Oncologic Benefits of Neoadjuvant Treatment versus Upfront Surgery in Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Definition and Study Endpoint
2.4. Quality Assessment and Data Extraction
2.5. Data Synthesis and Statistical Analysis
3. Results
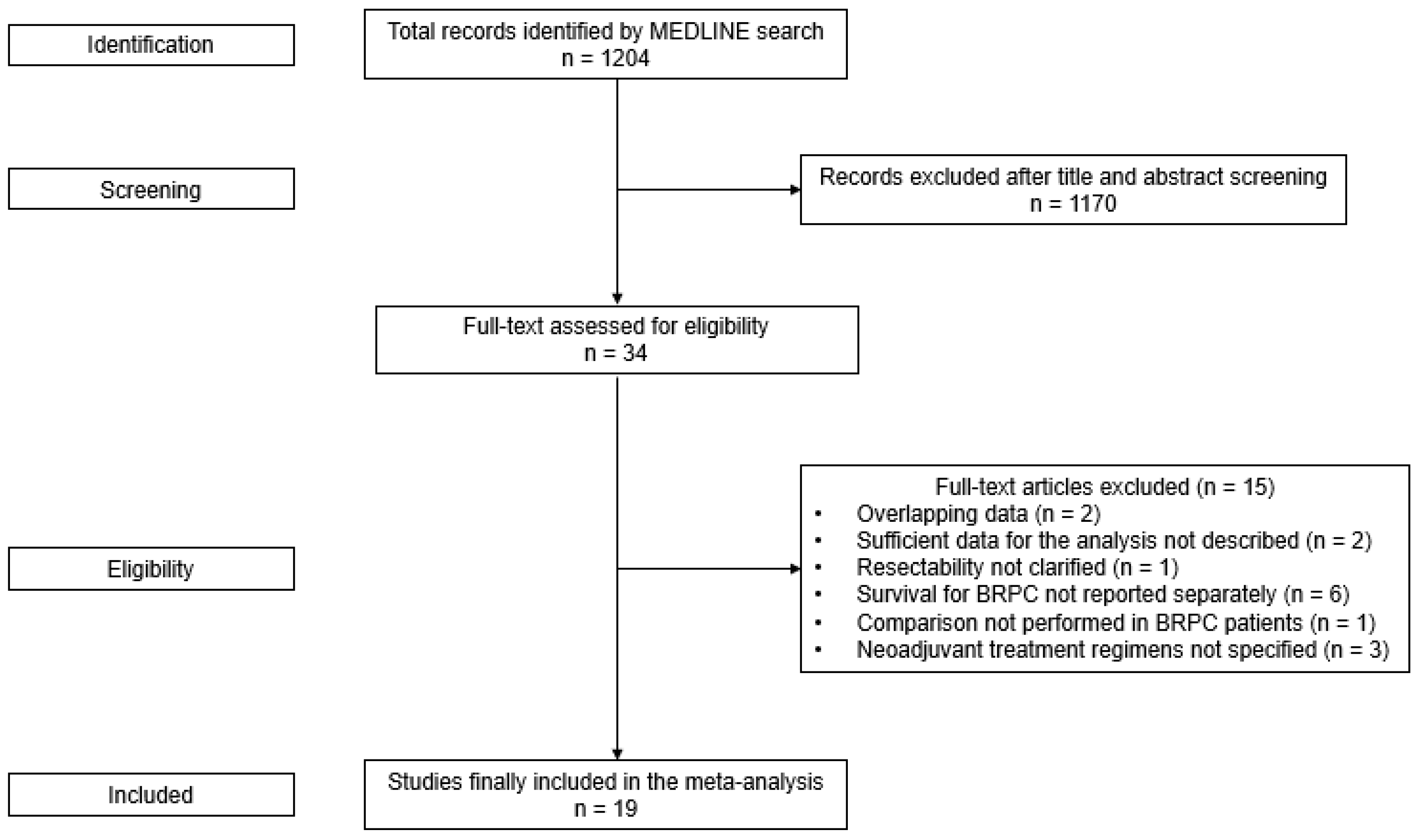
3.1. Study Selection
3.2. Study Characteristics
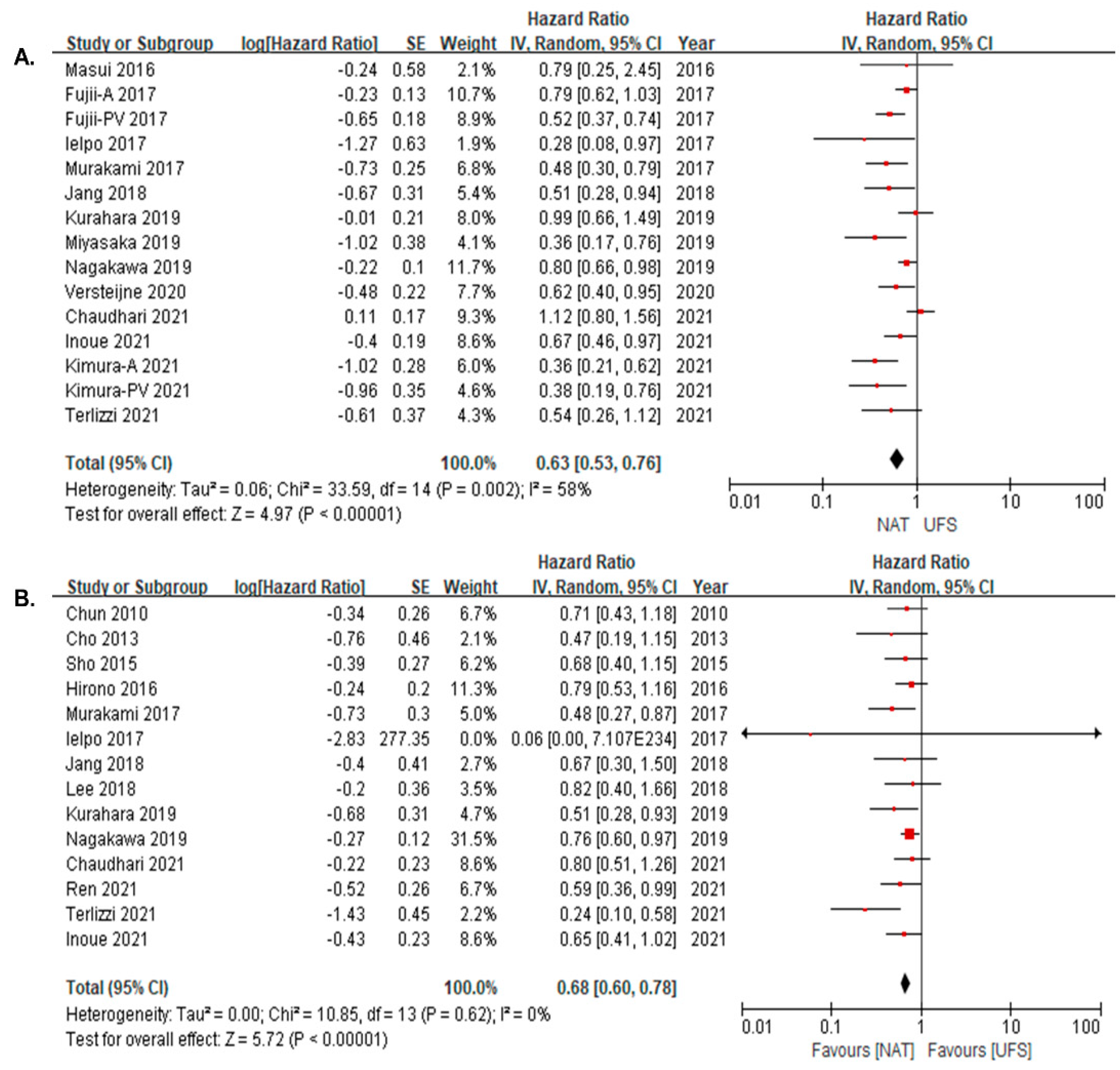
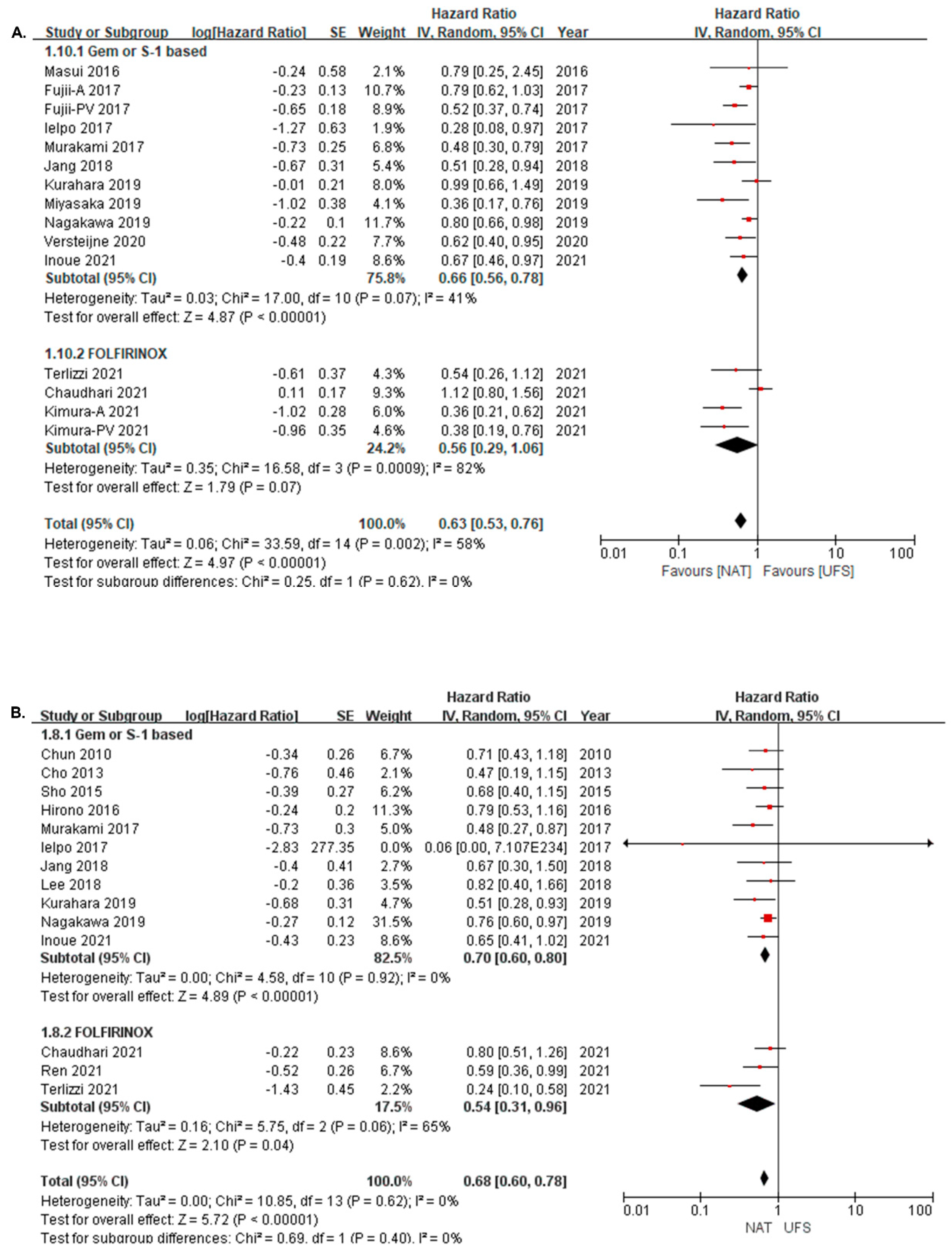
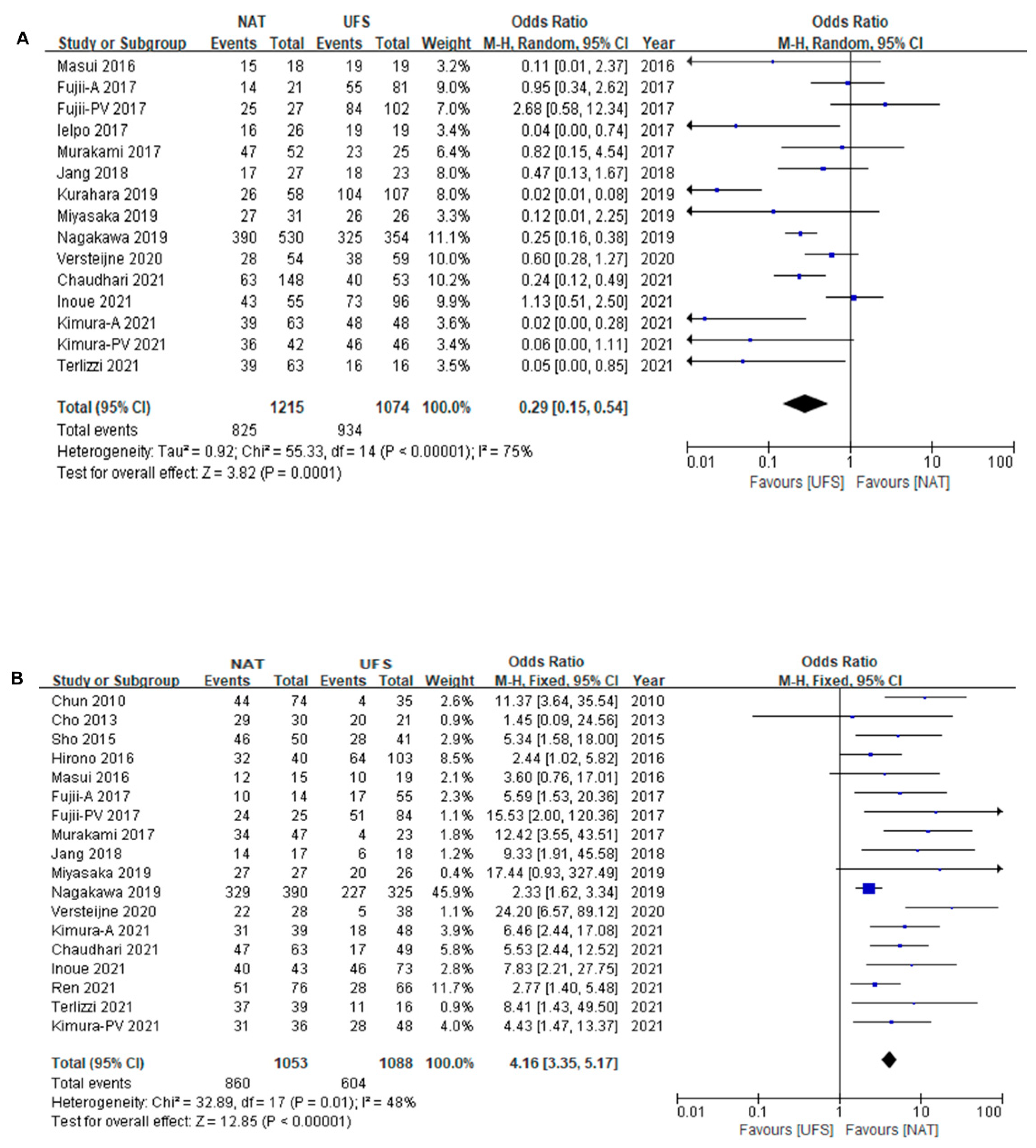
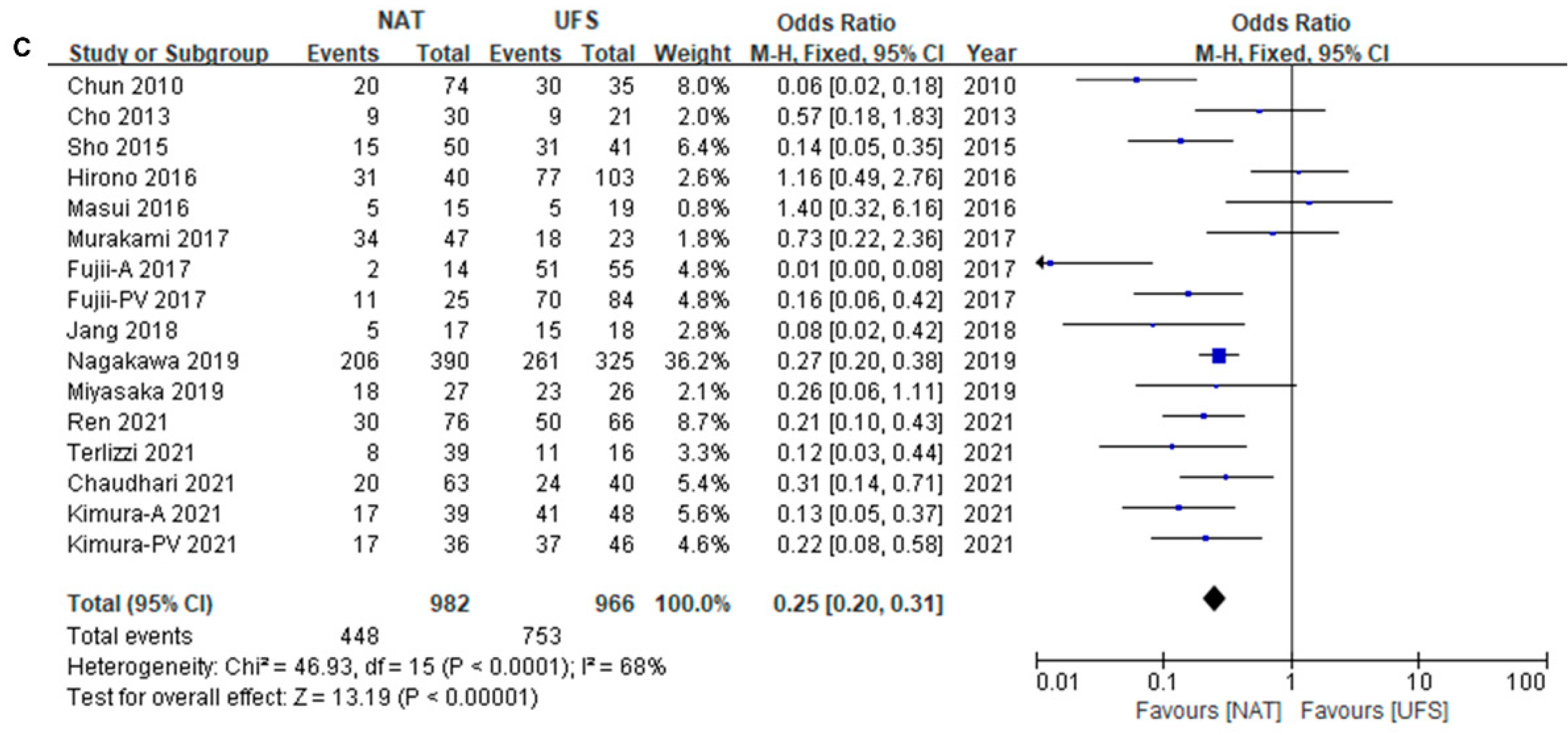
3.3. Survival Difference between NAT and UFS Groups
3.4. Survival Difference between NAT and UFS Groups
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Shaib, Y.; Davila, J.; Naumann, C.; El-Serag, H. The impact of curative intent surgery on the survival of pancreatic cancer patients: A U.S. Population-based study. Am. J. Gastroenterol. 2007, 102, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, S.V.; Barreto, S.G. Surgery for pancreatic carcinoma: State of the art. Indian J. Surg. 2012, 74, 79–86. [Google Scholar] [CrossRef][Green Version]
- Ielpo, B.; Caruso, R.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ferri, V.; Alvarez, R.; Cubillo, A.; Plaza, C.; et al. A comparative study of neoadjuvant treatment with gemcitabine plus nab-paclitaxel versus surgery first for pancreatic adenocarcinoma. Surg. Oncol. 2017, 26, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Sohal, D.P.; Walsh, R.M.; Ramanathan, R.K.; Khorana, A.A. Pancreatic adenocarcinoma: Treating a systemic disease with systemic therapy. J. Natl. Cancer Inst. 2014, 106, dju011. [Google Scholar] [CrossRef]
- Hirono, S.; Kawai, M.; Okada, K.I.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Ueno, M.; Yamaue, H. Treatment Strategy for Borderline Resectable Pancreatic Cancer With Radiographic Artery Involvement. Pancreas 2016, 45, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Doi, R.; Kawaguchi, Y.; Sato, A.; Nakano, K.; Ito, T.; Anazawa, T.; Takaori, K.; Uemoto, S. Concurrent gemcitabine+S-1 neoadjuvant chemotherapy contributes to the improved survival of patients with small borderline-resectable pancreatic cancer tumors. Surg. Today 2016, 46, 1282–1289. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, C.M.; Bang, S.M.; Choi, J.Y.; Seong, J.S.; Hwang, H.K.; Choi, S.H.; Lee, W.J. The Role of Neoadjuvant Chemoradiation Therapy in Patients With Borderline Resectable Pancreatic Cancer With Isolated Venous Vascular Involvement. Medicine 2015, 94, e1233. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G.; et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Pan, L.; Fang, J.; Tong, C.; Chen, M.; Zhang, B.; Juengpanich, S.; Wang, Y.; Cai, X. Survival benefits of neoadjuvant chemo(radio)therapy versus surgery first in patients with resectable or borderline resectable pancreatic cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Milestone, B.N.; Watson, J.C.; Cohen, S.J.; Burtness, B.; Engstrom, P.F.; Haluszka, O.; Tokar, J.L.; Hall, M.J.; Denlinger, C.S.; et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann. Surg. Oncol. 2010, 17, 2832–2838. [Google Scholar] [CrossRef]
- Cho, I.R.; Chung, M.J.; Bang, S.; Park, S.W.; Chung, J.B.; Song, S.Y.; Seong, J.; Hwang, H.K.; Kang, C.M.; Lee, W.J.; et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology 2013, 13, 539–543. [Google Scholar] [CrossRef]
- Sho, M.; Akahori, T.; Tanaka, T.; Kinoshita, S.; Nagai, M.; Nishiwada, S.; Tamamoto, T.; Nishiofuku, H.; Ohbayashi, C.; Hasegawa, M.; et al. Optimal indication of neoadjuvant chemoradiotherapy for pancreatic cancer. Langenbecks Arch. Surg. 2015, 400, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Satoi, S.; Yamada, S.; Murotani, K.; Yanagimoto, H.; Takami, H.; Yamamoto, T.; Kanda, M.; Yamaki, S.; Hirooka, S.; et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: An observational study using inverse probability of treatment weighting. J. Gastroenterol. 2017, 52, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Takahashi, S.; Sueda, T. Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother. Pharmacol. 2017, 79, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Jang, J.Y.; Kang, J.S.; Kim, J.R.; Han, Y.; Kim, E.; Kwon, W.; Kim, S.W. Recent treatment patterns and survival outcomes in pancreatic cancer according to clinical stage based on single-center large-cohort data. Ann. Hepatobiliary Pancreat Surg. 2018, 22, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, H.; Shinchi, H.; Ohtsuka, T.; Miyasaka, Y.; Matsunaga, T.; Noshiro, H.; Adachi, T.; Eguchi, S.; Imamura, N.; Nanashima, A.; et al. Significance of neoadjuvant therapy for borderline resectable pancreatic cancer: A multicenter retrospective study. Langenbecks Arch. Surg. 2019, 404, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Ohtsuka, T.; Kimura, R.; Matsuda, R.; Mori, Y.; Nakata, K.; Kakihara, D.; Fujimori, N.; Ohno, T.; Oda, Y.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann. Surg. Oncol. 2019, 26, 1528–1534. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Sahara, Y.; Hosokawa, Y.; Murakami, Y.; Yamaue, H.; Satoi, S.; Unno, M.; Isaji, S.; Endo, I.; Sho, M.; et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann. Surg. Oncol. 2019, 26, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Inoue, Y.; Saiura, A.; Oba, A.; Ono, Y.; Mise, Y.; Ito, H.; Sasaki, T.; Ozaka, M.; Sasahira, N.; Takahashi, Y. Neoadjuvant gemcitabine and nab-paclitaxel for borderline resectable pancreatic cancers: Intention-to-treat analysis compared with upfront surgery. J. Hepatobiliary Pancreat Sci. 2021, 28, 143–155. [Google Scholar] [CrossRef]
- Chaudhari, V.A.; Mitra, A.; Gupta, V.; Ostwal, V.; Ramaswamy, A.; Engineer, R.; Sirohi, B.; Shetty, N.; Bal, M.; DeSouza, A.; et al. Neoadjuvant therapy in borderline resectable pancreatic cancer: Outcomes in the era of changing practices and evolving evidence. Surgery 2021, 171, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Yamada, S.; Takami, H.; Murotani, K.; Yoshioka, I.; Shibuya, K.; Sonohara, F.; Hoshino, Y.; Hirano, K.; Watanabe, T.; et al. Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer. Cancers 2020, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xourafas, D.; Ashley, S.W.; Clancy, T.E. Prognostic Factors in Patients With Borderline Resectable Pancreatic Ductal Adenocarcinoma Undergoing Resection. Am. Surg. 2022, 88, 1172–1180. [Google Scholar] [CrossRef]
- Terlizzi, M.; Buscail, E.; Boussari, O.; Adgie, S.; Leduc, N.; Terrebonne, E.; Smith, D.; Blanc, J.F.; Lapuyade, B.; Laurent, C.; et al. Neoadjuvant treatment for borderline resectable pancreatic adenocarcinoma is associated with higher R0 rate compared to upfront surgery. Acta Oncol. 2021, 60, 1114–1121. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.-J.; Sheen, S.-S.; Hahn, S.; Jang, B.-H.; Son, H.-J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Coene, P.; van Eijck, C.H.J.; de Hingh, I.; Karsten, T.M.; van der Kolk, M.B.; et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): Study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021, 21, 300. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Shi, Q.; Meyers, J.P.; Herman, J.M.; Choung, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.d.W.; Schwartz, L.H.; Behr, S.; et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J. Clin. Oncol. 2021, 39, 377. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Rangelova, E.; Halimi, A.; Ateeb, Z.; Scandavini, C.; Valente, R.; Segersvärd, R.; Arnelo, U.; Verbeke, C.S. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB 2019, 21, 219–225. [Google Scholar] [CrossRef]
- Hank, T.; Sandini, M.; Ferrone, C.R.; Rodrigues, C.; Weniger, M.; Qadan, M.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-del Castillo, C. Association Between Pancreatic Fistula and Long-term Survival in the Era of Neoadjuvant Chemotherapy. JAMA Surg. 2019, 154, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Masui, T.; Nagai, K.; Anazawa, T.; Yoshimura, M.; Uza, N.; Takaori, K.; Mizowaki, T.; Uemoto, S. Postoperative pancreatic fistulas decrease the survival of pancreatic cancer patients treated with surgery after neoadjuvant chemoradiotherapy: A retrospective analysis. Surg. Oncol. 2020, 35, 527–532. [Google Scholar] [CrossRef]
| Author | Country | Year | Study Period | Study Design | Resectability | Definition | Analysis | NAT Regimen |
|---|---|---|---|---|---|---|---|---|
| Chun [17] | US | 2010 | 1990–2009 | Retrospective | BR | Ishikawa classification | Resected | Gemcitabine-based CRT 5-FU based CRT |
| Cho [18] | Korea | 2013 | 2002–2011 | Retrospective | BR | MDACC | Resected | Gemcitabine-based CCRT Capecitabine in some patients |
| Sho [19] | Japan | 2015 | 2006–2013 | Retrospective | R+BR | NCCN | Resected | Gemcitabine-based CCRT |
| Hirono [9] | Japan | 2016 | 2000–2013 | Retrospective | BR | NCCN | Resected | S-1-based CCRT S-1 + Gemcitabine |
| Masui [10] | Japan | 2016 | 2005–2010 | Prospective | BR | Modified criteria of the study group | ITT | Gemcitabine + S-1 |
| Fujii-Artery [20] | Japan | 2017 | 2001–2013 | Retrospective | R+BR | NCCN | ITT | S-1-based CCRT |
| Fujii-PV [20] | ITT | S-1-based CCRT | ||||||
| Ielpo [6] | Spain | 2017 | 2007–2016 | Retrospective + Prospective | R+BR | NCCN | ITT | Gemcitabine + Nab-paclitaxel IMRT since 2013 |
| Murakami [21] | Japan | 2017 | 2002–2015 | Retrospective | BR | NCCN | ITT | Gemcitabine + S-1 |
| Jang [12] | Korea | 2018 | 2012–2014 | RCT | BR | NCCN | ITT | Gemcitabine-based CCRT |
| Lee [22] | Korea | 2018 | 2007–2014 | Retrospective | R+BR+LA+M | NCCN | Resected | Gemcitabine- or 5-FU-based CCRT FOLFIRINOX |
| Kurahara [23] | Japan | 2019 | 2010–2014 | Retrospective | BR | NCCN | ITT | Gemcitabine- or S-1-based chemotherapy S-1-based CCRT |
| Miyasaka [24] | Japan | 2019 | 2010–2017 | Retrospective | BR | NCCN | ITT | Gemcitabine + Nab-paclitaxel |
| Nagakawa [25] | Japan | 2019 | 2011–2013 | Retrospective | BR | Japanese Classification of Pancreatic Carcinoma | ITT | Gemcitabine and/or S-1 RT (n = 319) |
| Versteijne [26] | Netherland | 2020 | 2013–2017 | RCT | R+BR | Dutch pancreatic cancer group criteria | ITT | Gemcitabine + Nab-paclitaxel |
| Inoue [27] | Japan | 2021 | 2008–2017 | Retrospective | BR | NCCN | ITT | Gemcitabine + Nab-paclitaxel |
| Chaudhari [28] | India | 2021 | 2007–2019 | Retrospective | BR | AHPBA/SSAO/SSO | ITT | FOLFIRINOX Gemcitabine-based RT (n = 89) |
| Kimura-A [29] | Japan | 2021 | 2002–2018 | Retrospective | BR | Japanese Classification of Pancreatic Carcinoma | ITT | GnP FOLFIRINOX Gemcitabine + S-1 and/or RT |
| Kimura-PV [29] | ITT | GnP FOLFIRINOX Gemcitabine + S-1 and/or RT | ||||||
| Ren [30] | US | 2021 | 2008–2018 | Retrospective | BR+LA | AHPBA/SSAO/SSO | Resected | FOLFIRINOX and/or RT |
| Terlizzi [31] | France | 2021 | 2010–2017 | Retrospective | BR | NCCN | ITT | FOLFIRINOX and/or RT |
| Author | No. of Patients | ITT OS (Month) | Resected OS (Month) | Resection Rate | R0 Rate | LN Metastasis |
|---|---|---|---|---|---|---|
| Chun [17] | NAT 74 UFS 35 | NA | 23.0 15.0 | 74/74 (100.0%) 35/35 (100.0%) | 44/74 (59.5%) 4/35 (11.4%) | 20/74 (27.0%) 30/35 (85.7%) |
| Cho [18] | NAT 30 UFS 21 | NA | 45.0 23.5 | 30/30 (100.0%) 21/21 (100.0%) | 29/30 (96.7%) 20/21 (95.2%) | 9/30 (30.0%) 9/21 (42.9%) |
| Sho [19] | NAT 50 UFS 41 | NA | 24.8 16.4 | 50/50 (100.0%) 41/41 (100.0%) | 46/50 (92.0%) 28/41 (68.3%) | 15/50 (30.0%) 31/41 (75.6%) |
| Hirono [9] | NAT 40 UFS 103 | NA | 19.3 13.7 | 40/40 (100.0%) 103/103 (100.0%) | 32/40 (80.0%) 64/103 (62.1%) | 31/40 (77.5%) 77/103 (74.8%) |
| Masui [10] | NAT 18 UFS 19 | 21.7 21.1 | NA | 15/18 (83.3%) 19/19 (100.0%) | 12/15 (80.0%) 10/19 (52.6%) | 5/15 (33.3%) 5/19 (26.3%) |
| Fujii-Artery [20] | NAT 21 UFS 81 | 18.1 10.0 | NA | 14/21 (66.7%) 55/81 (67.9%) | 10/14 (71.4%) 17/55 (30.9%) | 2/14 (14.3%) 51/55 (92.7%) |
| Fujii-PV [20] | NAT 27 UFS 102 | 28.4 20.1 | NA | 25/27 (92.6%) 84/102 (82.4%) | 24/25 (96.0%) 51/84 (60.7%) | 11/25 (44.0%) 70/84 (83.3%) |
| Ielpo [6] | NAT 26 UFS 19 | 18.9 13.5 | 43.6 13.5 | 16/26 (61.5%) 19/19 (100.0%) | NA | NA |
| Murakami [21] | NAT 52 UFS 25 | 27.1 11.6 | 27.2 11.6 | 47/52 (90.45) 23/25 (92.0%) | 34/47 (72.3%) 4/23 (17.4%) | 34/47 (72.3%) 18/23 (78.3%) |
| Jang [12] | NAT 30 UFS 28 | 21.0 12.0 | 22.0 19.5 | 17/27 (63.0%) 18/23 (78.3%) | 14/17 (82.4%) 6/18 (33.3%) | 5/17 (29.4%) 15/18 (83.3%) |
| Lee [22] | NAT 28 UFS 45 | NA | NA | 28/28 (100.0%) 45/45 (100.0%) | NA | NA |
| Kurahara [23] | NAT 58 UFS 107 | 22.0 16.7 | 53.7 17.8 | 26/58 (44.8%) 104/107 (97.2%) | NA | NA |
| Miyasaka [24] | NAT 31 UFS 26 | 27.9 12.4 | NA | 27/31 (87.1%) 26/26 (100.0%) | 27/27 (100.0%) 20/26 (76.9%) | 18/27 (66.7%) 23/26 (88.5%) |
| Nagakawa [25] | NAT 530 UFS 354 | 25.7 19.0 | 29.8 21.5 | 390/530 (73.6%) 325/354 (91.8%) | 329/390 (84.4%) 227/325 (69.8%) | 206/390 (52.8%) 261/325 (80.3%) |
| Versteijne [26] | NAT 54 UFS 59 | 17.6 13.2 | NA | 28/54 (51.9%) 38/59 (64.4%) | 22/28 (78.6%) 5/38 (13.2%) | NA |
| Inoue [27] | NAT 55 UFS 96 | 31.9 18.1 | 38.4 18.8 | 43/55 (78.2%) 73/96 (76.0%) | 40/43 (93.0%) 46/73 (63.0%) | 28/43 (65%) 57/73 (78%) |
| Chaudhari [28] | NAT 148 UFS 53 | 15 18 | 22 19 | 63/148 (42.6%) 40/53 (75.5%) | 47/63 (74.6%) 17/40 (42.5%) | 20/63 (31.7%) 24/40 (60%) |
| Kimura-A [29] | NAT 63 UFS 48 | 35.4 14.3 | NA | 39/63 (61.9%) 48/48 (100.0%) | 31/39 (79.5%) 18/48 (37.5%) | 17/39 (43.6%) 41/48 (85.4%) |
| Kimura-PV [29] | NAT 42 UFS 46 | 22.8 16.1 | NA | 36/42 (85.7%) 46/46 (100.0%) | 31/36 (86.1%) 28/46 (60.9%) | 17/36 (47.2%) 37/46 (80.4%) |
| Ren [30] | NAT 76 UFS 66 | NA | 35.8 27.8 | 76/76 (100.0%) 66/66 (100.0%) | 51/76 (67.1%) 28/66 (42.4%) | 30/76 (39.5%) 50/66 (75.8%) |
| Terlizzi [31] | NAT 63 UFS 16 | 29.0 27.2 | 63.1 27.2 | 39/63 (61.9%) 16/16 (100.0%) | 37/39 (94.9%) 11/16 (68.8%) | 8/39 (20.5%) 11/16 (68.8%) |
| Total | NAT 1516 UFS 1390 | - | - | NAT 67.9% UFS 81.4% | NAT 81.7% UFS 58.7% | NAT 46.4% UFS 78.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.-S.; Kim, H.S.; Kang, J.S.; Kang, Y.H.; Sohn, H.J.; Byun, Y.; Han, Y.; Yun, W.-G.; Cho, Y.J.; Lee, M.; et al. Oncologic Benefits of Neoadjuvant Treatment versus Upfront Surgery in Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4360. https://doi.org/10.3390/cancers14184360
Jung H-S, Kim HS, Kang JS, Kang YH, Sohn HJ, Byun Y, Han Y, Yun W-G, Cho YJ, Lee M, et al. Oncologic Benefits of Neoadjuvant Treatment versus Upfront Surgery in Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(18):4360. https://doi.org/10.3390/cancers14184360
Chicago/Turabian StyleJung, Hye-Sol, Hyeong Seok Kim, Jae Seung Kang, Yoon Hyung Kang, Hee Ju Sohn, Yoonhyeong Byun, Youngmin Han, Won-Gun Yun, Young Jae Cho, Mirang Lee, and et al. 2022. "Oncologic Benefits of Neoadjuvant Treatment versus Upfront Surgery in Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 18: 4360. https://doi.org/10.3390/cancers14184360
APA StyleJung, H.-S., Kim, H. S., Kang, J. S., Kang, Y. H., Sohn, H. J., Byun, Y., Han, Y., Yun, W.-G., Cho, Y. J., Lee, M., Kwon, W., & Jang, J.-Y. (2022). Oncologic Benefits of Neoadjuvant Treatment versus Upfront Surgery in Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(18), 4360. https://doi.org/10.3390/cancers14184360






