPB (Oxford) 2020, 22, 595–602. [Google Scholar] [CrossRef]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977.e2. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; D’Erasmo, L.; Bini, S.; Pastori, D.; Angelico, F.; Del Ben, M.; Arca, M.; Di Costanzo, A. Heterogeneity of non-alcoholic fatty liver disease (NAFLD): Implication for cardiovascular risk stratification. Atherosclerosis 2022, 357, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Patman, G.L.; Leathart, J.B.; Piguet, A.C.; Burt, A.D.; Dufour, J.F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Manjunath, H.; Yopp, A.C.; Beg, M.S.; Marrero, J.A.; Gopal, P.; Waljee, A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 325–334. [Google Scholar] [CrossRef]
- Pirola, C.J.; Sookoian, S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: A meta-analysis. Hepatology 2015, 62, 1742–1756. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. In Vivo 2019, 33, 1411–1420. [Google Scholar] [CrossRef]
- Honda, T.; Miyaaki, H.; Ichikawa, T.; Taura, N.; Miuma, S.; Shibata, H.; Isomoto, H.; Takeshima, F.; Nakao, K. Clinical characteristics of hepatocellular carcinoma in elderly patients. Oncol Lett. 2011, 2, 851–854. [Google Scholar] [CrossRef]
- Kaibori, M.; Matsui, K.; Ishizaki, M.; Saito, T.; Kitade, H.; Matsui, Y.; Kwon, A.H. Hepatic resection for hepatocellular carcinoma in the elderly. J. Surg. Oncol. 2009, 99, 154–160. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. The Short- and Long-Term Outcomes in Elderly Patients with Hepatocellular Carcinoma after Curative Surgery: A Case-Controlled Study with Propensity Score Matching. Eur. Surg. Res. 2018, 59, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Joliat, G.R.; Allemann, P.; Labgaa, I.; Demartines, N.; Halkic, N. Treatment and outcomes of recurrent hepatocellular carcinomas. Langenbecks Arch. Surg. 2017, 402, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Meniconi, R.L.; Komatsu, S.; Perdigao, F.; Boëlle, P.Y.; Soubrane, O.; Scatton, O. Recurrent hepatocellular carcinoma: A Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery 2015, 157, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Lafaro, K.; Grandhi, M.S.; Herman, J.M.; Pawlik, T.M. The importance of surgical margins in primary malignancies of the liver. J. Surg. Oncol. 2016, 113, 296–303. [Google Scholar] [CrossRef]
- An, H.J.; Shin, W.Y.; Lee, K.Y.; Ahn, S.I. A comparison of the risk factors of intrahepatic recurrence, early recurrence, and multiple recurrences after resection for single nodular hepatocellular carcinoma. Korean J. Hepatobiliary Pancreat Surg. 2015, 19, 89–97. [Google Scholar] [CrossRef][Green Version]
- Hwang, S.; Lee, Y.J.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Lee, S.G. The Impact of Tumor Size on Long-Term Survival Outcomes After Resection of Solitary Hepatocellular Carcinoma: Single-Institution Experience with 2558 Patients. J. Gastrointest. Surg. 2015, 19, 1281–1290. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Bagante, F.; Moris, D.; Hyer, J.M.; Sahara, K.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; Soubrane, O.; et al. Recurrence Patterns and Outcomes after Resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann. Surg. Oncol. 2020, 27, 2321–2331. [Google Scholar] [CrossRef]
- Brozzetti, S.; Bini, S.; De Lio, N.; Lombardo, C.; Boggi, U. Surgical-only treatment of pancreatic and extra-pancreatic metastases from renal cell carcinoma—Quality of life and survival analysis. BMC Surg. 2020, 20, 101. [Google Scholar] [CrossRef]
- Ganz, P.A.; Moinpour, C.M.; Cella, D.F.; Fettimg, J.H. Quality-of-life assessment in cancer clinical trials: A status report. J. Natl. Cancer Inst. 1992, 84, 994–995. [Google Scholar] [CrossRef][Green Version]
- Moinpour, C.M. Measuring quality of life: An emerging science. Semin. Oncol. 1994, 21, 48–60. [Google Scholar]
- Nayfield, S.G.; Ganz, P.A.; Moinpour, C.M.; Cella, D.F.; Hailey, B.J. Report from a National Cancer Institute (USA) workshop on quality of life assessment in cancer clinical trials. Qual. Life Res. 1992, 1, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shun, S.C.; Chen, C.H.; Sheu, J.C.; Liang, J.D.; Jang, J.C.; Lai, Y.H. Quality of life and its associated factors in patients with hepatocellular carcinoma receiving one course of transarterial chemoembolization treatment: A longitudinal study. Oncologist 2012, 17, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Satou, S.; Ishizawa, T.; Kaneko, J.I.; Aoki, T.; Hasegawa, K.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Impact of Surgery on Quality of Life in Patients with Hepatocellular Carcinoma. World J. Surg. 2014, 38, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www1.ordinemediciroma.it/new-commissioni/elenco-commissioni/29503-gruppo-lavoro-sub-commissione-onco-geriatrica-dedicata-al-paziente-anziano-con-malattie-cronico-degenerative-in-collaborazione-con-gogi-onlus-gruppo-italiano-di-oncologia-geriatrica.html (accessed on 24 June 2022).
| Variable | n, median (IQR) | % |
|---|---|---|
| Age, years, | 73.2 (67–81) | |
| Male | 125 | 67.2 |
| Female | 61 | 32.8 |
| BMI, Kg/m2, median (IQR) | 26.10 (25.65–26.5) | |
| Etiology | ||
| HBV | 27 | 14.5 |
| HCV | 131 | 70.4 |
| HBV + HCV | 7 | 3.8 |
| NAFLD/NASH | 19 | 10.2 |
| Alcohol | 2 | 1.1 |
| Hemocromatosis/Wilson | 0 | 0 |
| Comorbidities | ||
| Cardiovascular | 34 | 18% |
| Hypertension | 105 | 56.5 |
| Pulmonary | 61 | 32.8 |
| Renal disease | 1 | 0.5 |
| Metabolic disease | ||
| Diabetes | 29 | 15.6 |
| Metabolic syndrome | 3 | 1.6 |
| Malnutrition | 4 | 2.2 |
| ASA Score | ||
| I | 44 | 23.7 |
| II | 85 | 45.7 |
| III | 57 | 30.6 |
| MGA | ||
| Fit | 102 | 54.8 |
| Intermediate | 84 | 45.2 |
| Frail | 0 | 0 |
| Child-Turcotte-Pugh score | ||
| A | 174 | 93.55 |
| B | 12 | 6.45 |
| C | 0 | 0 |
| MELD, median (IQR) | 7 (5–8) | |
| METAVIR | ||
| F0-1 | 42 | 22.6 |
| F2 | 90 | 48.4 |
| F3 | 30 | 16.1 |
| F4 | 24 | 12.9 |
| Steatosis | 37 | 19.9 |
| Platelets, 10⁹/L, median (Range) | 147 (50–362) | |
| AFP UI/L, median (IQR) | 4.5 (2.8–9.32) | |
| Varices % | ||
| F0 | 76 | 40.86 |
| F1 | 100 | 53.76 |
| F2 | 10 | 5.38 |
| F3 | 0 | 0 |
| Stage 0 (n = 9) | Stage A (n = 99) | Stage AB (n = 20) | Stage B (n = 58) | p Value | |
|---|---|---|---|---|---|
| Nodules resected/ablated, n (%) | |||||
| 1 | 9 (100) | 60 (60.6) | 20 (100) | 0 | |
| 2 | 0 (0) | 30 (30.3)/ 10 * (10.1) | 0 (0) | 40 (51.3)/ 10 *(12.82) | |
| 3 | 0 (0) | 9 (9.1)/ 5 * (5.05) | 0 (0) | 18 (23)/ 13 * (16.7) | |
| HCC size (cm), median, (Range) | 1.7 (1.3–2) | 3.2 (0.8–4.5) | 7.5 (5–11) | 2.6 (0.8-4.5) | |
| Type of resection, n (%) | 0.00008 | ||||
| Extended Right Hepatectomy | 0 (0) | 0 (0) | 1 (5) | 0 (0) | |
| Right Hepatectomy | 0 (0) | 1 (1) | 0 (0) | 9 (15.51) | |
| Left hepatectomy | 0 (0) | 0 (0) | 1 (5) | 2 (3.45) | |
| Bi-Segmentectomy | 0 (0) | 52 (52.5) | 17 (85) | 26 (44.83) | |
| Segmentectomy | 9 (100) | 45 (45.4) | 1 (5) | 20 (34.48) | |
| Wedge | 0 (0) | 2 (2%) | 0 (0) | 4 (6.9) | |
| Morbidity n, (%) | 0.54 | ||||
| I-II (Clavien-Dindo) | 1 (11.1) | 22 (22.2) | 3 (15) | 17 (29.31) | |
| III (Clavien-Dindo) | 0 (0) | 1 (1.01) | 0 (0) | 1 (1.72) | |
| Length of hospital stay, mean (range) | 6 (5–8) | 7 (6–15) | 7 (6–10) | 8 (6–15) | |
| ICU length of stay, mean (range) | 0.5 (0–1) | 1.2 (0–3) | 1 (0–1) | 1.3 (0–3) | |
| 90-days mortality | 0 (0) | 0 (0) | 0 (0) | (0) | |
| I recurrence treatment, n (%) | 6 (66.66) | 86 (86.87) | 12 (60) | 58 (100) | |
| Curative Treatments | 6 (100) | 47 (54.65) | 11 (91.67) | 23 (39.66) | 0.003 |
| Palliative Treatments | 0 | 39 (45.35) | 1 (8.33) | 35 (60.34) | |
| II recurrence treatments, n (%) | 3 (33.33) | 38 (38.4) | 6 (30) | 24 (41.38) | |
| Curative | 3 (100) | 9 (23.7) | 4 (66.67) | 5 (20.83) | 0.013 |
| Palliative | 0 (0) | 29 (76.3) | 2 (33.33) | 19 (79.17) | |
| III recurrence treatments, n (%) | 1 (11.11) | 19 (19.2) | 0 (0) | 9 (15.5) | |
| Curative | 1 (100) | 6 (31.6) | 0 (0) | 2 (22.22) | 0.6 |
| Palliative | 0 (0) | 13(68.4) | 0 (0) | 7 (77.78) |
| Stage 0 | Stage A | Stage AB | Stage B | p Value | |
|---|---|---|---|---|---|
| 1-yr OS, survival % (IC 95%) | 100% [IC: 1–1] | 100% [IC:1–1] | 100% [IC: 1–1] | 100% [IC: 1–1] | 0.2 |
| 3-yrs OS, survival % (IC 95%) | 100% [IC: 1–1] | 96.03% [IC: 0.917–0.999] | 95.21% [IC: 0.813–0.946] | 97.43% [IC: 0.891–0.992] | 0.2 |
| 5-yrs OS, survival % (IC 95%) | 88.9% [IC: 0.706–1] | 80.8% [IC: 0.589–0.779] | 78.7% [IC: 0.532–0.755] | 67.2% [IC: 0.507–0.718] | 0.2 |
| 10.yrs OS, survival % (IC 95%) | 66.66% [IC: 0.507–0.718] | 62.2% [IC: 0.542–0.753] | 58.3% [IC: 0.492–0.723] | 50.3% [IC: 0.464–0.690] | 0.2 |
| Death, n (%) | 3 (33.33) | 37 (37.4) | 7 (35) | 28 (48.28) | 0.015 |
| HCC | 0(0) | 6 (16.2%) | 2 (28.57) | 15 (53.571) | |
| Liver disease/Cirrhosis | 0 (0) | 21 (56.8) | 3 (42.86) | 10 (35.71) | |
| Other causes | 3 (100) | 10 (27) | 2 (28.57) | 3 (10.71) |
| Variable | Resected pts n = 58 n, median (IQR) (%) | TACE pts n = 40 n, median (IQR) (%) |
|---|---|---|
| Age, years, | 70 (65–77) | 76 (68–83) |
| Male | 39 (67.24) | 35 (87.5) |
| Female | 19 (32.76) | 5 (12.5) |
| Etiology, n (%) | ||
| HBV | 9 (15.52) | 3 (7.5) |
| HCV | 46 (79.31) | 8 (20) |
| HBV + HCV | 3 (5.17) | 0 (0) |
| NAFLD/NASH | 0 (0) | 4 (10) |
| Alcohol | 0 (0) | 18 (45) |
| Hemochromatosis/or Wilson’s disease. | 0 (0) | 0 |
| Mixed etiology | 0 (0) | 1 (2.5) |
| Cryptogenetic | 0 (0) | 6 (15) |
| Comorbidities, n (%) | ||
| Cardiovascular | 10 (17.24) | 27 (67.5) |
| Hypertension | 32 (55.2) | 30 (75) |
| Pulmonary | 8 (13.8) | 11 (27.5) |
| Renal disease | 0 (0) | 2 (5) |
| Metabolic disease | ||
| Diabetes | 8 (13.8) | 14 (35) |
| Metabolic syndrome | 0 (0) | 10 (25) |
| Malnutrition, n (%) | 0 (0) | 6 (15) |
| ASA Score, n (%) | p = 0.0008 | |
| I | 14 (24.1) | 0 (0) |
| II | 26 (44.8) | 12 (30) |
| III | 18 (32.1) | 27 (67.5) |
| IV | 0 (0) | 1 (2.5) |
| MGA, n (%) | p < 0.00001 | |
| Fit | 25 (43.1) | 0 (0) |
| Intermediate | 33 (56.9) | 32 (80) |
| Frail | 0 (0) | 8 (20) |
| Child-Turcotte-Pugh score, n (%) | p < 0.0001 | |
| A | 54 (93.1) | 19 (47.5) |
| B | 4 (6.9) | 21 (52.5) |
| C | 0 (0) | 0 (0) |
| MELD, median [IQR] | 7 [5–8] | 10 [8–13] |
| METAVIR, n (%) | p < 0.0001 | |
| F0-1 | 6 (10.34) | 0 (0) |
| F2 | 27 (46.55) | 2 (5) |
| F3 | 14 (24.14) | 14 (35) |
| F4 | 11 (18.97) | 24 (60) |
| Platelets, 10⁹/L, median (Range) | 147 (50–362) | 109 (64–154) |
| AFP UI/mL, median [IQR] | 5.2 [3.3–9.8] | 9 [6.1–51] |
| Varices % | p = 0.0007 | |
| F0 | 23 (39.65) | 15 (37.5) |
| F1 | 31 (53.45) | 9 (22.5) |
| F2 | 4 (6.9) | 11 (27.5) |
| F3 | 0 (0) | 5 (12.5) |
| SR/RF 58 pts, n 134 HCC (%) | TACE 40 pts, n 69 HCC (%) | p Value | |
|---|---|---|---|
| Number of nodules n, (%) | 134 target HCC 108/26 * | 69 target HCC | <0.00001 |
| 2 | 40/10 * (68.97) | 11 (27.5) | |
| 3 | 18/13 * (31.03) | 9 (22.5) | |
| >3 | 0 | 20 (50) | |
| HCC site | <0.00001 | ||
| Unilobar | 55 (94.83) | 18 (45) | |
| Bilobar | 3 (5.17) | 22 (55) | |
| HCC nodules/pts, n (range) | 2 (2–3) | 4 (2–9) | |
| HCC, Median size (cm), (Range) | 2.6 (0.8–4.5) | 2.4 (0.7–12) |
| Surgical Resection | TACE | p | |
|---|---|---|---|
| <0.05 | |||
| Length of hospital stay median (range) | 8 (6–15) | 3 (3–15) | |
| ICU length of stay, median (range) | 1 (0–3) | 0 (0) | |
| 90-days mortality | 0 (0) | 0 (0) | |
| Perioperative adverse events, n (%) | 18 (31) | 7 (17.5) | 0.043 |
| Type of adverse events | |||
| 0.018 | |||
| Ascites n. Volume (cc) | 9 (200–400) | 0 | |
| Liver failure | 0 | 0 | |
| Bile leak | 0 | 0 | |
| Abdominal collection | 0 | 0 | |
| Bleeding | 1 | 0 | |
| Pleural effusion | 7 | 1 | |
| Wound infection | 0 | 0 | |
| Portal thrombosis | 0 | 0 | |
| Thromboembolic events | 0 | 0 | |
| Fever | 0 | 4 | |
| Other | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brozzetti, S.; D’Alterio, C.; Bini, S.; Antimi, J.; Rocco, B.; Fassari, A.; Lucatelli, P.; Nardis, P.; Di Martino, M.; De Sanctis, G.M.; et al. Surgical Resection Is Superior to TACE in the Treatment of HCC in a Well Selected Cohort of BCLC-B Elderly Patients—A Retrospective Observational Study. Cancers 2022, 14, 4422. https://doi.org/10.3390/cancers14184422
Brozzetti S, D’Alterio C, Bini S, Antimi J, Rocco B, Fassari A, Lucatelli P, Nardis P, Di Martino M, De Sanctis GM, et al. Surgical Resection Is Superior to TACE in the Treatment of HCC in a Well Selected Cohort of BCLC-B Elderly Patients—A Retrospective Observational Study. Cancers. 2022; 14(18):4422. https://doi.org/10.3390/cancers14184422
Chicago/Turabian StyleBrozzetti, Stefania, Chiara D’Alterio, Simone Bini, Jessica Antimi, Bianca Rocco, Alessia Fassari, Pierleone Lucatelli, Piergiorgio Nardis, Michele Di Martino, Giuseppe Maria De Sanctis, and et al. 2022. "Surgical Resection Is Superior to TACE in the Treatment of HCC in a Well Selected Cohort of BCLC-B Elderly Patients—A Retrospective Observational Study" Cancers 14, no. 18: 4422. https://doi.org/10.3390/cancers14184422
APA StyleBrozzetti, S., D’Alterio, C., Bini, S., Antimi, J., Rocco, B., Fassari, A., Lucatelli, P., Nardis, P., Di Martino, M., De Sanctis, G. M., Corona, M., Bagni, O., Cortesi, E., Bezzi, M., & Catalano, C. (2022). Surgical Resection Is Superior to TACE in the Treatment of HCC in a Well Selected Cohort of BCLC-B Elderly Patients—A Retrospective Observational Study. Cancers, 14(18), 4422. https://doi.org/10.3390/cancers14184422






