From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
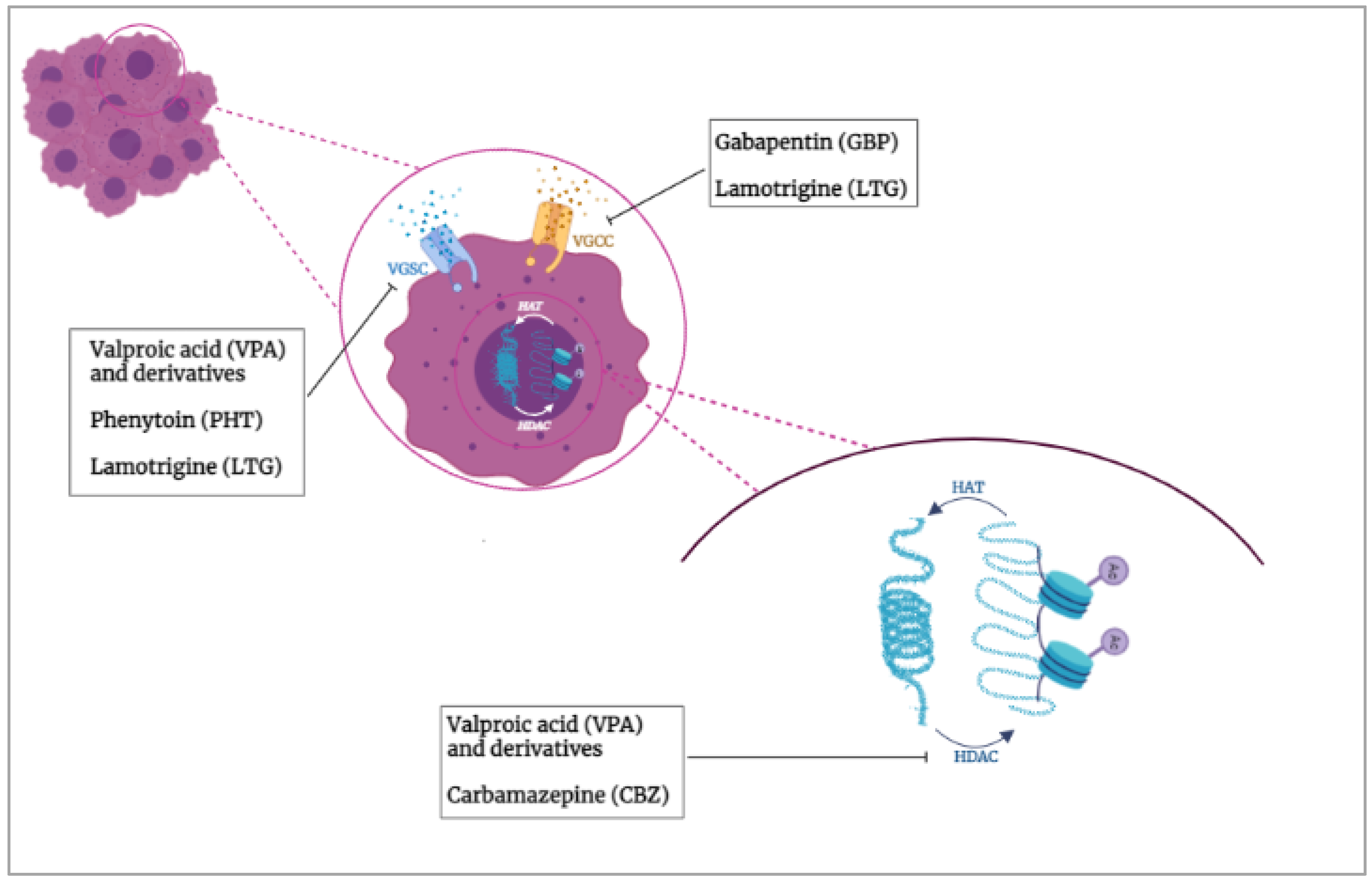
2. HDACs and VGICs Potential Functional Interaction in Cancer
3. AEDs in Breast Cancer
3.1. VGICs and HDACs as Prognostic Markers and Therapeutic Targets in Breast Cancer
3.2. Valproic Acid (VPA)
3.2.1. VPA Derivatives
3.2.2. Clinical Trials
3.3. Phenytoin (PHT)
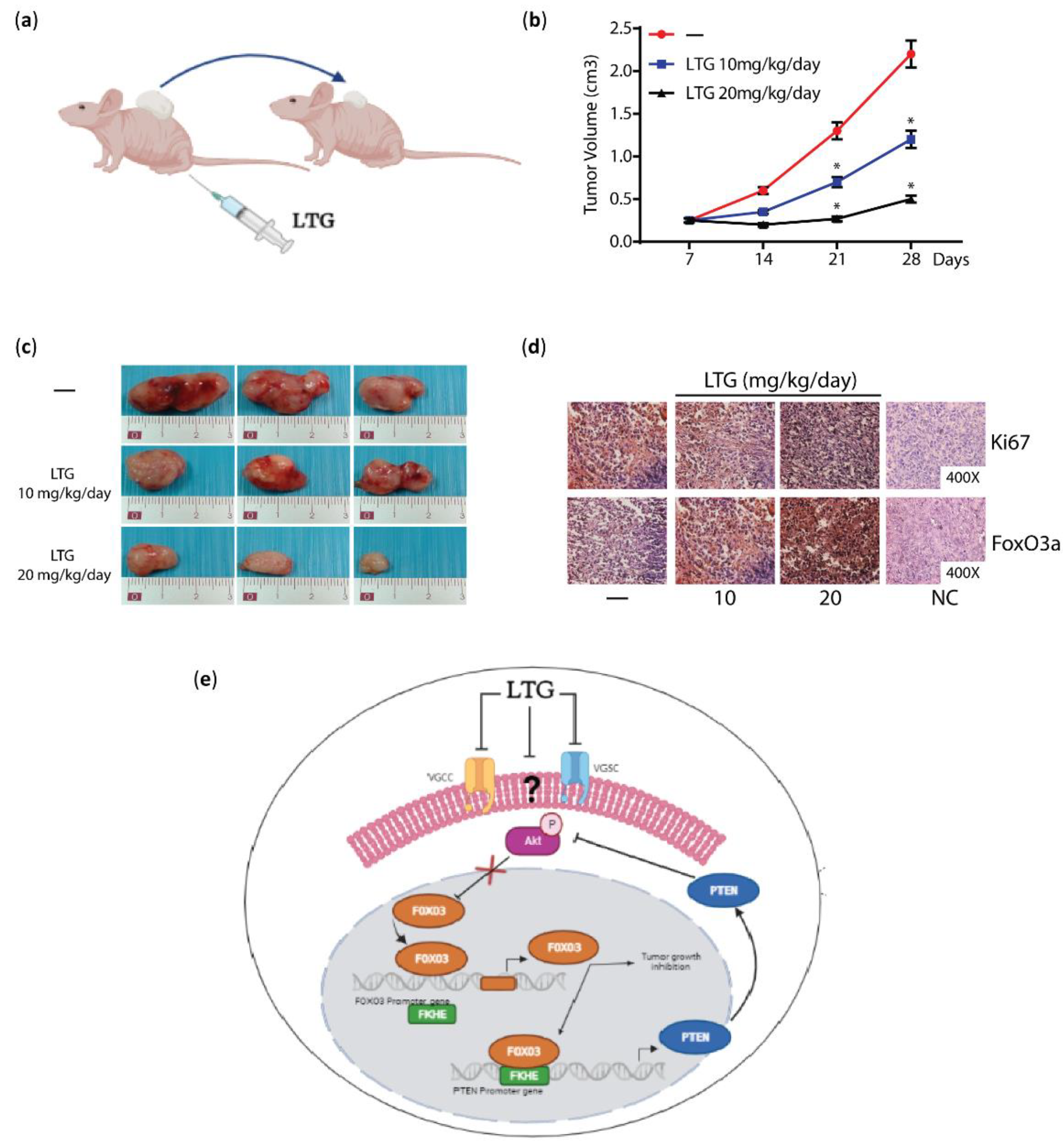
3.4. Lamotrigine (LTG)
3.5. Carbamazepine
4. AEDs and Prostate Cancer
4.1. Prostate Cancer: Diagnostic Markers and Therapeutic Management
4.2. VPA and Derivatives in PCa
4.2.1. VPA Anti-Proliferative Activity
4.2.2. VPA in PCa Progression
4.2.3. VPA and Neuroendocrine Transdifferentiation (NET) of PCa Cells
4.2.4. VPA in Combination Therapy for PCa Treatment
Combination of VPA and the Mammalian Target of Rapamycin (mTOR) Inhibitors in PCa
Combination of VPA and the Hypoglycemic Drug Metformin (MET) in PCa
Other Proposed VPA Combination Therapies in PCa
4.2.5. VPA in PCa Clinical Studies
4.3. Other AEDs in PCa
4.4. AEDs Users and PCa Risk
5. AEDs in Other Tumor Types
5.1. VGICs and HDACs Prognostic and Therapeutic Role in Other Tumors
5.2. VPA, Other AEDs and Drug Combinations in Other Tumors
6. Drug Delivery Systems (DDSs) Development to Overcome Toxicity and Low Solubility of AEDs and Their Derivatives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Olgen, S. Overview on Anticancer Drug Design and Development. Curr. Med. Chem. 2018, 25, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Meussen-Elholm, E.T.; Røste, L.S.; Taubøll, E. Antiepileptic drugs inhibit cell growth in the human breast cancer cell line MCF7. Mol. Cell. Endocrinol. 2004, 213, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, L.; Moini Zanjani, T.; Sabetkasaei, M. Comparison of Anticancer Effects of Carbamazepine and Valproic Acid. Iran. Red Crescent Med. J. 2016, 18, e37230. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Han, H.R.; Ahn, S.; Ryu, C.H.; Jeun, S.S. Combination treatment with VPA and MSCs-TRAIL could increase anti-tumor effects against intracranial glioma. Oncol. Rep. 2021, 45, 869–878. [Google Scholar] [CrossRef]
- Li, W.; Ma, L. Synergistic antitumor activity of oridonin and valproic acid on HL-60 leukemia cells. J. Cell. Biochem. 2019, 120, 5620–5627. [Google Scholar] [CrossRef]
- Vecht, C.; Royer-Perron, L.; Houillier, C.; Huberfeld, G. Seizures and Anticonvulsants in Brain Tumours: Frequency, Mechanisms and Anti-Epileptic Management. Curr. Pharm. Des. 2017, 23, 6464–6487. [Google Scholar] [CrossRef]
- Brito, B.E.; García, M.A. Concomitant Antihyperalgesic and Antitumor Effects of Gabapentin in a Murine Cancer Pain Model. Int. J. Mol. Sci. 2021, 22, 9671. [Google Scholar] [CrossRef]
- Rudà, R.; Mo, F.; Pellerino, A. Epilepsy in brain metastasis: An emerging entity. Curr. Treat. Options Neurol. 2020, 22, 6. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Oh, J. Neuropathic cancer pain: Prevalence, pathophysiology, and management. Korean J. Intern. Med. 2018, 33, 1058–1069. [Google Scholar] [CrossRef]
- Scarborough, B.M.; Smith, C.B. Optimal pain management for patients with cancer in the modern era. Cancer J. Clin. 2018, 68, 182–196. [Google Scholar] [CrossRef] [PubMed]
- White, H.S.; Smith, M.D.; Wilcox, K.S. Mechanisms of action of antiepileptic drugs. Int. Rev. Neurobiol. 2007, 81, 85–110. [Google Scholar] [CrossRef] [PubMed]
- Yellen, G. The voltage-gated potassium channels and their relatives. Nature 2002, 419, 35–42. [Google Scholar] [CrossRef]
- Tikhonov, D.B.; Zhorov, B.S. Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J. Gen. Physiol. 2017, 149, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, G.M.; Fozzard, H.A. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol. Pharmacol. 2010, 78, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.M.; Snutch, T.P. Voltage-Gated Calcium Channels in Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; Bethesda: Rockville, MD, USA, 2012. [Google Scholar]
- Catterall, W.A.; Swanson, T.M. Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Mol. Pharmacol. 2015, 88, 141–150. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Mechanisms of action of antiepileptic drugs: The search for synergy. Curr. Opin. Neurol. 2010, 23, 157–163. [Google Scholar] [CrossRef]
- Berzingi, S.; Newman, M.; Yu, H.G. Altering bioelectricity on inhibition of human breast cancer cells. Cancer Cell Int. 2016, 16, 72. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Fraser, S.P.; Pardo, L.A. Ion channels: Functional expression and therapeutic potential in cancer. Colloquium on Ion Channels and Cancer. EMBO Rep. 2008, 9, 512–515. [Google Scholar] [CrossRef]
- Djamgoz, M.B.; Coombes, R.C.; Schwab, A. Ion transport and cancer: From initiation to metastasis. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2014, 369, 20130092. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, A.; Crociani, O.; Lastraioli, E.; Masi, A.; Pillozzi, S.; Becchetti, A. Targeting ion channels in cancer: A novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009, 16, 66–93. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Novillo, C.; Capera, J.; Colomer-Molera, M.; Condom, E.; Ferreres, J.C.; Felipe, A. Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers 2019, 11, 287. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, J.; Körner, H.; Jiang, Y.; Ying, S. The Emerging Role of Voltage-Gated Sodium Channels in Tumor Biology. Front. Oncol. 2019, 9, 124. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. [Google Scholar] [CrossRef]
- Patel, F.; Brackenbury, W.J. Dual roles of voltage-gated sodium channels in development and cancer. Int. J. Dev. Biol. 2015, 59, 357–366. [Google Scholar] [CrossRef]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Rubino, R.; Besson, P.; Labbal, F.; Chevalier, S.; et al. NaV1.5 Na(+) channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef]
- House, C.D.; Vaske, C.J.; Schwartz, A.M.; Obias, V.; Frank, B.; Luu, T.; Sarvazyan, N.; Irby, R.; Strausberg, R.L.; Hales, T.G.; et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010, 70, 6957–6967. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Pukkanasut, P.; Velu, S.E.; Brackenbury, W.J.; Hales, T.G.; Besson, P.; Gomora, J.C.; Roger, S. Pharmacological and nutritional targeting of voltage-gated sodium channels in the treatment of cancers. iScience 2021, 24, 102270. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lai, M.D.; Phan, N.N.; Sun, Z.; Lin, Y.C. Meta-Analysis of Public Microarray Datasets Reveals Voltage-Gated Calcium Gene Signatures in Clinical Cancer Patients. PLoS ONE 2015, 10, e0125766. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Perez-Neut, M.; Kaja, S.; Gentile, S. Voltage-gated ion channels in cancer cell proliferation. Cancers 2015, 7, 849–875. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewska, B.; Gray, L.S.; Dziegielewski, J. T-type calcium channels blockers as new tools in cancer therapies. Pflug. Arch. Eur. J. Physiol. 2014, 466, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef]
- Alza, L.; Visa, A.; Herreros, J.; Cantí, C. T-type channels in cancer cells: Driving in reverse. Cell Calcium 2022, 105, 102610. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Pitt, G.S.; Matsui, M.; Cao, C. Voltage-Gated Calcium Channels in Nonexcitable Tissues. Annu. Rev. Physiol. 2021, 83, 183–203. [Google Scholar] [CrossRef]
- Nelson, M.; Yang, M.; Dowle, A.A.; Thomas, J.R.; Brackenbury, W.J. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol. Cancer 2015, 14, 13. [Google Scholar] [CrossRef]
- Takada, M.; Fujimoto, M.; Motomura, H.; Hosomi, K. Inverse Association between Sodium Channel-Blocking Antiepileptic Drug Use and Cancer: Data Mining of Spontaneous Reporting and Claims Databases. Int. J. Med. Sci. 2016, 13, 48–59. [Google Scholar] [CrossRef]
- Heers, H.; Stanislaw, J.; Harrelson, J.; Lee, M.W. Valproic acid as an adjunctive therapeutic agent for the treatment of breast cancer. Eur. J. Pharmacol. 2018, 835, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Ott, M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Micheau, O. Epigenetic Regulation of TRAIL Signaling: Implication for Cancer Therapy. Cancers 2019, 11, 850. [Google Scholar] [CrossRef]
- Hai, R.; Yang, D.; Zheng, F.; Wang, W.; Han, X.; Bode, A.M.; Luo, X. The emerging roles of HDACs and their therapeutic implications in cancer. Eur. J. Pharmacol. 2022, 931, 175216. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kaur, P.; Singh, P.; Singh, S. Differential molecular mechanistic behavior of HDACs in cancer progression. Med. Oncol. 2022, 39, 171. [Google Scholar] [CrossRef]
- Kaur, S.; Rajoria, P.; Chopra, M. HDAC6: A unique HDAC family member as a cancer target. Cell. Oncol. 2022. [Google Scholar] [CrossRef]
- Pramanik, S.D.; Kumar Halder, A.; Mukherjee, U.; Kumar, D.; Dey, Y.N.; Mogana, R. Potential of histone deacetylase inhibitors in the control and regulation of prostate, breast and ovarian cancer. Front. Chem. 2022, 10, 948217. [Google Scholar] [CrossRef]
- Martin, F.; Ufodiama, C.; Watt, I.; Bland, M.; Brackenbury, W.J. Therapeutic Value of Voltage-Gated Sodium Channel Inhibitors in Breast, Colorectal, and Prostate Cancer: A Systematic Review. Front. Pharmacol. 2015, 6, 273. [Google Scholar] [CrossRef]
- Eyal, S.; Yagen, B.; Sobol, E.; Altschuler, Y.; Shmuel, M.; Bialer, M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 2004, 45, 737–744. [Google Scholar] [CrossRef]
- Renzini, A.; D’Onghia, M.; Coletti, D.; Moresi, V. Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs. Front. Physiol. 2022, 13, 706003. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Seto, E. Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance. Cell. Mol. Life Sci. 2021, 78, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Korfei, M.; Mahavadi, P. Targeting Histone Deacetylases in Idiopathic Pulmonary Fibrosis: A Future Therapeutic Option. Cells 2022, 11, 1626. [Google Scholar] [CrossRef]
- Kamarulzaman, N.S.; Dewadas, H.D.; Leow, C.Y.; Yaacob, N.S.; Mokhtar, N.F. The role of REST and HDAC2 in epigenetic dysregulation of Nav1.5 and nNav1.5 expression in breast cancer. Cancer Cell Int. 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.T.; Villaronga, M.Á.; Rodrigo, J.P.; Álvarez-Teijeiro, S.; Urdinguio, R.G.; Fraga, M.F.; Suárez, C.; García-Pedrero, J.M. HERG1A potassium channel is the predominant isoform in head and neck squamous cell carcinomas: Evidence for regulation by epigenetic mechanisms. Sci. Rep. 2016, 6, 19666. [Google Scholar] [CrossRef]
- Ohya, S.; Kanatsuka, S.; Hatano, N.; Kito, H.; Matsui, A.; Fujimoto, M.; Matsuba, S.; Niwa, S.; Zhan, P.; Suzuki, T.; et al. Downregulation of the Ca(2+)-activated K(+) channel KC a3.1 by histone deacetylase inhibition in human breast cancer cells. Pharmacol. Res. Perspect. 2016, 4, e00228. [Google Scholar] [CrossRef]
- Vikram, A.; Lewarchik, C.M.; Yoon, J.Y.; Naqvi, A.; Kumar, S.; Morgan, G.M.; Jacobs, J.S.; Li, Q.; Kim, Y.R.; Kassan, M.; et al. Sirtuin 1 regulates cardiac electrical activity by deacetylating the cardiac sodium channel. Nat. Med. 2017, 23, 361–367. [Google Scholar] [CrossRef]
- Zhang, X.; Patel, D. Differences in Functional Expression of Connexin43 and Na(V)1.5 by Pan- and Class-Selective Histone Deacetylase Inhibition in Heart. Int. J. Mol. Sci. 2018, 19, 2288. [Google Scholar] [CrossRef]
- Xu, Q.; Patel, D.; Zhang, X.; Veenstra, R.D. Changes in cardiac Nav1.5 expression, function, and acetylation by pan-histone deacetylase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1139–H1149. [Google Scholar] [CrossRef]
- Jang, S.; Jeong, H.S. Data for the effect of histone deacetylase inhibitors on voltage- and ligand-gated ion channel gene expression in neurogenic induced-human adipose tissue-derived mesenchymal stem cells. Data Brief 2018, 17, 1314–1319. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Yu, H. Depolarization or hyperpolarization: Emerging role of altered bioelectricity in breast cancer metastasis. EBioMedicine 2022, 76, 103853. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Diss, J.K.; Chioni, A.M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Besson, P.; Le Guennec, J.Y. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim. Et Biophys. Acta 2003, 1616, 107–111. [Google Scholar] [CrossRef]
- Deuis, J.R.; Mueller, A.; Israel, M.R.; Vetter, I. The pharmacology of voltage-gated sodium channel activators. Neuropharmacology 2017, 127, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Voltage-gated sodium channel as a target for metastatic risk reduction with re-purposed drugs. F1000Research 2015, 4, 297. [Google Scholar] [CrossRef]
- Yang, M.; Kozminski, D.J.; Wold, L.A.; Modak, R.; Calhoun, J.D.; Isom, L.L.; Brackenbury, W.J. Therapeutic potential for phenytoin: Targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res. Treat. 2012, 134, 603–615. [Google Scholar] [CrossRef]
- Nelson, M.; Yang, M.; Millican-Slater, R.; Brackenbury, W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination In Vivo. Oncotarget 2015, 6, 32914–32929. [Google Scholar] [CrossRef]
- Pera, E.; Kaemmerer, E.; Milevskiy, M.J.G.; Yapa, K.; O’Donnell, J.S.; Brown, M.A.; Simpson, F.; Peters, A.A.; Roberts-Thomson, S.J.; Monteith, G.R. The voltage gated Ca(2+)-channel Cav3.2 and therapeutic responses in breast cancer. Cancer Cell Int. 2016, 16, 24. [Google Scholar] [CrossRef]
- Bhargava, A.; Saha, S. T-Type voltage gated calcium channels: A target in breast cancer? Breast Cancer Res. Treat. 2019, 173, 11–21. [Google Scholar] [CrossRef]
- Kanwar, N.; Carmine-Simmen, K.; Nair, R.; Wang, C.; Moghadas-Jafari, S.; Blaser, H.; Tran-Thanh, D.; Wang, D.; Wang, P.; Wang, J.; et al. Amplification of a calcium channel subunit CACNG4 increases breast cancer metastasis. EBioMedicine 2020, 52, 102646. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Le Guennec, J.Y.; Besson, P. Particular sensitivity to calcium channel blockers of the fast inward voltage-dependent sodium current involved in the invasive properties of a metastastic breast cancer cell line. Br. J. Pharmacol. 2004, 141, 610–615. [Google Scholar] [CrossRef][Green Version]
- Ohkubo, T.; Yamazaki, J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int. J. Oncol. 2012, 41, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Batcioglu, K.; Uyumlu, A.B.; Satilmis, B.; Yildirim, B.; Yucel, N.; Demirtas, H.; Onkal, R.; Guzel, R.M.; Djamgoz, M.B. Oxidative stress in the in vivo DMBA rat model of breast cancer: Suppression by a voltage-gated sodium channel inhibitor (RS100642). Basic Clin. Pharmacol. Toxicol. 2012, 111, 137–141. [Google Scholar] [CrossRef]
- Ari, F.; Napieralski, R.; Akgun, O.; Magdolen, V.; Ulukaya, E. Epigenetic modulators combination with chemotherapy in breast cancer cells. Cell Biochem. Funct. 2021, 39, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.P.; Dawood, S.; Mitchell, M.; Debeb, B.G.; Bloom, E.; Gonzalez-Angulo, A.M.; Sulman, E.P.; Buchholz, T.A.; Woodward, W.A. Antiepileptic drug use improves overall survival in breast cancer patients with brain metastases in the setting of whole brain radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 117, 308–314. [Google Scholar] [CrossRef]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]
- Wawruszak, A.; Halasa, M.; Okon, E.; Kukula-Koch, W.; Stepulak, A. Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers 2021, 13, 3409. [Google Scholar] [CrossRef]
- Fortunati, N.; Bertino, S.; Costantino, L.; Bosco, O.; Vercellinatto, I.; Catalano, M.G.; Boccuzzi, G. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008, 259, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, N.; Bertino, S.; Costantino, L.; De Bortoli, M.; Compagnone, A.; Bandino, A.; Catalano, M.G.; Boccuzzi, G. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol. Cell. Endocrinol. 2010, 314, 17–22. [Google Scholar] [CrossRef]
- Li, G.F.; Qian, T.L.; Li, G.S.; Yang, C.X.; Qin, M.; Huang, J.; Sun, M.; Han, Y.Q. Sodium valproate inhibits MDA-MB-231 breast cancer cell migration by upregulating NM23H1 expression. Genet. Mol. Res. GMR 2012, 11, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Terranova-Barberio, M.; Roca, M.S.; Zotti, A.I.; Leone, A.; Bruzzese, F.; Vitagliano, C.; Scogliamiglio, G.; Russo, D.; D’Angelo, G.; Franco, R.; et al. Valproic acid potentiates the anticancer activity of capecitabine in vitro and in vivo in breast cancer models via induction of thymidine phosphorylase expression. Oncotarget 2016, 7, 7715–7731. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of Interactions between Cisplatin and Two Histone Deacetylase Inhibitors in MCF7, T47D and MDA-MB-231 Human Breast Cancer Cell Lines—An Isobolographic Analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef] [PubMed]
- Aztopal, N.; Erkisa, M.; Erturk, E.; Ulukaya, E.; Tokullugil, A.H.; Ari, F. Valproic acid, a histone deacetylase inhibitor, induces apoptosis in breast cancer stem cells. Chem.-Biol. Interact. 2018, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Prestegui-Martel, B.; Bermudez-Lugo, J.A.; Chavez-Blanco, A.; Duenas-Gonzalez, A.; Garcia-Sanchez, J.R.; Perez-Gonzalez, O.A.; Padilla, M., II; Fragoso-Vazquez, M.J.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; et al. N-(2-hydroxyphenyl)-2-propylpentanamide, a valproic acid aryl derivative designed in silico with improved anti-proliferative activity in HeLa, rhabdomyosarcoma and breast cancer cells. J. Enzym. Inhib. Med. Chem. 2016, 31, 140–149. [Google Scholar] [CrossRef]
- Tarasenko, N.; Chekroun-Setti, H.; Nudelman, A.; Rephaeli, A. Comparison of the anticancer properties of a novel valproic acid prodrug to leading histone deacetylase inhibitors. J. Cell. Biochem. 2018, 119, 3417–3428. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Fragoso-Vázquez, M.J.; Martel, B.P.; Chávez-Blanco, A.; Dueñas-González, A.; García-Sánchez, J.R.; Bello, M.; Romero-Castro, A.; Correa-Basurto, J. Targeting Breast Cancer Cells with G4 PAMAM Dendrimers and Valproic Acid Derivative Complexes. Anti-Cancer Agents Med. Chem. 2020, 20, 1857–1872. [Google Scholar] [CrossRef]
- Cai, Z.; Lim, D.; Liu, G.; Chen, C.; Jin, L.; Duan, W.; Ding, C.; Sun, Q.; Peng, J.; Dong, C.; et al. Valproic Acid-Like Compounds Enhance and Prolong the Radiotherapy Effect on Breast Cancer by Activating and Maintaining Anti-Tumor Immune Function. Front. Immunol. 2021, 12, 646384. [Google Scholar] [CrossRef]
- University of California; H. Lee Moffitt Cancer Center and Research Institute. Valproic Acid in Combination with FEC100 for Primary Therapy in Patients with Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT01010854 (accessed on 2 September 2022).
- National Institute of Cancerología; National Council of Science and Technology; Psicofarma S.A. de C.V. Hydralazine and Valproate Added to Chemotherapy for Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00395655 (accessed on 14 July 2022).
- M.D. Anderson Cancer Center; National Cancer Institute (NCI). Bevacizumab and Temsirolimus Alone or in Combination with Valproic Acid or Cetuximab in Treating Patients with Advanced or Metastatic Malignancy or Other Benign Disease. Available online: https://ClinicalTrials.gov/show/NCT01552434 (accessed on 14 July 2022).
- Ragsdale, D.S.; Avoli, M. Sodium channels as molecular targets for antiepileptic drugs. Brain Res. Brain Res. Rev. 1998, 26, 16–28. [Google Scholar] [CrossRef]
- Mohammed, F.H.; Khajah, M.A.; Yang, M.; Brackenbury, W.J.; Luqmani, Y.A. Blockade of voltage-gated sodium channels inhibits invasion of endocrine-resistant breast cancer cells. Int. J. Oncol. 2016, 48, 73–83. [Google Scholar] [CrossRef]
- Keppel Hesselink, J.M.; Kopsky, D.J. Phenytoin: 80 years young, from epilepsy to breast cancer, a remarkable molecule with multiple modes of action. J. Neurol. 2017, 264, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Fadiel, A.; Song, J.; Tivon, D.; Hamza, A.; Cardozo, T.; Naftolin, F. Phenytoin is an estrogen receptor alpha-selective modulator that interacts with helix 12. Reprod. Sci. 2015, 22, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Oral SM-88 in Patients with Metastatic HR+/HER2- Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT04720664 (accessed on 15 July 2022).
- Stefani, A.; Spadoni, F.; Siniscalchi, A.; Bernardi, G. Lamotrigine inhibits Ca2+ currents in cortical neurons: Functional implications. Eur. J. Pharmacol. 1996, 307, 113–116. [Google Scholar] [CrossRef]
- Stefani, A.; Spadoni, F.; Bernardi, G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: Implications for neuroprotective strategies. Exp. Neurol. 1997, 147, 115–122. [Google Scholar] [CrossRef]
- Xie, X.; Hagan, R.M. Cellular and molecular actions of lamotrigine: Possible mechanisms of efficacy in bipolar disorder. Neuropsychobiology 1998, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.B.; O’Reilly, A.; Duclohier, H.; Wallace, B.A. Binding of the anticonvulsant drug lamotrigine and the neurotoxin batrachotoxin to voltage-gated sodium channels induces conformational changes associated with block and steady-state activation. J. Biol. Chem. 2003, 278, 10675–10682. [Google Scholar] [CrossRef]
- Leng, Y.; Fessler, E.B.; Chuang, D.M. Neuroprotective effects of the mood stabilizer lamotrigine against glutamate excitotoxicity: Roles of chromatin remodelling and Bcl-2 induction. Int. J. Neuropsychopharmacol. 2013, 16, 607–620. [Google Scholar] [CrossRef]
- Ookubo, M.; Kanai, H.; Aoki, H.; Yamada, N. Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: Brain region specific changes. J. Psychiatr. Res. 2013, 47, 1204–1214. [Google Scholar] [CrossRef]
- Pellegrino, M.; Rizza, P.; Nigro, A.; Ceraldi, R.; Ricci, E.; Perrotta, I.; Aquila, S.; Lanzino, M.; Ando, S.; Morelli, C.; et al. FoxO3a Mediates the Inhibitory Effects of the Antiepileptic Drug Lamotrigine on Breast Cancer Growth. Mol. Cancer Res. MCR 2018, 16, 923–934. [Google Scholar] [CrossRef]
- Yano, S.; Tokumitsu, H.; Soderling, T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 1998, 396, 584–587. [Google Scholar] [CrossRef]
- Danciu, T.E.; Adam, R.M.; Naruse, K.; Freeman, M.R.; Hauschka, P.V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003, 536, 193–197. [Google Scholar] [CrossRef]
- Kourti, M.; Liaropoulou, D.; Paschou, M.; Giagklisi, I.; Paschalidi, M.; Petani, E.; Papazafiri, P. Enhanced Ca(2+) Entry Sustains the Activation of Akt in Glucose Deprived SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 1386. [Google Scholar] [CrossRef]
- Rim, H.K.; Lee, H.W.; Choi, I.S.; Park, J.Y.; Choi, H.W.; Choi, J.H.; Cho, Y.W.; Lee, J.Y.; Lee, K.T. T-type Ca2+ channel blocker, KYS05047 induces G1 phase cell cycle arrest by decreasing intracellular Ca2+ levels in human lung adenocarcinoma A549 cells. Bioorganic Med. Chem. Lett. 2012, 22, 7123–7126. [Google Scholar] [CrossRef]
- Valerie, N.C.; Dziegielewska, B.; Hosing, A.S.; Augustin, E.; Gray, L.S.; Brautigan, D.L.; Larner, J.M.; Dziegielewski, J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharmacol. 2013, 85, 888–897. [Google Scholar] [CrossRef]
- Pellegrino, M.; Rizza, P.; Donà, A.; Nigro, A.; Ricci, E.; Fiorillo, M.; Perrotta, I.; Lanzino, M.; Giordano, C.; Bonofiglio, D. FoxO3a as a Positive Prognostic Marker and a Therapeutic Target in Tamoxifen-Resistant Breast Cancer. Cancers 2019, 11, 1858. [Google Scholar] [CrossRef]
- Beutler, A.S.; Li, S.; Nicol, R.; Walsh, M.J. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005, 76, 3107–3115. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, X.; Sun, L.; Zhao, C.; Sui, G.; Cai, L. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol. Cell. Biochem. 2011, 348, 165–171. [Google Scholar] [CrossRef]
- Teichmann, M.; Kretschy, N.; Kopf, S.; Jarukamjorn, K.; Atanasov, A.G.; Viola, K.; Giessrigl, B.; Saiko, P.; Szekeres, T.; Mikulits, W.; et al. Inhibition of tumour spheroid-induced prometastatic intravasation gates in the lymph endothelial cell barrier by carbamazepine: Drug testing in a 3D model. Arch. Toxicol. 2014, 88, 691–699. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular mechanisms and genetic alterations in prostate cancer: From diagnosis to targeted therapy. Cancer Lett. 2022, 534, 215619. [Google Scholar] [CrossRef]
- He, Y.; Xu, W.; Xiao, Y.-T.; Huang, H.; Gu, D.; Ren, S. Targeting signaling pathways in prostate cancer: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Brunello, S.; Salvarese, N. A Review on the Current State and Future Perspectives of [(99m)Tc]Tc-Housed PSMA-i in Prostate Cancer. Molecules 2022, 27, 2617. [Google Scholar] [CrossRef]
- Graça, I.; Pereira-Silva, E.; Henrique, R.; Packham, G.; Crabb, S.J.; Jerónimo, C. Epigenetic modulators as therapeutic targets in prostate cancer. Clin. Epigenet. 2016, 8, 98. [Google Scholar] [CrossRef]
- Kan, Y.; Li, B.; Yang, D.; Liu, Y.; Liu, J.; Yang, C.; Mao, L. Emerging Roles of Long Non-coding RNAs as Novel Biomarkers in the Diagnosis and Prognosis of Prostate Cancer. Discov. Med. 2021, 32, 29–37. [Google Scholar]
- Sedky, N.K.; Hamdan, A.A.; Emad, S.; Allam, A.L.; Ali, M.; Tolba, M.F. Insights into the therapeutic potential of histone deacetylase inhibitor/immunotherapy combination regimens in solid tumors. Clin. Transl. Oncol. 2022, 24, 1262–1273. [Google Scholar] [CrossRef]
- Sikes, R.A.; Walls, A.M.; Brennen, W.N.; Anderson, J.D.; Choudhury-Mukherjee, I.; Schenck, H.A.; Brown, M.L. Therapeutic approaches targeting prostate cancer progression using novel voltage-gated ion channel blockers. Clin. Prostate Cancer 2003, 2, 181–187. [Google Scholar] [CrossRef]
- Silvestri, R.; Pucci, P.; Venalainen, E.; Matheou, C.; Mather, R.; Chandler, S.; Aceto, R.; Rigas, S.H.; Wang, Y.; Rietdorf, K.; et al. T-type calcium channels drive the proliferation of androgen-receptor negative prostate cancer cells. Prostate 2019, 79, 1580–1586. [Google Scholar] [CrossRef]
- Shapovalov, G.; Skryma, R.; Prevarskaya, N. Calcium channels and prostate cancer. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 18–26. [Google Scholar] [CrossRef]
- Tran, L.N.K.; Kichenadasse, G.; Sykes, P.J. Combination Therapies Using Metformin and/or Valproic Acid in Prostate Cancer: Possible Mechanistic Interactions. Curr. Cancer Drug Targets 2019, 19, 368–381. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Saghari, Y.; Zarrabi, A.; Hamblin, M.R.; Entezari, M.; Hashemi, M.; Aref, A.R.; Hushmandi, K.; Kumar, A.P.; et al. Transforming growth factor-beta (TGF-β) in prostate cancer: A dual function mediator? Int. J. Biol. Macromol. 2022, 206, 435–452. [Google Scholar] [CrossRef]
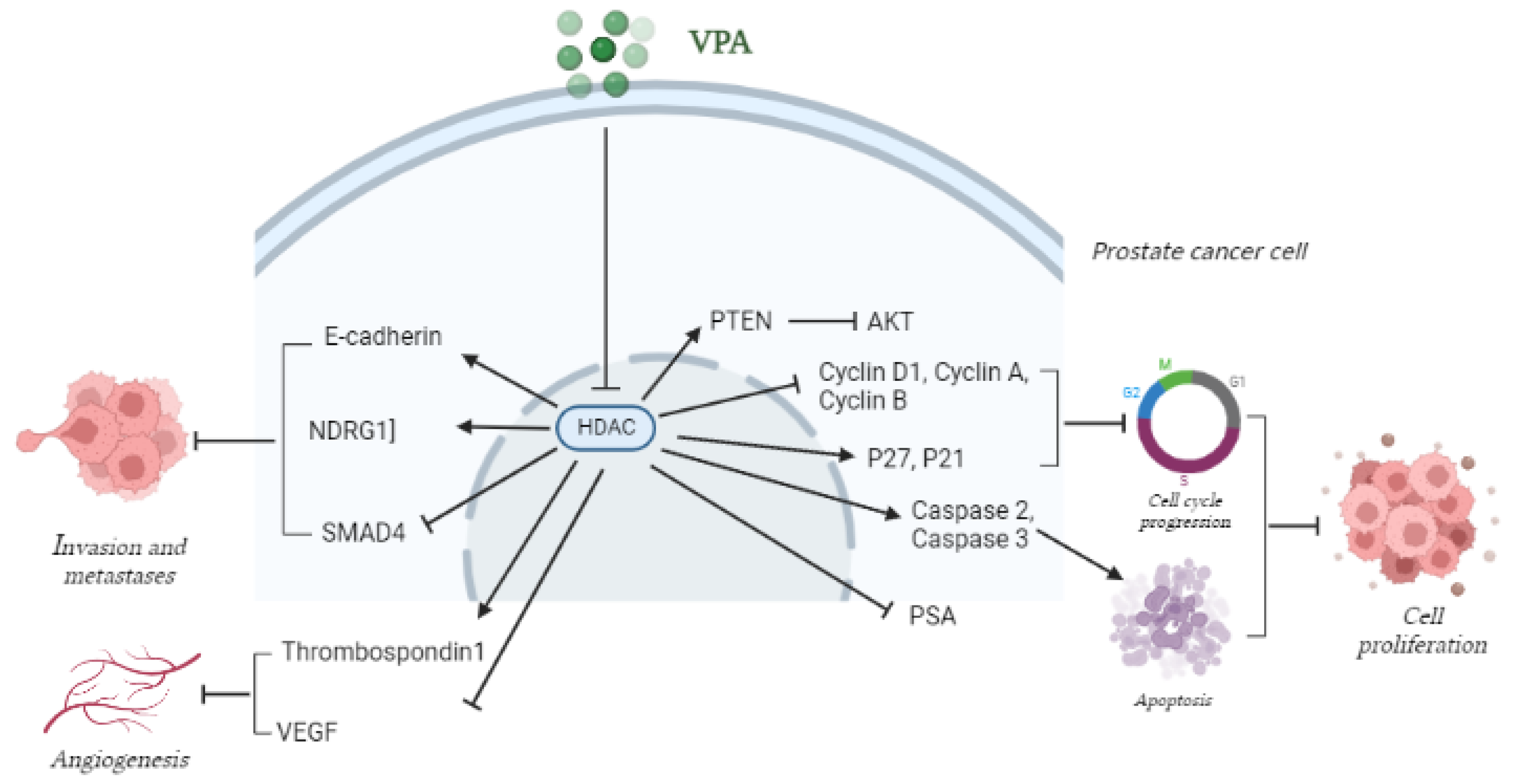
- Thelen, P.; Schweyer, S.; Hemmerlein, B.; Wuttke, W.; Seseke, F.; Ringert, R.H. Expressional changes after histone deacetylase inhibition by valproic acid in LNCaP human prostate cancer cells. Int. J. Oncol. 2004, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Sung, J.; Chowdhury, W.; Chen, C.L.; Höti, N.; Shabbeer, S.; Carducci, M.; Rodriguez, R. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006, 66, 7237–7244. [Google Scholar] [CrossRef] [PubMed]
- Kortenhorst, M.S.; Isharwal, S.; van Diest, P.J.; Chowdhury, W.H.; Marlow, C.; Carducci, M.A.; Rodriguez, R.; Veltri, R.W. Valproic acid causes dose- and time-dependent changes in nuclear structure in prostate cancer cells in vitro and in vivo. Mol. Cancer Ther. 2009, 8, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.W.; Chaturvedi, N.K.; Ouyang, S.; Lin, F.F.; Kaushik, D.; Wang, J.; Kim, I.; Lin, M.F. Histone deacetylase inhibitor valproic acid suppresses the growth and increases the androgen responsiveness of prostate cancer cells. Cancer Lett. 2011, 311, 177–186. [Google Scholar] [CrossRef]
- Angelucci, A.; Valentini, A.; Millimaggi, D.; Gravina, G.L.; Miano, R.; Dolo, V.; Vicentini, C.; Bologna, M.; Federici, G.; Bernardini, S. Valproic acid induces apoptosis in prostate carcinoma cell lines by activation of multiple death pathways. Anti-Cancer Drugs 2006, 17, 1141–1150. [Google Scholar] [CrossRef]
- Giordano, F.; Naimo, G.D.; Nigro, A.; Romeo, F.; Paolì, A.; De Amicis, F.; Vivacqua, A. Valproic Acid Addresses Neuroendocrine Differentiation of LNCaP Cells and Maintains Cell Survival. Drug Des. Dev. Ther. 2019, 13, 4265–4274. [Google Scholar] [CrossRef]
- Witt, D.; Burfeind, P.; von Hardenberg, S.; Opitz, L.; Salinas-Riester, G.; Bremmer, F.; Schweyer, S.; Thelen, P.; Neesen, J.; Kaulfuss, S. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis 2013, 34, 1115–1124. [Google Scholar] [CrossRef]
- Ouyang, D.Y.; Xu, L.H.; He, X.H.; Zhang, Y.T.; Zeng, L.H.; Cai, J.Y.; Ren, S. Autophagy is differentially induced in prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing profiles of ATG5. Autophagy 2013, 9, 20–32. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, Y.; Jiang, W.; Huang, Z.; Wang, M.; Rodriguez, R.; Jin, X. Valproic acid induces autophagy by suppressing the Akt/mTOR pathway in human prostate cancer cells. Oncol. Lett. 2016, 12, 1826–1832. [Google Scholar] [CrossRef]
- Shabbeer, S.; Kortenhorst, M.S.; Kachhap, S.; Galloway, N.; Rodriguez, R.; Carducci, M.A. Multiple Molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo. Prostate 2007, 67, 1099–1110. [Google Scholar] [CrossRef]
- Sidana, A.; Wang, M.; Shabbeer, S.; Chowdhury, W.H.; Netto, G.; Lupold, S.E.; Carducci, M.; Rodriguez, R. Mechanism of growth inhibition of prostate cancer xenografts by valproic acid. J. Biomed. Biotechnol. 2012, 2012, 180363. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xia, Q.; Lv, J.; Zhang, H. Chronic administration of valproic acid inhibits PC3 cell growth by suppressing tumor angiogenesis in vivo. Int. J. Urol. 2007, 14, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Chelluri, R.; Caza, T.; Woodford, M.R.; Reeder, J.E.; Bratslavsky, G.; Byler, T. Valproic Acid Alters Angiogenic and Trophic Gene Expression in Human Prostate Cancer Models. Anticancer Res. 2016, 36, 5079–5086. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhang, J.; Zhang, X.; Yuan, J.; Shi, Y.; Qiao, L. Inhibition of lipogenesis and induction of apoptosis by valproic acid in prostate cancer cells via the C/EBPα/SREBP-1 pathway. Acta Biochim. Biophys. Sin. 2021, 53, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, F.; Urbano, R.; Graziani, G.; Muzi, A.; Navarra, P.; Sica, G. Valproic acid activity in androgen-sensitive and -insensitive human prostate cancer cells. Int. J. Oncol. 2008, 32, 1293–1303. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wang, L.; Song, C.; Wang, X.; Kang, J. Valproic acid inhibits prostate cancer cell migration by up-regulating E-cadherin expression. Die Pharm. 2011, 66, 614–618. [Google Scholar]
- Lee, J.E.; Kim, J.H. Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1. Genet. Mol. Biol. 2015, 38, 527–533. [Google Scholar] [CrossRef]
- Jiang, W.; Zheng, Y.; Huang, Z.; Wang, M.; Zhang, Y.; Wang, Z.; Jin, X.; Xia, Q. Role of SMAD4 in the mechanism of valproic acid’s inhibitory effect on prostate cancer cell invasiveness. Int. Urol. Nephrol. 2014, 46, 941–946. [Google Scholar] [CrossRef]
- Lan, X.; Lu, G.; Yuan, C.; Mao, S.; Jiang, W.; Chen, Y.; Jin, X.; Xia, Q. Valproic acid (VPA) inhibits the epithelial-mesenchymal transition in prostate carcinoma via the dual suppression of SMAD4. J. Cancer Res. Clin. Oncol. 2016, 142, 177–185. [Google Scholar] [CrossRef]
- Qi, G.; Lu, G.; Yu, J.; Zhao, Y.; Wang, C.; Zhang, H.; Xia, Q. Up-regulation of TIF1γ by valproic acid inhibits the epithelial mesenchymal transition in prostate carcinoma through TGF-β/Smad signaling pathway. Eur. J. Pharmacol. 2019, 860, 172551. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Mo, J.; Pang, M.; Chen, Z.; Feng, F.; Xie, P.; Yang, B. Identification of HCG18 and MCM3AP-AS1 That Associate with Bone Metastasis, Poor Prognosis and Increased Abundance of M2 Macrophage Infiltration in Prostate Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033821990064. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Biancolella, M.; Amati, F.; Gravina, P.; Miano, R.; Chillemi, G.; Farcomeni, A.; Bueno, S.; Vespasiani, G.; Desideri, A.; et al. Valproic acid induces neuroendocrine differentiation and UGT2B7 up-regulation in human prostate carcinoma cell line. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, A.; Muzi, P.; Cristiano, L.; Millimaggi, D.; Cimini, A.; Dolo, V.; Miano, R.; Vicentini, C.; Cerù, M.P.; Bologna, M. Neuroendocrine transdifferentiation induced by VPA is mediated by PPARgamma activation and confers resistance to antiblastic therapy in prostate carcinoma. Prostate 2008, 68, 588–598. [Google Scholar] [CrossRef]
- Sidana, A.; Wang, M.; Chowdhury, W.H.; Toubaji, A.; Shabbeer, S.; Netto, G.; Carducci, M.; Lupold, S.E.; Rodriguez, R. Does valproic acid induce neuroendocrine differentiation in prostate cancer? J. Biomed. Biotechnol. 2011, 2011, 607480. [Google Scholar] [CrossRef]
- Wedel, S.; Hudak, L.; Seibel, J.M.; Juengel, E.; Tsaur, I.; Wiesner, C.; Haferkamp, A.; Blaheta, R.A. Inhibitory effects of the HDAC inhibitor valproic acid on prostate cancer growth are enhanced by simultaneous application of the mTOR inhibitor RAD001. Life Sci. 2011, 88, 418–424. [Google Scholar] [CrossRef]
- Wedel, S.; Hudak, L.; Seibel, J.M.; Makarević, J.; Juengel, E.; Tsaur, I.; Wiesner, C.; Haferkamp, A.; Blaheta, R.A. Impact of combined HDAC and mTOR inhibition on adhesion, migration and invasion of prostate cancer cells. Clin. Exp. Metastasis 2011, 28, 479–491. [Google Scholar] [CrossRef]
- Thelen, P.; Krahn, L.; Bremmer, F.; Strauss, A.; Brehm, R.; Loertzer, H. Synergistic effects of histone deacetylase inhibitor in combination with mTOR inhibitor in the treatment of prostate carcinoma. Int. J. Mol. Med. 2013, 31, 339–346. [Google Scholar] [CrossRef]
- Makarević, J.; Rutz, J.; Juengel, E.; Maxeiner, S.; Tsaur, I.; Chun, F.K.; Bereiter-Hahn, J.; Blaheta, R.A. Influence of the HDAC Inhibitor Valproic Acid on the Growth and Proliferation of Temsirolimus-Resistant Prostate Cancer Cells In Vitro. Cancers 2019, 11, 566. [Google Scholar] [CrossRef]
- Makarević, J.; Rutz, J.; Juengel, E.; Maxeiner, S.; Mani, J.; Vallo, S.; Tsaur, I.; Roos, F.; Chun, F.K.; Blaheta, R.A. HDAC Inhibition Counteracts Metastatic Re-Activation of Prostate Cancer Cells Induced by Chronic mTOR Suppression. Cells 2018, 7, 129. [Google Scholar] [CrossRef]
- Tran, L.N.K.; Kichenadasse, G.; Butler, L.M.; Centenera, M.M.; Morel, K.L.; Ormsby, R.J.; Michael, M.Z.; Lower, K.M.; Sykes, P.J. The Combination of Metformin and Valproic Acid Induces Synergistic Apoptosis in the Presence of p53 and Androgen Signaling in Prostate Cancer. Mol. Cancer Ther. 2017, 16, 2689–2700. [Google Scholar] [CrossRef]
- Tran, L.N.K.; Kichenadasse, G.; Morel, K.L.; Lavranos, T.C.; Klebe, S.; Lower, K.M.; Ormsby, R.J.; Elliot, D.J.; Sykes, P.J. The Combination of Metformin and Valproic Acid Has a Greater Anti-tumoral Effect on Prostate Cancer Growth In Vivo than Either Drug Alone. In Vivo 2019, 33, 99–108. [Google Scholar] [CrossRef]
- Ouyang, D.Y.; Ji, Y.H.; Saltis, M.; Xu, L.H.; Zhang, Y.T.; Zha, Q.B.; Cai, J.Y.; He, X.H. Valproic acid synergistically enhances the cytotoxicity of gossypol in DU145 prostate cancer cells: An iTRTAQ-based quantitative proteomic analysis. J. Proteom. 2011, 74, 2180–2193. [Google Scholar] [CrossRef]
- Sargazi, S.; Saravani, R.; Zavar Reza, J.; Jaliani, H.Z.; Mirinejad, S.; Rezaei, Z.; Zarei, S. Induction of apoptosis and modulation of homologous recombination DNA repair pathway in prostate cancer cells by the combination of AZD2461 and valproic acid. EXCLI J. 2019, 18, 485–498. [Google Scholar] [CrossRef]
- Hudak, L.; Tezeeh, P.; Wedel, S.; Makarević, J.; Juengel, E.; Tsaur, I.; Bartsch, G.; Wiesner, C.; Haferkamp, A.; Blaheta, R.A. Low dosed interferon alpha augments the anti-tumor potential of histone deacetylase inhibition on prostate cancer cell growth and invasion. Prostate 2012, 72, 1719–1735. [Google Scholar] [CrossRef]
- Iannelli, F.; Roca, M.S.; Lombardi, R.; Ciardiello, C.; Grumetti, L.; De Rienzo, S.; Moccia, T.; Vitagliano, C.; Sorice, A.; Costantini, S.; et al. Synergistic antitumor interaction of valproic acid and simvastatin sensitizes prostate cancer to docetaxel by targeting CSCs compartment via YAP inhibition. J. Exp. Clin. Cancer Res. 2020, 39, 213. [Google Scholar] [CrossRef]
- Pacheco, M.B.; Camilo, V.; Lopes, N.; Moreira-Silva, F. Hydralazine and Panobinostat Attenuate Malignant Properties of Prostate Cancer Cell Lines. Pharmaceuticals 2021, 14, 670. [Google Scholar] [CrossRef]
- Wedel, S.; Hudak, L.; Seibel, J.M.; Juengel, E.; Oppermann, E.; Haferkamp, A.; Blaheta, R.A. Critical analysis of simultaneous blockage of histone deacetylase and multiple receptor tyrosine kinase in the treatment of prostate cancer. Prostate 2011, 71, 722–735. [Google Scholar] [CrossRef]
- Chen, X.; Wong, J.Y.; Wong, P.; Radany, E.H. Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis. Mol. Cancer Res. MCR 2011, 9, 448–461. [Google Scholar] [CrossRef]
- Gavrilov, V.; Leibovich, Y.; Ariad, S.; Lavrenkov, K.; Shany, S. A combined pretreatment of 1,25-dihydroxyvitamin D3 and sodium valproate enhances the damaging effect of ionizing radiation on prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2010, 121, 391–394. [Google Scholar] [CrossRef]
- Sharma, S.; Symanowski, J.; Wong, B.; Dino, P.; Manno, P.; Vogelzang, N. A Phase II Clinical Trial of Oral Valproic Acid in Patients with Castration-Resistant Prostate Cancers Using an Intensive Biomarker Sampling Strategy. Transl. Oncol. 2008, 1, 141–147. [Google Scholar] [CrossRef][Green Version]
- Valproic Acid in Treating Patients with Progressive, Non-Metastatic Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT00670046 (accessed on 21 July 2022).
- Wheler, J.J.; Janku, F.; Falchook, G.S.; Jackson, T.L.; Fu, S.; Naing, A.; Tsimberidou, A.M.; Moulder, S.L.; Hong, D.S.; Yang, H.; et al. Phase I study of anti-VEGF monoclonal antibody bevacizumab and histone deacetylase inhibitor valproic acid in patients with advanced cancers. Cancer Chemother. Pharmacol. 2014, 73, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.Z.; Butler, P.M.; Kurowski, D.; Perloff, M.D. Beyond neuropathic pain: Gabapentin use in cancer pain and perioperative pain. Clin. J. Pain 2014, 30, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Mahon, S. Hot flash management: Update of the evidence for patients with cancer. Clin. J. Oncol. Nurs. 2014, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Ginex, P.K.; Michaud, L.B.; Fernández-Ortega, P.; Leibelt, J.; Mahon, S. ONS Guidelines™ for Cancer Treatment-Related Hot Flashes in Women with Breast Cancer and Men with Prostate Cancer. Oncol. Nurs. Forum 2020, 47, 374–399. [Google Scholar] [CrossRef] [PubMed]
- Tran-Van-Minh, A.; Dolphin, A.C. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 12856–12867. [Google Scholar] [CrossRef]
- Warnier, M.; Roudbaraki, M.; Derouiche, S.; Delcourt, P.; Bokhobza, A.; Prevarskaya, N.; Mariot, P. CACNA2D2 promotes tumorigenesis by stimulating cell proliferation and angiogenesis. Oncogene 2015, 34, 5383–5394. [Google Scholar] [CrossRef]
- Bugan, I.; Karagoz, Z.; Altun, S.; Djamgoz, M.B. Gabapentin, an Analgesic Used Against Cancer-Associated Neuropathic Pain: Effects on Prostate Cancer Progression in an In Vivo Rat Model. Basic Clin. Pharmacol. Toxicol. 2016, 118, 200–207. [Google Scholar] [CrossRef]
- Fraser, S.P.; Salvador, V.; Manning, E.A.; Mizal, J.; Altun, S.; Raza, M.; Berridge, R.J.; Djamgoz, M.B. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. Lateral motility. J. Cell. Physiol. 2003, 195, 479–487. [Google Scholar] [CrossRef]
- Yildirim, S.; Altun, S.; Gumushan, H.; Patel, A.; Djamgoz, M.B.A. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012, 323, 58–61. [Google Scholar] [CrossRef]
- Diss, J.K.; Archer, S.N.; Hirano, J.; Fraser, S.P.; Djamgoz, M.B. Expression profiles of voltage-gated Na(+) channel alpha-subunit genes in rat and human prostate cancer cell lines. Prostate 2001, 48, 165–178. [Google Scholar] [CrossRef]
- Diss, J.K.; Stewart, D.; Pani, F.; Foster, C.S.; Walker, M.M.; Patel, A.; Djamgoz, M.B. A potential novel marker for human prostate cancer: Voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005, 8, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.; Hoosein, N. Inhibition by anticonvulsants of prostate-specific antigen and interleukin-6 secretion by human prostate cancer cells. Anticancer Res. 2001, 21, 2045–2048. [Google Scholar] [PubMed]
- Gartrell, B.A.; Roach, M., III; Retter, A.; Sokol, G.H.; Del Priore, G.; Scher, H.I. Phase II trial of SM-88, a cancer metabolism based therapy, in non-metastatic biochemical recurrent prostate cancer. Investig. New Drugs 2021, 39, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Stettner, M.; Krämer, G.; Strauss, A.; Kvitkina, T.; Ohle, S.; Kieseier, B.C.; Thelen, P. Long-term antiepileptic treatment with histone deacetylase inhibitors may reduce the risk of prostate cancer. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2012, 21, 55–64. [Google Scholar] [CrossRef]
- Salminen, J.K.; Kuoppamäki, V.; Talala, K. Antiepileptic drugs and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Int. J. Cancer 2021, 149, 307–315. [Google Scholar] [CrossRef]
- Salminen, J.K.; Mehtola, A.; Talala, K.; Taari, K.; Mäkinen, J.; Peltola, J.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Anti-epileptic drugs and prostate cancer-specific mortality compared to non-users of anti-epileptic drugs in the Finnish Randomized Study of Screening for Prostate Cancer. Br. J. Cancer 2022, 127, 704–711. [Google Scholar] [CrossRef]
- Diaz, D.; Delgadillo, D.M.; Hernández-Gallegos, E.; Ramírez-Domínguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J. Cell. Physiol. 2007, 210, 469–478. [Google Scholar] [CrossRef]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef]
- Altamura, C.; Greco, M.R. Emerging Roles for Ion Channels in Ovarian Cancer: Pathomechanisms and Pharmacological Treatment. Cancers 2021, 13, 668. [Google Scholar] [CrossRef]
- Brummelhuis, I.S.; Fiascone, S.J.; Hasselblatt, K.T.; Frendl, G.; Elias, K.M. Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer. Cancers 2021, 13, 5437. [Google Scholar] [CrossRef]
- Gao, R.; Shen, Y.; Cai, J.; Lei, M.; Wang, Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol. Rep. 2010, 23, 1293–1299. [Google Scholar] [CrossRef]
- House, C.D.; Wang, B.D.; Ceniccola, K.; Williams, R.; Simaan, M.; Olender, J.; Patel, V.; Baptista-Hon, D.T.; Annunziata, C.M.; Gutkind, J.S.; et al. Voltage-gated Na+ Channel Activity Increases Colon Cancer Transcriptional Activity and Invasion via Persistent MAPK Signaling. Sci. Rep. 2015, 5, 11541. [Google Scholar] [CrossRef]
- Guzel, R.M.; Ogmen, K.; Ilieva, K.M.; Fraser, S.P.; Djamgoz, M.B.A. Colorectal cancer invasiveness in vitro: Predominant contribution of neonatal Nav1.5 under normoxia and hypoxia. J. Cell. Physiol. 2019, 234, 6582–6593. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lv, Y.; Xu, J.; Mao, X.; Chen, Z.; Lu, W. Over-expression of Nav1.6 channels is associated with lymph node metastases in colorectal cancer. World J. Surg. Oncol. 2019, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Peng, J.; Han, K.; Lin, J.; Zhang, R.; Ou, Q.; Qin, J.; Deng, Y.; Zhou, W.; Kong, L.; et al. Voltage-gated sodium channel Na(v)1.5 promotes tumor progression and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cancer Lett. 2021, 500, 119–131. [Google Scholar] [CrossRef]
- Lastraioli, E.; Fraser, S.P.; Guzel, R.M.; Iorio, J.; Bencini, L.; Scarpi, E. Neonatal Nav1.5 Protein Expression in Human Colorectal Cancer: Immunohistochemical Characterization and Clinical Evaluation. Cancers 2021, 13, 3832. [Google Scholar] [CrossRef]
- Xia, J.; Huang, N.; Huang, H.; Sun, L.; Dong, S.; Su, J.; Zhang, J.; Wang, L.; Lin, L.; Shi, M.; et al. Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int. J. Cancer 2016, 139, 2553–2569. [Google Scholar] [CrossRef]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef]
- Campbell, T.M.; Main, M.J.; Fitzgerald, E.M. Functional expression of the voltage-gated Na⁺-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J. Cell Sci. 2013, 126, 4939–4949. [Google Scholar] [CrossRef]
- Arcangeli, A.; Pillozzi, S.; Becchetti, A. Targeting ion channels in leukemias: A new challenge for treatment. Curr. Med. Chem. 2012, 19, 683–696. [Google Scholar] [CrossRef]
- Moufarrij, S.; Dandapani, M.; Arthofer, E.; Gomez, S.; Srivastava, A.; Lopez-Acevedo, M.; Villagra, A.; Chiappinelli, K.B. Epigenetic therapy for ovarian cancer: Promise and progress. Clin. Epigenet. 2019, 11, 7. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.; Cai, H.; Li, Y.; Zhang, Y.; Zhang, X.; Zhao, D.; Li, Z.; Ma, H.; Wang, J.; et al. HDAC as a therapeutic target for treatment of endometrial cancers. Curr. Pharm. Des. 2014, 20, 1847–1856. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Ramakrishnan, R.K. Histone Modification in NSCLC: Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 11701. [Google Scholar] [CrossRef]
- Mamdani, H.; Jalal, S.I. Histone Deacetylase Inhibition in Non-small Cell Lung Cancer: Hype or Hope? Front. Cell Dev. Biol. 2020, 8, 582370. [Google Scholar] [CrossRef]
- Eyüpoglu, I.Y.; Savaskan, N.E. Epigenetics in Brain Tumors: HDACs Take Center Stage. Curr. Neuropharmacol. 2016, 14, 48–54. [Google Scholar] [CrossRef]
- Mehrpouri, M.; Pourbagheri-Sigaroodi, A.; Bashash, D. The contributory roles of histone deacetylases (HDACs) in hematopoiesis regulation and possibilities for pharmacologic interventions in hematologic malignancies. Int. Immunopharmacol. 2021, 100, 108114. [Google Scholar] [CrossRef]
- Imai, Y.; Hirano, M.; Kobayashi, M.; Futami, M.; Tojo, A. HDAC Inhibitors Exert Anti-Myeloma Effects through Multiple Modes of Action. Cancers 2019, 11, 475. [Google Scholar] [CrossRef]
- Lipska, K.; Gumieniczek, A.; Filip, A.A. Anticonvulsant valproic acid and other short-chain fatty acids as novel anticancer therapeutics: Possibilities and challenges. Acta Pharm. 2020, 70, 291–301. [Google Scholar] [CrossRef]
- Wu, K.C.; Liao, K.S.; Yeh, L.R.; Wang, Y.K. Drug Repurposing: The Mechanisms and Signaling Pathways of Anti-Cancer Effects of Anesthetics. Biomedicines 2022, 10, 1589. [Google Scholar] [CrossRef]
- Cucchiara, F.; Pasqualetti, F.; Giorgi, F.S.; Danesi, R.; Bocci, G. Epileptogenesis and oncogenesis: An antineoplastic role for antiepileptic drugs in brain tumours? Pharmacol. Res. 2020, 156, 104786. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lai, H.Y.; Chiu, A.; Chan, S.H.; Hsiao, L.P.; Lee, S.T. The effects of antiepileptic drugs on the growth of glioblastoma cell lines. J. Neuro-Oncol. 2016, 127, 445–453. [Google Scholar] [CrossRef]
- Han, W.; Guan, W. Valproic Acid: A Promising Therapeutic Agent in Glioma Treatment. Front. Oncol. 2021, 11, 687362. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Sorrentino, S.; Proietti, G.; Lama, G.; Dobrowolny, G.; Catizone, A.; Binda, E.; Larocca, L.M.; Sica, G. Levetiracetam enhances the temozolomide effect on glioblastoma stem cell proliferation and apoptosis. Cancer Cell Int. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Min, K.L.; Chang, M.J. Effect of anti-epileptic drugs on the survival of patients with glioblastoma multiforme: A retrospective, single-center study. PLoS ONE 2019, 14, e0225599. [Google Scholar] [CrossRef] [PubMed]
- Julie, D.A.R.; Ahmed, Z.; Karceski, S.C.; Pannullo, S.C.; Schwartz, T.H.; Parashar, B.; Wernicke, A.G. An overview of anti-epileptic therapy management of patients with malignant tumors of the brain undergoing radiation therapy. Seizure 2019, 70, 30–37. [Google Scholar] [CrossRef]
- Lai, Z.Y.; Li, D.Y.; Huang, C.Y.; Tung, K.C.; Yang, C.C.; Liu, H.M.; Chou, F.I.; Chuang, Y.J. Valproic Acid Enhances Radiosensitization via DNA Double-strand Breaks for Boronophenylalanine-mediated Neutron Capture Therapy in Melanoma Cells. Anticancer Res. 2022, 42, 3413–3426. [Google Scholar] [CrossRef] [PubMed]
- Chodurek, E.; Kulczycka, A.; Orchel, A.; Aleksander-Konert, E.; Dzierzewicz, Z. Effect of valproic acid on the proliferation and apoptosis of the human melanoma G-361 cell line. Acta Pol. Pharm. 2014, 71, 917–921. [Google Scholar]
- Sanaei, M.; Kavoosi, F.; Roustazadeh, A.; Shahsavani, H. In Vitro Effect of the Histone Deacetylase Inhibitor Valproic Acid on Viability and Apoptosis of the PLC/PRF5 Human Hepatocellular Carcinoma Cell Line. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2507–2510. [Google Scholar] [CrossRef]
- Han, B.R.; You, B.R.; Park, W.H. Valproic acid inhibits the growth of HeLa cervical cancer cells via caspase-dependent apoptosis. Oncol. Rep. 2013, 30, 2999–3005. [Google Scholar] [CrossRef]
- Anirudh, B.V.M.; Ezhilarasan, D. Reactive Oxygen Species-Mediated Mitochondrial Dysfunction Triggers Sodium Valproate-Induced Cytotoxicity in Human Colorectal Adenocarcinoma Cells. J. Gastrointest. Cancer 2021, 52, 899–906. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Huang, L.; Alston, N.; Ouyang, N.; Vrankova, K.; Mattheolabakis, G.; Constantinides, P.P.; Rigas, B. Targeting mitochondrial STAT3 with the novel phospho-valproic acid (MDC-1112) inhibits pancreatic cancer growth in mice. PLoS ONE 2013, 8, e61532. [Google Scholar] [CrossRef]
- Luo, D.; Fraga-Lauhirat, M.; Millings, J.; Ho, C.; Villarreal, E.M.; Fletchinger, T.C.; Bonfiglio, J.V.; Mata, L.; Nemesure, M.D.; Bartels, L.E.; et al. Phospho-valproic acid (MDC-1112) suppresses glioblastoma growth in preclinical models through the inhibition of STAT3 phosphorylation. Carcinogenesis 2019, 40, 1480–1491. [Google Scholar] [CrossRef]
- Luo, D.; Digiovanni, M.G.; Wei, R.; Lacomb, J.F.; Williams, J.L.; Rigas, B.; Mackenzie, G.G. Phospho-valproic acid (MDC-1112) reduces pancreatic cancer growth in patient-derived tumor xenografts and KPC mice: Enhanced efficacy when combined with gemcitabine. Carcinogenesis 2020, 41, 927–939. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Wang, R.; Rigas, B.; Mackenzie, G.G. Phospho-valproic acid inhibits pancreatic cancer growth in mice: Enhanced efficacy by its formulation in poly-(L)-lactic acid-poly(ethylene glycol) nanoparticles. Int. J. Oncol. 2017, 51, 1035–1044. [Google Scholar] [CrossRef]
- Dodurga, Y.; Gundogdu, G.; Tekin, V.; Koc, T.; Satiroglu-Tufan, N.L.; Bagci, G.; Kucukatay, V. Valproic acid inhibits the proliferation of SHSY5Y neuroblastoma cancer cells by downregulating URG4/URGCP and CCND1 gene expression. Mol. Biol. Rep. 2014, 41, 4595–4599. [Google Scholar] [CrossRef]
- Michaelis, M.; Suhan, T.; Cinatl, J.; Driever, P.H.; Cinatl, J., Jr. Valproic acid and interferon-alpha synergistically inhibit neuroblastoma cell growth in vitro and in vivo. Int. J. Oncol. 2004, 25, 1795–1799. [Google Scholar] [PubMed]
- Shah, R.D.; Jagtap, J.C.; Mruthyunjaya, S.; Shelke, G.V.; Pujari, R.; Das, G.; Shastry, P. Sodium valproate potentiates staurosporine-induced apoptosis in neuroblastoma cells via Akt/survivin independently of HDAC inhibition. J. Cell. Biochem. 2013, 114, 854–863. [Google Scholar] [CrossRef]
- Park, H.K.; Han, B.R.; Park, W.H. Combination of Arsenic Trioxide and Valproic Acid Efficiently Inhibits Growth of Lung Cancer Cells via G2/M-Phase Arrest and Apoptotic Cell Death. Int. J. Mol. Sci. 2020, 21, 2649. [Google Scholar] [CrossRef]
- Hakami, T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef]
- Pal, R.; Singh, K.; Khan, S.A.; Chawla, P.; Kumar, B.; Akhtar, M.J. Reactive metabolites of the anticonvulsant drugs and approaches to minimize the adverse drug reaction. Eur. J. Med. Chem. 2021, 226, 113890. [Google Scholar] [CrossRef] [PubMed]
- Luna-Palencia, G.R.; Correa-Basurto, J.; Trujillo-Ferrara, J.; Meraz-Ríos, M.A.; Vásquez- Moctezuma, I. Epigenetic Evaluation of N-(2-hydroxyphenyl)-2-Propylpentanamide, a Valproic Acid Aryl Derivative with Activity Against HeLa Cells. Curr. Mol. Pharmacol. 2021, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, H.; Kühne, M.; Grune, C.; Warncke, P.; Hofmann, S.; Koschella, A.; Godmann, M.; Fischer, D.; Heinzel, T.; Heinze, T. Polysaccharide Nanoparticles Bearing HDAC Inhibitor as Nontoxic Nanocarrier for Drug Delivery. Macromol. Biosci. 2020, 20, e2000039. [Google Scholar] [CrossRef] [PubMed]
- Kühne, M.; Lindemann, H.; Grune, C.; Schröder, D.; Cseresnyés, Z.; Godmann, M.; Koschella, A.; Figge, M.T.; Eggeling, C.; Fischer, D.; et al. Biocompatible sulfated valproic acid-coupled polysaccharide-based nanocarriers with HDAC inhibitory activity. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 717–730. [Google Scholar] [CrossRef]
- Singh, A.K.; Banerjee, S.; Nair, A.V.; Ray, S.; Ojha, M.; Mondal, A.; Singh, N.D.P. Green Light-Activated Single-Component Organic Fluorescence-Based Nano-Drug Delivery System for Dual Uncaging of Anticancer Drugs. ACS Appl. Bio Mater. 2022, 5, 1202–1209. [Google Scholar] [CrossRef]
| Tumor Type | AED | Molecular Targets | Study Models | Biological Effects and Main Underlying Mechanisms | References |
|---|---|---|---|---|---|
| Breast | VPA and Derivatives | VGSCs HDACi |
In vitro and in vivo clinical studies | Inhibit proliferation, cell cycle, survival, migration and hormone receptor expression | [42,76,78,79,81,82,83,86,88,91,92] |
| PHT | VGSCs (Nav1.5) | In vitro and in vivo | Inhibit cells migration, invasion and metastasis | [14,40,64,94,95] | |
| LTG | VGSCs VGCCs HDACi | In vitro and in vivo | Anti-proliferative effect and inhibition of breast tumor growth | [14,15,16,17,98,99,100,101,102,103,104] | |
| CBZ | HDACi CCIDs | In vitro | Anti-metastatic potential by inducing HER2 proteasomal degradation; inhibition of cell proliferation. Inactivation of MLC2, MYPT1 and FAK mobility proteins | [111,112,113] | |
| Prostate | VPA and Derivatives | HDACi C/EBPα/SREBP-1 E-cadherin mTOR |
In vitro and in vivo clinical studies | PSA down-regulation; caspase-3 up regulation; cell cycle arrest, apoptosis, autophagy and suppression of tumor angiogenesis. Cell growth Inhibition by reducing lipogenesis. Inhibition of cells migration. Reduction of tumor growth in vivo. | [127,128,131,132,133,134,135,136,137,138,139,140,141,150] |
| GBP | Calcium channel α2δ2 subunit | In vitro | Inhibition of tumor cell growth | [171] | |
| CBZ | VGSCs | In vitro | Reduction of cell motility; inhibition of PSA secretion and of cell growth in matrigel | [173,177] | |
| PHT | VGSCs | In vitro | Inhibition of PSA secretion and of cell growth in matrigel | [173,177] | |
| Brain | VPA and Derivatives | HDACi URG4/URGCP and CCND1 | In vitro and in vivo | Inhibition of proliferation; apoptosis; growth suppression through STAT3 phosphorylation inhibition; cell cycle arrest | [206,207,219,222,223,224] |
| OXC | VGSCs | In vitro | Inhibition of proliferation; apoptosis | [207] | |
| LTG | HDACs PI3k/AKT VGSCs and VGCCs? | In vitro | Inhibition of proliferation; apoptosis | [207,208] | |
| GBP | VGSCs | In vitro | Inhibition of proliferation; apoptosis | [207,208] | |
| TGB | GAT-1 | In vitro | Inhibition of proliferation; apoptosis | [207,208] | |
| PHT | VGSCs | In vitro | Inhibition of proliferation; apoptosis | [207,208] | |
| Hepatocellular carcinoma | VPA | HDACi | In vitro | Inhibition of proliferation; apoptosis | [215] |
| Cervical | VPA | HDACi ROS | In vitro | Inhibition of proliferation via caspase-dependent apoptosis | [216] |
| Pancreatic | VPA and Derivatives | mitochondrial STAT3 | In vitro and in vivo | Inhibition of proliferation; apoptosis | [218,219,221] |
| Melanoma | VPA | increasing DNA DSBs | In vitro and in vivo | Inhibition of proliferation; apoptosis | [213,214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, M.; Ricci, E.; Ceraldi, R.; Nigro, A.; Bonofiglio, D.; Lanzino, M.; Morelli, C. From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer. Cancers 2022, 14, 4401. https://doi.org/10.3390/cancers14184401
Pellegrino M, Ricci E, Ceraldi R, Nigro A, Bonofiglio D, Lanzino M, Morelli C. From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer. Cancers. 2022; 14(18):4401. https://doi.org/10.3390/cancers14184401
Chicago/Turabian StylePellegrino, Michele, Elena Ricci, Rosangela Ceraldi, Alessandra Nigro, Daniela Bonofiglio, Marilena Lanzino, and Catia Morelli. 2022. "From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer" Cancers 14, no. 18: 4401. https://doi.org/10.3390/cancers14184401
APA StylePellegrino, M., Ricci, E., Ceraldi, R., Nigro, A., Bonofiglio, D., Lanzino, M., & Morelli, C. (2022). From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer. Cancers, 14(18), 4401. https://doi.org/10.3390/cancers14184401









