Systematic Review of the Role of Alpha-Protein Kinase 1 in Cancer and Cancer-Related Inflammatory Diseases
Abstract
Simple Summary
Abstract
1. Introduction
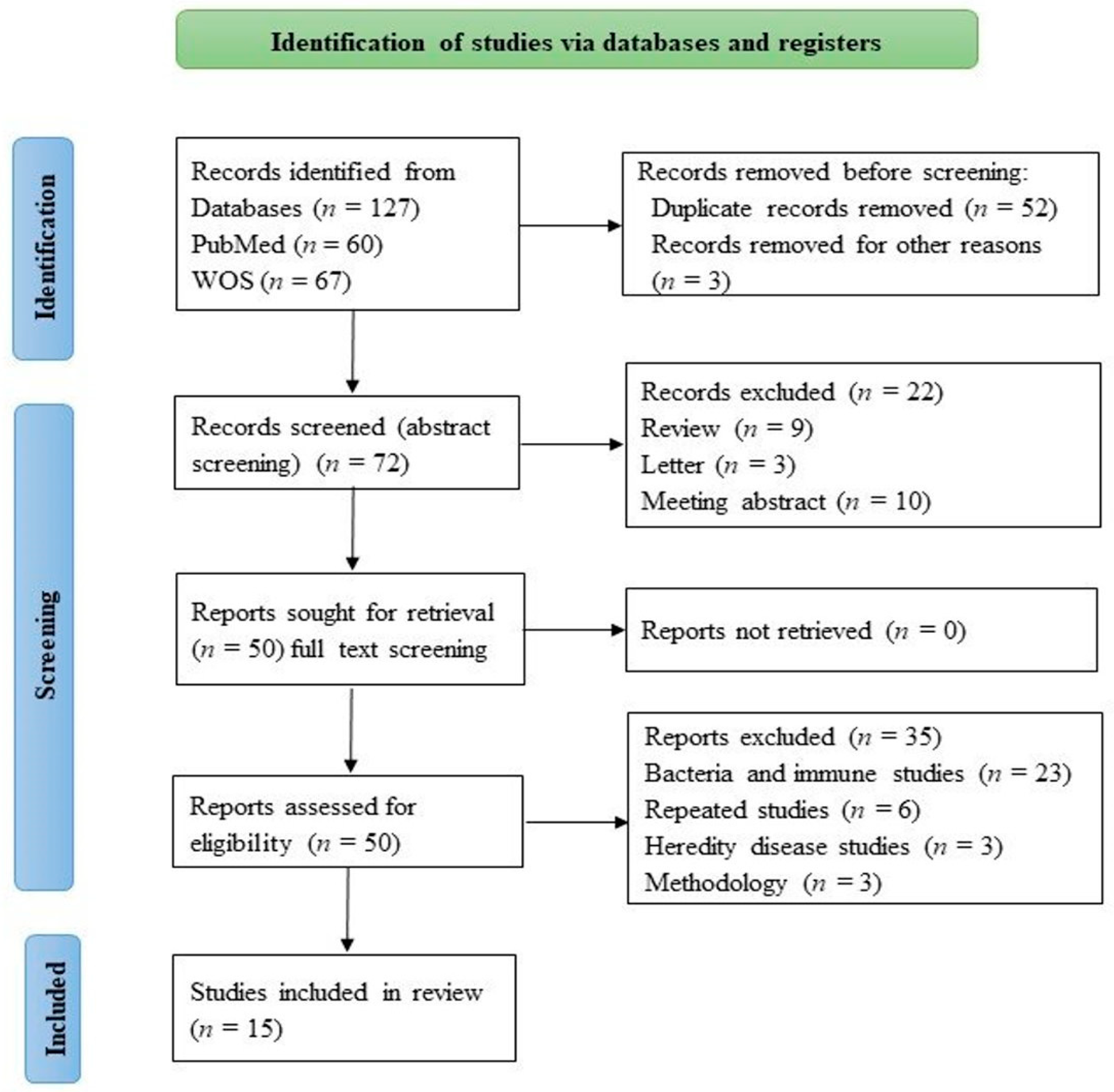
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
3. Results
3.1. ALPK1 Associated with Cancer Development
3.2. ALPK1 Associated with Gout, Chronic Kidney Disease, and Diabetes
4. Discussion
4.1. ALPK1 Is Associated with Cancer Development and Metastasis
4.1.1. ALPK1 and Cancer in Human Models
4.1.2. ALPK1 and Cancer in Experimental Cell Models
4.2. ALPK1 Is Associated with Gout, Chronic Kidney Disease, and Diabetes
4.2.1. ALPK1 and Gout
4.2.2. ALPK1 and Chronic Kidney Disease and Diabetes
4.3. Gout, Chronic Kidney Disease, and Diabetes Increase Cancer Risk as Shown by Meta-Analysis of Epidemiological Studies
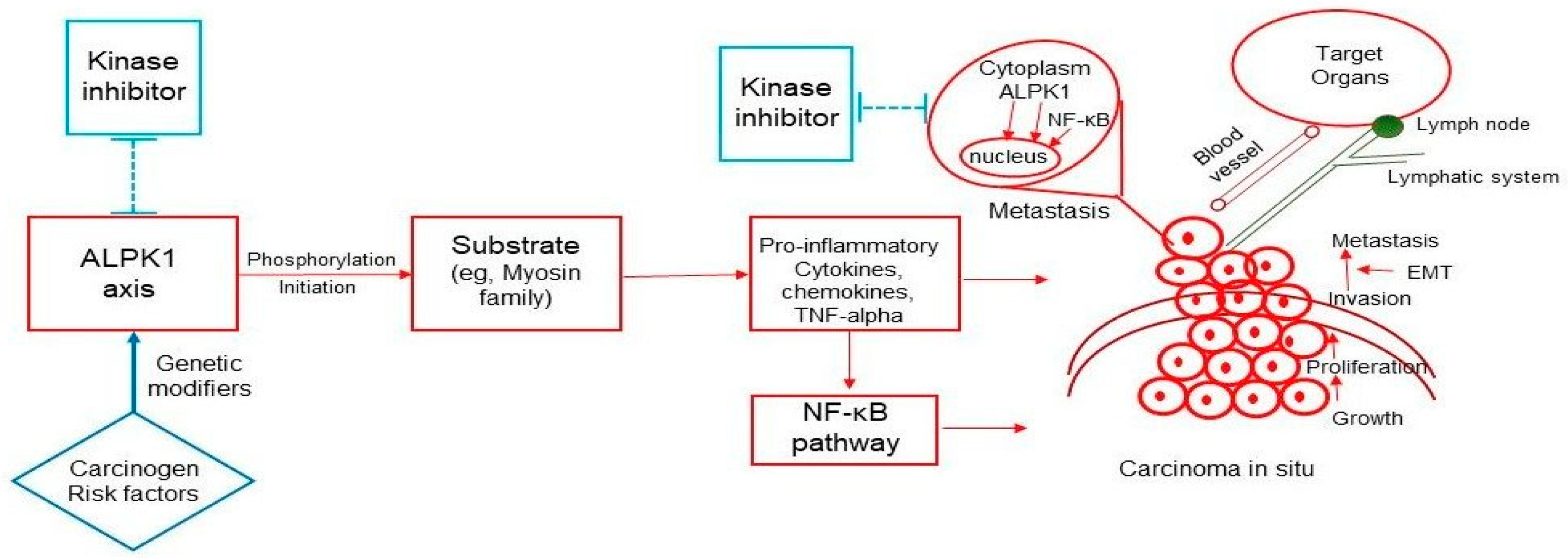
4.4. Possible Mechanisms and Pathways of ALPK1-Related Cancer Development and Metastasis
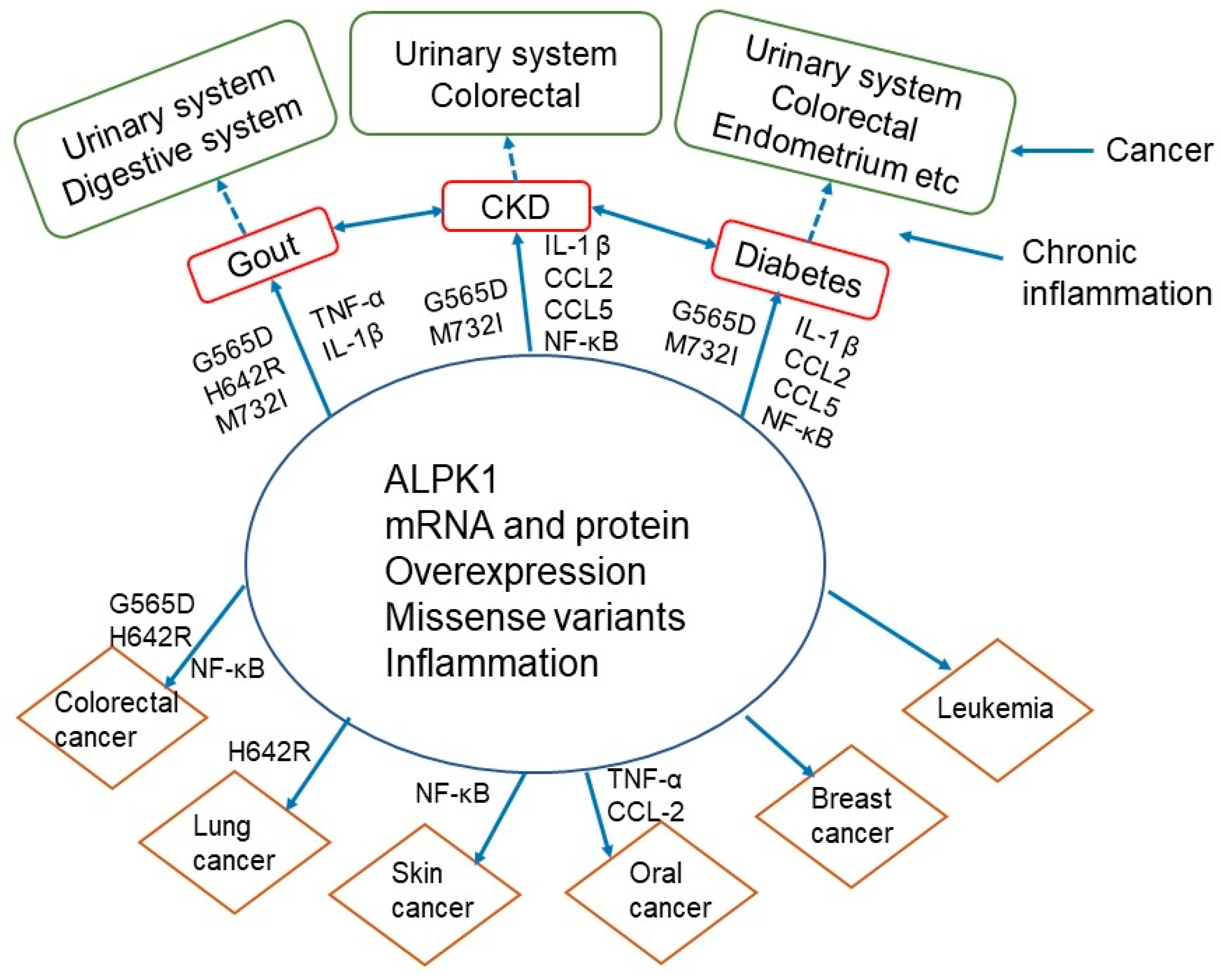
4.5. Interrelationships of ALPK1 with Gout, Chronic Kidney Disease, Diabetes, and Cancer
4.6. Clinical Implications of ALPK1 and the Development of Preventative and Therapeutic Drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Vaillancourt, J.P.; Lyons, C.; Côté, G.P. Identification of two phosphorylated threonines in the tail region of Dictyostelium myosin II. J. Biol. Chem. 1988, 263, 10082–10087. [Google Scholar] [CrossRef]
- Futey, L.M.; Medley, Q.G.; Côté, G.P.; Egelhoff, T.T. Structural analysis of myosin heavy chain kinase A from Dictyostelium. Evidence for a highly divergent protein kinase domain, an amino-terminal coiled-coil domain, and a domain homologous to the beta-subunit of heterotrimeric G proteins. J. Biol. Chem. 1995, 270, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ryazanov, A.G.; Pavur, K.S.; Dorovkov, M.V. Alpha-kinases: A new class of protein kinases with a novel catalytic domain. Curr. Biol. 1999, 9, R43–R45. [Google Scholar] [CrossRef]
- Heine, M.; Cramm-Behrens, C.I.; Ansari, A.; Chu, H.-P.; Ryazanov, A.G.; Naim, H.Y.; Jacob, R. α-Kinase 1, a new component in apical proteintransport. J. Biol. Chem. 2005, 280, 25637–25643. [Google Scholar] [CrossRef] [PubMed]
- McGuffin, L.J.; Bryson, K.; Jones, D.T.J.B. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zhang, Y. Protein-protein complex structure predictions by multimeric threading and template recombination. Structure 2011, 19, 955–966. [Google Scholar] [CrossRef]
- Cheng, L.S.-C.; Chiang, S.-L.; Tu, H.-P.; Chang, S.-J.; Wang, T.-N.; Ko, A.M.-J.; Chakraborty, R.; Ko, Y.-C. Genomewide scan for gout in Taiwanese aborigines reveals linkage to chromosome 4q25. Am. J. Hum. Genet. 2004, 75, 498–503. [Google Scholar] [CrossRef]
- Wang, S.-J.; Tu, H.-P.; Ko, A.M.-S.; Chiang, S.-L.; Chiou, S.-J.; Lee, S.-S.; Tsai, Y.-S.; Lee, C.-P.; Ko, Y.-C. Lymphocyte α-kinase is a gout-susceptible gene involved in monosodium urate monohydrate-induced inflammatory responses. J. Mol. Med. 2011, 89, 1241–1251. [Google Scholar] [CrossRef]
- Shimokata, S.; Oguri, M.; Fujimaki, T.; Horibe, H.; Kato, K.; Yamada, Y. Association between polymorphisms of the α-kinase 1 gene and type 2 diabetes mellitus in community-dwelling individuals. Biomed. Rep. 2013, 1, 940–944. [Google Scholar] [CrossRef]
- Yamada, Y.; Nishida, T.; Ichihara, S.; Kato, K.; Fujimaki, T.; Oguri, M.; Horibe, H.; Yoshida, T.; Watanabe, S.; Satoh, K.; et al. Identification of chromosome 3q28 and ALPK1 as susceptibility loci for chronic kidney disease in Japanese individuals by a genome-wide association study. J. Med. Genet. 2013, 50, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T.; Horibe, H.; Oguri, M.; Kato, K.; Yamada, Y. Association of genetic variants of the α-kinase 1 gene with myocardial infarction in community-dwelling individuals. Biomed. Rep. 2014, 2, 127–131. [Google Scholar] [CrossRef]
- Chiba, T.; Matsuo, H.; Sakiyama, M.; Nakayama, A.; Shimizu, S.; Wakai, K.; Suma, S.; Nakashima, H.; Sakurai, Y.; Shimizu, T.; et al. Common variant of ALPK1 is not associated with gout: A replication study. Hum. Cell 2014, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Nordenvall, C.; Nyrén, O.; Ye, W. A prospective study of gout and cancer. Eur. J. Cancer Prev. 2009, 18, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Chang, T.-C.; Chao, T.-Y.; Huang, M.-T.; Lin, H.-W. Risk of colorectal cancer in chronic kidney disease: A matched cohort study based on administrative data. Ann. Surg. Oncol. 2013, 20, 3885–3891. [Google Scholar] [CrossRef]
- Christensson, A.; Savage, C.; Sjoberg, D.D.; Cronin, A.M.; O’Brien, M.F.; Lowrance, W.; Nilsson, P.M.; Vickers, A.; Russo, P.; Lilja, H. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int. J. Cancer 2013, 133, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- de Vivar Chevez, A.R.; Finke, J.; Bukowski, R. The role of inflammation in kidney cancer. Adv. Exp. Med. Biol. 2014, 816, 197–234. [Google Scholar] [CrossRef]
- Ge, Z.; Ben, Q.; Qian, J.; Wang, Y.; Li, Y. Diabetes mellitus and risk of gastric cancer: A systematic review and meta-analysis of observational studies. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1127–1135. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef]
- Krämer, H.U.; Schöttker, B.; Raum, E.; Brenner, H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur. J. Cancer 2012, 48, 1269–1282. [Google Scholar] [CrossRef]
- Sciacca, L.; Vigneri, R.; Tumminia, A.; Frasca, F.; Squatrito, S.; Frittitta, L. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-F.; Lee, H.-H.; Chang, Y.-S.; Lin, C.-L.; Liu, T.-Y.; Chen, Y.-C.; Yen, J.-C.; Lee, Y.-T.; Lin, C.-Y.; Wu, S.-H.; et al. Down-regulated and Commonly mutated ALPK1 in Lung and Colorectal Cancers. Sci. Rep. 2016, 6, 27350. [Google Scholar] [CrossRef] [PubMed]
- Strietz, J.; Stepputtis, S.S.; Preca, B.-T.; Vannier, C.; Kim, M.M.; Castro, D.J.; Au, Q.; Boerries, M.; Busch, H.; Aza-Blanc, P.; et al. ERN1 and ALPK1 inhibit differentiation of bi-potential tumor-initiating cells in human breast cancer. Oncotarget 2016, 7, 83278–83293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Kuang, L.; Zhu, B.; Chen, J.; Wang, X.; Huang, X. Identification of prognostic risk factors of acute lymphoblastic leukemia based on mRNA expression profiling. Neoplasma 2017, 64, 494–501. [Google Scholar] [CrossRef]
- Ji, C.; Lin, S.; Yao, D.; Li, M.; Chen, W.; Zheng, S.; Zhao, Z. Identification of promising prognostic genes for relapsed acute lymphoblastic leukemia. Blood Cells Mol. Dis. 2019, 77, 113–119. [Google Scholar] [CrossRef]
- Rashid, M.; Van Der Horst, M.; Mentzel, T.; Butera, F.; Ferreira, I.; Pance, A.; Rütten, A.; Luzar, B.; Marusic, Z.; Aubain, N.D.S.; et al. ALPK1 hotspot mutation as a driver of human spiradenoma and spiradenocarcinoma. Nat. Commun. 2019, 10, 2213. [Google Scholar] [CrossRef]
- Chen, P.-K.; Hua, C.-H.; Hsu, H.-T.; Kuo, T.-M.; Chung, C.-M.; Lee, C.-P.; Tsai, M.-H.; Yeh, K.-T.; Ko, Y.-C. ALPK1 Expression Is Associated with Lymph Node Metastasis and Tumor Growth in Oral Squamous Cell Carcinoma Patients. Am. J. Pathol. 2019, 189, 190–199. [Google Scholar] [CrossRef]
- Lee, C.-P.; Ko, A.M.-S.; Nithiyanantham, S.; Lai, C.-H.; Ko, Y.-C. Long noncoding RNA HAR1A regulates oral cancer progression through the alpha-kinase 1, bromodomain 7, and myosin IIA axis. J. Mol. Med. 2021, 99, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef]
- Ko, A.M.-S.; Tu, H.-P.; Liu, T.-T.; Chang, J.-G.; Yuo, C.-Y.; Chiang, S.-L.; Chang, S.-J.; Liu, Y.-F.; Ko, A.M.-J.; Lee, C.-H.; et al. ALPK1 genetic regulation and risk in relation to gout. Int. J. Epidemiol. 2013, 42, 466–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamada, Y.; Matsui, K.; Takeuchi, I.; Fujimaki, T. Association of genetic variants with dyslipidemia and chronic kidney disease in a longitudinal population-based genetic epidemiological study. Int. J. Mol. Med. 2015, 35, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsui, K.; Takeuchi, I.; Oguri, M.; Fujimaki, T. Association of genetic variants of the α-kinase 1 gene with type 2 diabetes mellitus in a longitudinal population-based genetic epidemiological study. Biomed. Rep. 2015, 3, 347–354. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-P.; Chiang, S.-L.; Ko, A.M.-S.; Liu, Y.-F.; Ma, C.; Lu, C.-Y.; Huang, C.-M.; Chang, J.-G.; Kuo, T.-M.; Chen, C.-L.; et al. ALPK1 phosphorylates myosin IIA modulating TNF-α trafficking in gout flares. Sci. Rep. 2016, 6, 25740. [Google Scholar] [CrossRef]
- Natsuko, P.-D.; Laura, S.-C.; Denise, C.-C.; Lucio, V.-R.; Carlos, A.-S.; Fausto, S.-M.; Ambar, L.-M. Differential gene expression of ABCG2, SLC22A12, IL-1β, and ALPK1 in peripheral blood leukocytes of primary gout patients with hyperuricemia and their comorbidities: A case–control study. Eur. J. Med. Res. 2022, 27, 62. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-M.; Hsu, H.-T.; Chung, C.-M.; Yeh, K.-T.; Wu, C.-T.; Lee, C.-P.; Chiang, S.-L.; Huang, C.-M.; Ko, Y.-C. Enhanced alpha-kinase 1 accelerates multiple early nephropathies in streptozotocin-induced hyperglycemic mice. Biochim. Biophys. Acta 2016, 1862, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-P.; Nithiyanantham, S.; Hsu, H.-T.; Yeh, K.-T.; Kuo, T.-M.; Ko, Y.-C. ALPK1 regulates streptozotocin-induced nephropathy through CCL2 and CCL5 expressions. J. Cell. Mol. Med. 2019, 23, 7699–7708. [Google Scholar] [CrossRef]
- Sanches, M.; Duffy, N.M.; Talukdar, M.; Thevakumaran, N.; Chiovitti, D.; Canny, M.D.; Lee, K.; Kurinov, I.; Uehling, D.; Al-Awar, R.; et al. Structure and mechanism of action of the hydroxy–aryl–aldehyde class of IRE1 endoribonuclease inhibitors. Nat. Commun. 2014, 5, 4202. [Google Scholar] [CrossRef]
- Koumenis, C. ER stress, hypoxia tolerance and tumor progression. Curr. Mol. Med. 2006, 6, 55–69. [Google Scholar] [CrossRef]
- Tapley, P.; Lamballe, F.; Barbacid, M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 1992, 7, 371–381. [Google Scholar]
- Humans IWGotEoCRt. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 85, 1–334. [Google Scholar]
- Matsuo, H.; Yamamoto, K.; Nakaoka, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Takahashi, A.; Nakamura, T.; Nakashima, H.; Takada, Y.; et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann. Rheum. Dis. 2016, 75, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Stiburkova, B.; Pavelcová, K.; Zavada, J.; Petru, L.; Simek, P.; Cepek, P.; Pavlikova, M.; Matsuo, H.; Merriman, T.R.; Pavelka, K. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology 2017, 56, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Nakaoka, H.; Nakayama, A.; Okada, Y.; Yamamoto, K.; Higashino, T.; Sakiyama, M.; Shimizu, T.; Ooyama, H.; Ooyama, K.; et al. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Ann. Rheum. Dis. 2019, 78, 1430–1437. [Google Scholar] [CrossRef]
- Tu, H.-P.; Ko, A.M.-S.; Lee, S.-S.; Lee, C.-P.; Kuo, T.-M.; Huang, C.-M.; Ko, Y.-C. Variants of ALPK1 with ABCG2, SLC2A9, and SLC22A12 increased the positive predictive value for gout. J. Hum. Genet. 2018, 63, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, D.; Wang, B.; Yan, S.; Wang, X.; Yin, Y.; Wang, X.; Sun, B.; Sun, X. Increased Risk of Cancer in relation to Gout: A Review of Three Prospective Cohort Studies with 50,358 Subjects. Mediat. Inflamm. 2015, 2015, 680853. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, P.; Liu, K.; Lin, S.; Wang, M.; Tian, T.; Dai, C.; Deng, Y.; Li, N.; Hao, Q.; et al. Hyperuricemia and gout are associated with cancer incidence and mortality: A meta-analysis based on cohort studies. J. Cell. Physiol. 2019, 234, 14364–14376. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Staplin, N.; Emberson, J.; Baigent, C.; Turner, R.; Chalmers, J.; Zoungas, S.; Pollock, C.; Cooper, B.; Harris, D.; et al. Chronic kidney disease and the risk of cancer: An individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016, 16, 488. [Google Scholar] [CrossRef]
- Komaki, Y.; Komaki, F.; Micic, D.; Ido, A.; Sakuraba, A. Risk of Colorectal Cancer in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2018, 52, 796–804. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Association of Type 2 Diabetes with Cancer: A Meta-analysis With Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care 2020, 43, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Morgado, M.; Sutton, M.N.; Simmons, M.; Warren, C.R.; Lu, Z.; Constantinou, P.E.; Liu, J.; Francis, L.L.; Conlan, R.S.; Bast, R.C., Jr.; et al. Tumor necrosis factor-α and interferon-γ stimulate MUC16 (CA125) expression in breast, endometrial and ovarian cancers through NFκB. Oncotarget 2016, 7, 14871–14884. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Shi, X.; Pei, X.; Wu, X. Tumor necrosis factor-alpha-308 gene promoter polymorphism associates with survival of cancer patients: A meta-analysis. Medicine 2018, 97, e13160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef]
- Tang, D.; Tao, D.; Fang, Y.; Deng, C.; Xu, Q.; Zhou, J. TNF-Alpha Promotes Invasion and Metastasis via NF-Kappa B Pathway in Oral Squamous Cell Carcinoma. Med. Sci. Monit. Basic Res. 2017, 23, 141–149. [Google Scholar] [CrossRef]
- Mazzolini, R.; Dopeso, H.; Mateo-Lozano, S.; Chang, W.; Rodrigues, P.; Bazzocco, S.; Alazzouzi, H.; Landolfi, S.; Hernández-Losa, J.; Andretta, E.; et al. Brush border Myosin Ia has tumor suppressor activity in the intestine. Proc. Natl. Acad. Sci. USA 2012, 109, 1530–1535. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Zhang, Y.; Yang, J. Myosin Heavy Chain 9: Oncogene or Tumor Suppressor Gene? Med. Sci. Monit. 2019, 25, 888–892. [Google Scholar] [CrossRef]
- Schramek, D.; Sendoel, A.; Segal, J.P.; Beronja, S.; Heller, E.; Oristian, D.; Reva, B.; Fuchs, E. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science 2014, 343, 309–313. [Google Scholar] [CrossRef]
- Conti, M.A.; Saleh, A.D.; Brinster, L.R.; Cheng, H.; Chen, Z.; Cornelius, S.; Liu, C.; Ma, X.; Van Waes, C.; Adelstein, R.S. Conditional deletion of nonmuscle myosin II-A in mouse tongue epithelium results in squamous cell carcinoma. Sci. Rep. 2015, 5, 14068. [Google Scholar] [CrossRef]
- Kas, S.M.; De Ruiter, J.R.; Schipper, K.; Annunziato, S.; Schut, E.; Klarenbeek, S.; Drenth, A.P.; Van Der Burg, E.; Klijn, C.; Hoeve, J.J.T.; et al. Insertional mutagenesis identifies drivers of a novel oncogenic pathway in invasive lobular breast carcinoma. Nat. Genet. 2017, 49, 1219–1230. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Huang, T.; Zhong, X.-M.; Zhang, H.-W.; Cong, X.-L.; Xu, H.; Lu, G.-X.; Yu, F.; Xue, S.-B.; Lv, Z.-W.; et al. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol. Cancer 2018, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qi, X.; Liu, J.; Zhou, R.; Lin, C.; Shangguan, J.; Zhang, Z.; Zhao, L.; Li, G. MYH9 Promotes Growth and Metastasis via Activation of MAPK/AKT Signaling in Colorectal Cancer. J. Cancer 2019, 10, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Teng, G.G.; Yuan, J.-M.; Koh, W.-P. Bidirectional Association between Diabetes and Gout: The Singapore Chinese Health Study. Sci. Rep. 2016, 6, 25766. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Maiorino, M.I.; Bellastella, G.; Giugliano, D.; Esposito, K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine 2016, 56, 231–239. [Google Scholar] [CrossRef]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
| Author (Reference) | Study Design | Sample | Association/Mechanism |
|---|---|---|---|
| Liao et al. 2016 [23] | Paired case–control study; cellular model | A total of 47 colon and lung cancers and adjacent tissue; colon and lines—primary, late stages | A total of 5–8 variants of ALPK1 in colon or lung cancer tissues; ALPK1 upregulated cancer cell migration in the late stage |
| Chen et al. 2019 [28] | Paired case–control study; cellular model | A total of 39 oral cancers and adjacent tissue; oral (pre)cancer lines—dysplasia, primary, metastatic stage | ALPK1 was associated with cancer metastasis; TNF-α was decreased in metastatic cells with depleted ALPK1 |
| Rashid et al. 2019 [27] | Paired case–control study; | A total of 42–57 spiradenomas and spiradenocarcinoma and adjacent tissues | A missense mutation of ALPK1 was associated with these benign and malignant skin cancers; NF-κB activation |
| Zhang et al. 2022 [30] | Paired case–control study; cellular model Fusobacterium nucleatum | A total of 98 colorectal cancers and adjacent tissues; colorectal cancer cell lines and endothelial cells | F. nucleatum—induced ALPK1/NF-κB/ICAM1 axis regulating colorectal cancer metastasis; shorter survival |
| Li et al. 2017 [25] | Observational study | Acute lymphoblastic leukemia cases; 114 poor prognosis cases and 59 good prognosis cases | A 70-month follow-up; ALPK1 and a cluster gene acted as prognosis risk factors |
| Ji et al. 2019 [26] | Observational study | Acute lymphoblastic leukemia cases; 114 poor prognosis cases and 59 good prognosis cases | A 70-month follow-up; ALPK1 and a cluster gene acted as prognosis risk factors |
| Strietz et al. 2016 [24] | Cellular model; inhibitor effect | Metastatic adenocarcinoma of the breast; a tyrosine kinase inhibitor | Restricting the expression of ALPK1 reduced tumorigenicity; kinase inhibitor decreased cancer cell growth |
| Lee et al. 2021 [29] | Cellular model; mechanical method | Oral (pre)cancer lines—dysplasia, primary stage; human monocytes | ALPK1 expression increased from 26 to 80% in dysplastic oral cell nucleus and oral cancer cell nucleus; TNF-α and CCL2 expression reduced following ALPK1 knockdown |
| Author (Reference) | Study Design | Sample | Association/Mechanism |
|---|---|---|---|
| Wang et al. [9] | Case–control study; cellular model | A total of 23 gout cases and 39 controls for ALPK1 expression; human monocytes and kidney cells | ALPK1 overexpression in patients with gout; ALPK1 knockdown resulted in decreased IL-1β, TNF-α, and IL-8 mRNA expression |
| Ko et al. [31] | Population-based case–control study; bioinformatics | Gout cases and controls: 511 and 840 Taiwanese and 104 and 407 Han Chinese, respecitvely | ALPK1 variants were related to excess risk in patients with gout; signal peak of NF-κB at ALPK1 transcription initiation site |
| Lee et al. [34] | Case–control study; cellular model; proteomic | A total of 20 gout cases and 10 controls; human monocytes | Gout patients expressed higher levels of ALPK1, myosin IIA, and plasma TNF-α; ALPK1 phosphorylated myosin IIA and increased TNF-α secretion in MSU-induced monocytes |
| Natsuko et al. [35] | Cross-sectional and observational study | A total of 36 gout cases and 52 controls; monocytes and leukocytes; Mexican | ALPK1 expression in gout patients was correlated with serum uric acid, creatinine, C-reactive protein, and IL-1β |
| Yamada et al. [32] | Population-based cohort study | A total of 655 CKD cases and 1457 controls; Japanese | ALPK1 variants were associated with excess risk and with serum creatinine level in patients with CKD |
| Yamada et al. [33] | Population-based cohort study | A total of 797 diabetes cases and 5230 controls; Japanese | ALPK1 variants were associated with excess risk in patients with diabetes |
| Kuo et al. [36] | Animal/cellular models | Three groups of mice; wild type; STZ-treated wild type, STZ-treated hALPK1 transgenic mice | ALPK1 accelerated nephropathy in STZ-induced hyperglycemic mice; levels of IL-1β and TGF-β1 were increased in hALPK1 transgenic mice |
| Lee et al. [37] | Animal/cellular models | Added hALPK1 transgenic mice group; human kidney cell lines, human monocytes | NF-κB, chemokine CCL2 and CCL5 expression was increased in STZ-treated diabetic nephropathy hALPK1 mice; glucose elevated ALPK1 expression in cells |
| Author (Reference) | Study Design | Participants | Site of Cancer and Incidence Pooled Relative Risk (95% CI) |
|---|---|---|---|
| Wang et al. [46] | Prospective cohort study | Three studies involving 50,358 individuals for gout and cancer | All: 1.42 (1.09–1.84); urology: 1.72 (1.30–2.26); digestive system: 1.39 (1.23–1.56); lung: 1.29 (1.01–1.65) |
| Xie et al. [47] | Prospective cohort study | Six studies involving 226,083 individuals for gout and cancer | All: 1.19 (1.12–1.25); urinary system: 1.28 (1.11–1.48); digestive system: 1.15 (1.07–1.24); lung: 1.11 (1.01–1.21) |
| Wong et al. [48] | Population-based cohort study and randomized controlled trial | Six studies involving 32,057 individuals for chronic kidney disease and cancer | Urinary eGFR <45: 1.66 (1.02–2.70); dialysis: 2.34 (1.31–4.18) |
| Komaki et al. [49] | Retrospective cohort study | A total of 54 studies involving 1,208,767 individuals for chronic kidney disease and cancer | Colorectum without kidney transplantation: 1.18 (1.01–1.37); after kidney transplantation: 1.40 (1.15–1.71) |
| Tsilidis et al. [50] | Reanalysis of previous meta-analysis with random effect and 95% prediction intervals | A total of 27 meta-analyses involving > 1 million individuals for type 2 diabetes and cancer | Breast: 1.20 (1.12–1.28); colorectum: 1.27 (1.21–1.34); endometrium: 1.97 (1.71–2.27); intrahepatic cholangiocarcinoma: 1.97 (1.57–2.46) |
| Liang et al. [51] | Reanalysis of previous meta-analysis with bias analysis | 151 cohorts comprising 32 million people for type 2 diabetes and cancer | Very likely causal relationship: liver, pancreatic, endometrium (100%); gallbladder (86%); kidney, colon, colorectal system (>60%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, A.M.-S.; Tu, H.-P.; Ko, Y.-C. Systematic Review of the Role of Alpha-Protein Kinase 1 in Cancer and Cancer-Related Inflammatory Diseases. Cancers 2022, 14, 4390. https://doi.org/10.3390/cancers14184390
Ko AM-S, Tu H-P, Ko Y-C. Systematic Review of the Role of Alpha-Protein Kinase 1 in Cancer and Cancer-Related Inflammatory Diseases. Cancers. 2022; 14(18):4390. https://doi.org/10.3390/cancers14184390
Chicago/Turabian StyleKo, Albert Min-Shan, Hung-Pin Tu, and Ying-Chin Ko. 2022. "Systematic Review of the Role of Alpha-Protein Kinase 1 in Cancer and Cancer-Related Inflammatory Diseases" Cancers 14, no. 18: 4390. https://doi.org/10.3390/cancers14184390
APA StyleKo, A. M.-S., Tu, H.-P., & Ko, Y.-C. (2022). Systematic Review of the Role of Alpha-Protein Kinase 1 in Cancer and Cancer-Related Inflammatory Diseases. Cancers, 14(18), 4390. https://doi.org/10.3390/cancers14184390







