Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethical Statement
2.3. Collection of Blood Samples and Plasma Preparation
2.4. Thrombin Generation Potential and Plasma Hemostatic Biomarkers
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Hematological and Hemostatic Parameters
3.3. 6-Month DP and Association with Hematological and Hemostatic Parameters
3.4. One-Year OS and Association with Hematological and Hemostatic Parameters
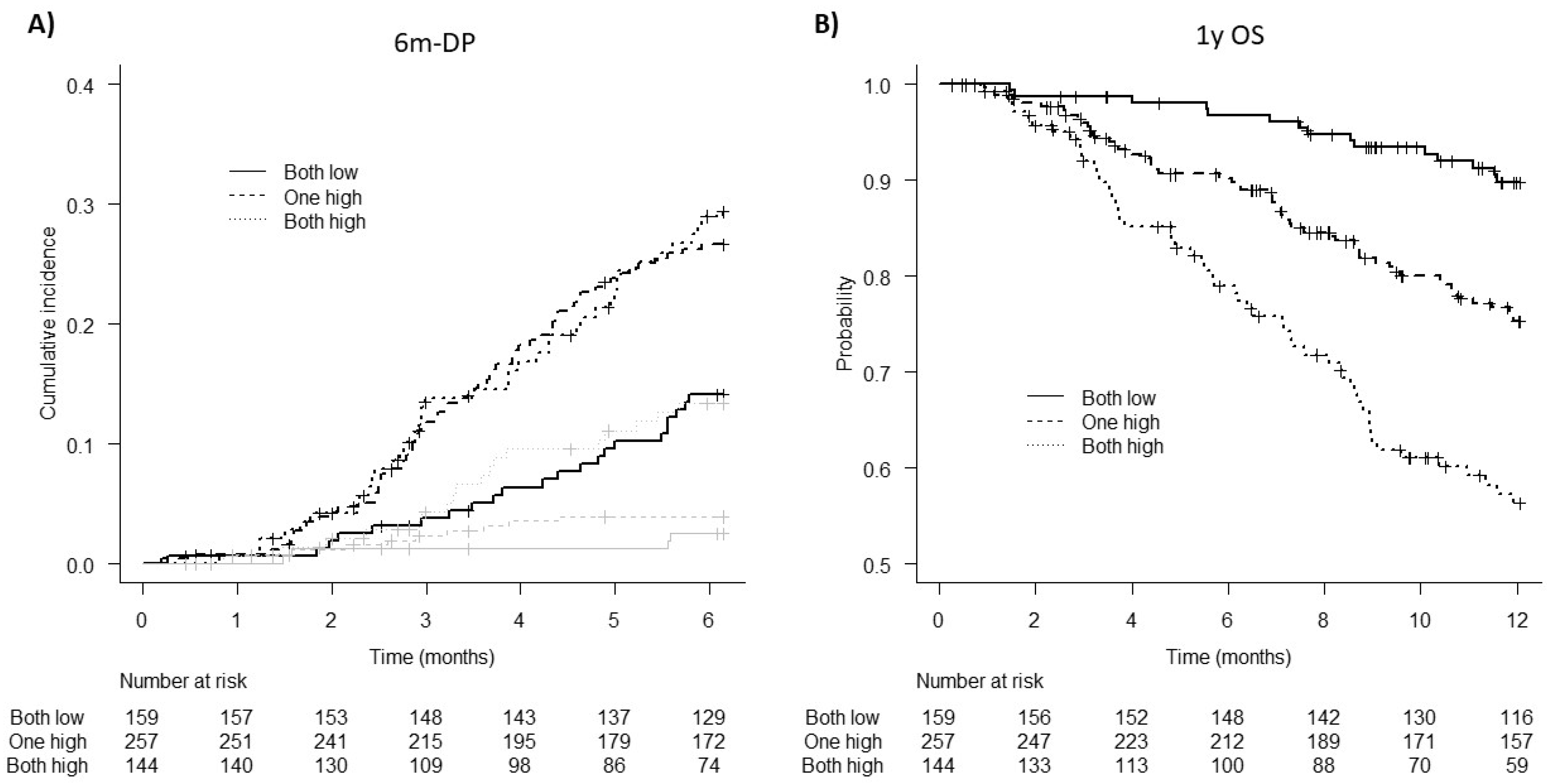
3.5. Patient Stratification for 6m-DP and 1y OS Risk According to D-Dimer and TG Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.M.; Himbert, C.; Holowatyj, A.N.; Hursting, S.D. Energy balance and gastrointestinal cancer: Risk, interventions, outcomes and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Schieppati, F.; Russo, D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin. Thromb. Hemost. 2015, 41, 756–764. [Google Scholar]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Russo, L.; Verzeroli, C. Mechanisms of thrombosis in cancer. Thromb. Res. 2013, 131 (Suppl. S1), S59–S62. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Russo, L. The mechanisms of cancer-associated thrombosis. Thromb. Res. 2015, 135 (Suppl. S1), S8–S11. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Hemostatic biomarkers in cancer progression. Thromb. Res. 2018, 164 (Suppl. S1), S54–S61. [Google Scholar] [CrossRef]
- Hong, T.; Shen, D.; Chen, X.; Wu, X.; Hua, D. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: A prospective cohort study. Cancer Biomark. 2017, 19, 103–111. [Google Scholar] [CrossRef]
- Tang, L.; Liu, K.; Wang, J.; Wang, C.; Zhao, P.; Liu, J. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J. Surg. Oncol. 2010, 102, 428–432. [Google Scholar] [CrossRef]
- Lee, S.; Huh, S.J.; Oh, S.Y.; Koh, M.S.; Kim, S.H.; Lee, J.H.; Han, J.Y.; Choi, H.J.; Kim, S.J.; Kim, H.J. Clinical significance of coagulation factors in operable colorectal cancer. Oncol. Lett. 2017, 13, 4669–4674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhao, S.; Jing, Z.; Li, J.; Shuanying, Y.; Zhang, N. Combination of D-dimer and carcinoembryonic antigen levels as a predictive and prognostic biomarker in advanced colorectal cancer patients. J. Cell. Biochem. 2018, 120, 8086–8092. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, K.W.; Kim, S.; Bae, J.M. Preoperative plasma fibrinogen levels in gastric cancer patients correlate with extent of tumor. Hepatogastroenterology 2004, 51, 1860–1863. [Google Scholar] [PubMed]
- Liu, L.; Zhang, X.; Yan, B.; Gu, Q.; Zhang, X.; Jiao, J.; Sun, D.; Wang, N.; Yue, X. Elevated plasma D-dimer levels correlate with long term survival of gastric cancer patients. PLoS ONE 2014, 9, e90547. [Google Scholar]
- Yu, X.; Hu, F.; Yao, Q.; Li, C.; Zhang, H.; Xue, Y. Serum fibrinogen levels are positively correlated with advanced tumor stage and poor survival in patients with gastric cancer undergoing gastrectomy: A large cohort retrospective study. BMC Cancer 2016, 16, 480. [Google Scholar] [CrossRef]
- Go, S.I.; Lee, M.J.; Lee, W.S.; Choi, H.J.; Lee, U.S.; Kim, R.B.; Kang, M.H.; Kim, H.G.; Lee, G.W.; Kang, J.H.; et al. D-Dimer Can Serve as a Prognostic and Predictive Biomarker for Metastatic Gastric Cancer Treated by Chemotherapy. Medicine 2015, 94, e951. [Google Scholar] [CrossRef]
- Marchetti, M.; Castoldi, E.; Spronk, H.M.; van Oerle, R.; Balducci, D.; Barbui, T.; Rosing, J.; Ten Cate, H.; Falanga, A. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood 2008, 112, 4061–4068. [Google Scholar] [CrossRef]
- Reitter, E.M.; Kaider, A.; Ay, C.A.; Quehenberger, P.; Marosi, C.; Zielinski, C.; Pabinger, I. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. J. Thromb. Haemost. 2016, 14, 294–305. [Google Scholar] [CrossRef]
- Ay, C.; Dunkler, D.; Simanek, R.; Thaler, J.; Koder, S.; Marosi, C.; Zielinski, C.; Pabinger, I. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2011, 29, 2099–2103. [Google Scholar] [CrossRef]
- Tinholt, M.; Viken, M.K.; Dahm, A.E.; Vollan, H.K.M.; Sahlberg, K.K.; Garred, Ø.; Børresen-Dale, A.L.; Jacobsen, A.F.; Kristensen, V.; Bukholm, I.; et al. Increased coagulation activity and genetic polymorphisms in the F5, F10 and EPCR genes are associated with breast cancer: A case-control study. BMC Cancer 2014, 14, 845. [Google Scholar] [CrossRef]
- Marchetti, M.; Giaccherini, C.; Masci, G.; Verzeroli, C.; Russo, L.; Celio, L.; Sarmiento, R.; Gamba, S.; Tartari, C.J.; Diani, E.; et al. Thrombin generation predicts early recurrence in breast cancer patients. J. Thromb. Haemost. 2020, 18, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Santoro, A.; Labianca, R.; De Braud, F.; Gasparini, G.; D’Alessio, A.; Barni, S.; Iacoviello, L.; Barcella, L.; Brevi, S.; et al. Hypercoagulation screening as an innovative tool for risk assessment, early diagnosis and prognosis in cancer: The HYPERCAN study. Thromb. Res. 2016, 140 (Suppl. S1), S55–S59. [Google Scholar] [CrossRef]
- D’Alessio, A.; Marchetti, M.; Tartari, C.J.; Russo, L.; Cecchini, S.; Lambregts, K.W.; di Mauro, D.; Falanga, A. Long Term Low Molecular Weight Heparin Anticoagulant Therapy Modulates Thrombin Generation and D-dimer in Patients with Cancer and Venous Thromboembolism. Cancer Investig. 2017, 35, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Tartari, C.J.; Russo, L.; Panova-Noeva, M.; Leuzzi, A.; Rambaldi, A.; Finazzi, G.; Woodhams, B.; Falanga, A. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am. J. Hematol. 2014, 89, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, R.; Kleinegris, M.C.; Loubele, S.T.B.G.; Pluijmen, P.H.M.; Fens, D.; Van Oerle, R.; Ten Cate, H.; Spronk, H.M.H. Preanalytic variables of thrombin generation: Towards a standard procedure and validation of the method. J. Thromb. Haemost. 2012, 10, 2544–2554. [Google Scholar] [CrossRef]
- Dargaud, Y.; Wolberg, A.S.; Luddington, R.; Regnault, V.; Spronk, H.; Baglin, T.; Lecompte, T.; Ten Cate, H.; Negrier, C. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: An international multicentre study. Thromb. Res. 2012, 130, 929–934. [Google Scholar] [CrossRef]
- Hemker, H.C. Calibrated automated thrombinography (CAT). Thromb. Res. 2005, 115, 255. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.; Falanga, A.; Khorana, A.A. A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality. Oncologist 2016, 21, 861–867. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, L.Q.; Wang, F.W.; Li, C.P.; Tian, X.P.; Huang, X.X.; Mai, S.J.; Liao, Y.J.; Deng, H.X.; Chen, Q.Y.; et al. Elevated levels of plasma D-dimer predict a worse outcome in patients with nasopharyngeal carcinoma. BMC Cancer 2014, 14, 583. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, C.; Li, J.; Jiao, S.; Hu, Y.; Tao, H.; Wu, B.; Li, A. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed. Pharmacother. 2016, 81, 210–217. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, K.; Ishikawa, M.; Katagiri, H.; Minamoto, T.; Ishibashi, T.; Ishikawa, N.; Sato, E.; Sanuki, K.; Yamashita, H.; et al. High Pre-Treatment Plasma D-Dimer Level as a Potential Prognostic Biomarker for Cervical Carcinoma. Anticancer Res. 2016, 36, 2933–2938. [Google Scholar] [PubMed]
- Stender, M.T.; Larsen, A.C.; Sall, M.; Thorlacius-Ussing, O. D-Dimer predicts prognosis and non-resectability in patients with pancreatic cancer: A prospective cohort study. Blood Coagul. Fibrinolysis 2016, 27, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, Z.; Fang, M. Impact of pretreatment plasma D-dimer levels and its perioperative change on prognosis in operable esophageal squamous cell carcinoma. Oncotarget 2017, 8, 79537–79545. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.; Yoldas, O.; Keskek, M.; Ertan, T.; Tez, M.; Gocmen, E.; Koc, M. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis. 2008, 10, 238–241. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, B.; Zhao, Y.; Liu, L.; Yang, C.; Yang, Y.; Zhong, H. High levels of D-dimer correlated with disease status and poor prognosis of inoperable metastatic colorectal cancer patients treated with bevacizumab. J. Cancer Res. Ther. 2014, 10, 246–251. [Google Scholar] [CrossRef]
- Rong, G.; Fan, W.; Shen, J. High pretreatment plasma D-dimer levels predict poor prognosis in gastrointestinal cancers: A meta-analysis. Medicine 2019, 98, e16520. [Google Scholar] [CrossRef]
- Gomez-Rosas, P.; Pesenti, M.; Verzeroli, C.; Giaccherini, C.; Russo, L.; Sarmiento, R.; Masci, G.; Celio, L.; Minelli, M.; Gamba, S.; et al. Validation of the Role of Thrombin Generation Potential by a Fully Automated System in the Identification of Breast Cancer Patients at High Risk of Disease Recurrence. TH Open 2021, 5, e56–e65. [Google Scholar]
- Pedrazzani, C.; Mantovani, G.; Salvagno, G.L.; Baldiotti, E.; Ruzzenente, A.; Iacono, C.; Lippi, G.; Guglielmi, A. Elevated fibrinogen plasma level is not an independent predictor of poor prognosis in a large cohort of Western patients undergoing surgery for colorectal cancer. World J. Gastroenterol. 2016, 22, 9994–10001. [Google Scholar] [CrossRef]
- Stender, M.T.; Larsen, T.B.; Sørensen, H.T.; Thorlacius-Ussing, O. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): A prospective clinical cohort study. J. Thromb. Haemost. 2012, 10, 2027–2031. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshinaga, K.; Matsuyama, A.; Iwasa, T.; Osoegawa, A.; Tsujita, E.; Yamashita, Y.; Tsutsui, S.; Ishida, T. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology 2012, 83, 10–15. [Google Scholar] [CrossRef]
- Cheng, F.; Zeng, C.; Zeng, L.; Chen, Y. Clinicopathological and prognostic value of preoperative plasma fibrinogen in gastric cancer patients: A meta-analysis. Medicine 2019, 98, e17310. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 626) | GC (n = 164) | CRC (n = 462) | p Value | |
|---|---|---|---|---|
| Gender (n) | 383 M/243 F | 105 M/59 F | 278 M/184 F | 0.385 |
| Age (years, median, min–max) | 66 (26–87) | 65 (26–84) | 67 (29–87) | 0.248 |
| ECOG-PS (n, %) | ||||
| 0 | 338 (54) | 77 (47) | 261 (56.5) | 0.015 |
| 1 | 191 (30.5) | 61 (37.2) | 130 (28.1) | |
| 2 | 35 (5.6) | 14 (8.5) | 21 (4.5) | |
| NA | 62 (9.9) | 12 (7.3) | 50 (10.8) | |
| Comorbidities (n, %) | ||||
| Yes | 429 (68.5) | 115 (70.1) | 314 (68.0) | 0.737 |
| No | 192 (30.7) | 49 (29.9) | 143 (31.0) | |
| NA | 5 (0.8) | - | 5 (1.1) | |
| Cardiovascular risk * (n, %) | ||||
| Yes | 299 (69.7) | 73 (63.5) | 226 (72.0) | 0.073 |
| No | 128 (29.8) | 42 (36.5) | 86 (27.4) | |
| NA | 2 (0.5) | - | 2 (0.6) | |
| Antiplatelet therapy (n, %) | ||||
| Yes | 77 (12.3) | 19 (11.6) | 58 (12.6) | 0.706 |
| No | 543 (86.7) | 145 (88.4) | 398 (86.1) | |
| NA | 6 (1.0) | - | 6 (1.3) | |
| Anticoagulant therapy (n, %) | ||||
| Yes | 32 (5.1) | 9 (5.5) | 23 (5.0) | 0.826 |
| No | 588 (93.9) | 155 (94.5) | 433 (93.7) | |
| NA | 6 (1.0) | - | 6 (1.3) | |
| History of venous thrombosis (n, %) | ||||
| Yes | 8 (1.3) | 4 (2.4) | 4 (0.9) | 0.122 |
| No | 520 (83.1) | 134 (81.7) | 386 (83.5) | |
| NA | 98 (15.7) | 26 (15.9) | 72 (15.6) | |
| History of arterial thrombosis (n, %) | ||||
| Yes | 9 (1.4) | 4 (2.4) | 5 (1.1) | 0.207 |
| No | 519 (82.9) | 134 (81.7) | 385 (83.3) | |
| NA | 98 (15.7) | 26 (15.9) | 72 (15.6) | |
| Number of metastatic sites (n, %) | ||||
| 1 | 305 (48.7) | 57 (34.8) | 248 (53.7) | <0.001 |
| >1 | 303 (48.4) | 103 (62.8) | 200 (43.3) | |
| NA | 18 (2.9) | 4 (2.4) | 14 (3.0) | |
| 6m-DP (n, %) | 148 (23.6) | 67 (40.9) | 81 (17.5) | <0.001 |
| Time to 6m-DP (days, median, 5th–95th) | 104 (40–175) | 90 (36–178) | 112 (46–171) | 0.341 |
| 1y-death (n, %) | 139 (22.2) | 67 (40.9) | 72 (15.6) | <0.001 |
| Time to 1y-death (days, median, 5th–95th) | 191 (45–351) | 195 (47–321) | 188 (33–355) | 0.958 |
| Total (n = 626) | GC (n= 164) | CRC (n = 462) | p Value | Ref | |
|---|---|---|---|---|---|
| WBC (109/L) | 7.1 (4.2–13.0) | 6.9 (3.9–12.6) | 7.2 (4.3–13.1) | 0.387 | 4.5–11 |
| RBC (1012/L) | 4.48 (3.54–5.35) | 4.33 (3.24–5.08) | 4.52 (3.61–5.40) | 0.010 | 3.9–5.0 |
| Hemoglobin (g/dl) | 12.3 (9.4–15.3) | 12.0 (9.1–15.0) | 12.3 (9.4–15.4) | 0.007 | 11.5–14.4 |
| Platelets (109/L) | 263 (134–522) | 252 (115–507) | 265 (140–523) | 0.149 | 150–450 |
| F 1 + 2 (pmol/L) | 343 (161–862) | 361 (151–1337) | 337 (163–745) | 0.181 | 215 (126–478) |
| D-dimer (ng/mL) | 375 (104–2108) | 462 (121–3114) | 350 (99–1872) | 0.003 | 108 (44–283) |
| Fibrinogen (mg/dl) | 410 (235–725) | 400 (207–726) | 410 (247–704) | 0.426 | 150–400 |
| TG lag time (min) | 3.1 (2.0–5.5) | 3.2 (2.0–5.8) | 3.1 (2.0–5.5) | 0.543 | 3.1 (2.2–4.45) |
| TG ETP (nM∙min ) | 1702 (962–2601) | 1689 (951–2602) | 1706 (963–2600) | 0.413 | 1516 (970–2168) |
| TG peak (nM) | 344 (117–524) | 360 (122–554) | 341 (115–521) | 0.253 | 237 (128–404) |
| TG ttPeak (min) | 5.7 (4.0–9.8)) | 5.7 (3.8–10.1) | 5.7 (4.0–9.8) | 0.498 | 6.7 (4.7–8.8) |
| 6m-DP | No 6m-DP | 1y Death | 1y OS | |
|---|---|---|---|---|
| WBC (109/L) | 7.6 | 7.0 * | 8.5 | 6.8 *** |
| (4.3–12.6) | (4.2–13.1) | (4.2–15.6) | (4.2–12.5) | |
| RBC (1012/L) | 4.41 | 4.49 | 4.30 | 4.53 ** |
| (3.47–5.39) | (3.57–5.34) | (3.20–5.27) | (3.60–5.42) | |
| Hemoglobin (g/dL) | 12.2 | 12.3 | 11.6 | 12.4 ** |
| (9.4–15.7) | (9.2–15.1) | (9.4–15.6) | (9.4–15.2) | |
| Platelets (109/L) | 270 | 262 | 294 | 259 |
| (148–597) | (133–508) | (133–507) | (138–526) | |
| F1 + 2 (pmol/L) | 358 | 337 | 382 | 337 ** |
| (166–1164) | (160–806) | (172–1298) | (160–813) | |
| D-dimer (ng/mL) | 460 | 360 ** | 584 | 342 *** |
| (141–2686) | (94–1868) | (154–3694) | (95–1578) | |
| Fibrinogen (mg/dL) | 418 | 408 | 443 | 402 * |
| (241–726) | (234–704) | (225–742) | (239–704) | |
| TG lag time (min) | 3.2 | 3.1 | 3.4 | 3.1 *** |
| (2.1–5.7) | (2.0–5.4) | (2.1–6.1) | (2.0–5.3) | |
| TG ETP (nM∙min) | 1783 | 1687 | 1885 | 1673 ** |
| (843–2595) | (962–2605) | (1052–3122) | (955–2535) | |
| TG peak (nM) | 362 | 339 | 378 | 337 ** |
| (104–527) | (115–525) | (127–590) | (112–502) | |
| TG ttPeak (min) | 5.9 | 5.7 | 5.9 | 5.7 |
| (4.2–10.2) | (4.0–9.7) | (4.3–9.8) | (4.0–9.8) |
| 6m-DP | 1y OS | |||||
|---|---|---|---|---|---|---|
| SHR | CI | p Value | HR | CI | p Value | |
| D-dimer > 420 ng/mL | 1.44 | 1.01–2.06 | 0.047 | 2.41 | 1.53–3.80 | <0.001 |
| TG ETP > 1700 nM∙min | 1.57 | 1.09–2.25 | 0.014 | 2.02 | 1.30–3.14 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaccherini, C.; Verzeroli, C.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Schieppati, F.; Ticozzi, C.; Sarmiento, R.; Celio, L.; et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers 2022, 14, 4347. https://doi.org/10.3390/cancers14184347
Giaccherini C, Verzeroli C, Russo L, Gamba S, Tartari CJ, Bolognini S, Schieppati F, Ticozzi C, Sarmiento R, Celio L, et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers. 2022; 14(18):4347. https://doi.org/10.3390/cancers14184347
Chicago/Turabian StyleGiaccherini, Cinzia, Cristina Verzeroli, Laura Russo, Sara Gamba, Carmen Julia Tartari, Silvia Bolognini, Francesca Schieppati, Chiara Ticozzi, Roberta Sarmiento, Luigi Celio, and et al. 2022. "Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer" Cancers 14, no. 18: 4347. https://doi.org/10.3390/cancers14184347
APA StyleGiaccherini, C., Verzeroli, C., Russo, L., Gamba, S., Tartari, C. J., Bolognini, S., Schieppati, F., Ticozzi, C., Sarmiento, R., Celio, L., Masci, G., Tondini, C., Petrelli, F., Giuliani, F., D’Alessio, A., De Braud, F., Santoro, A., Labianca, R., Gasparini, G., ... Falanga, A., on behalf of the HYPERCAN Investigators. (2022). Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers, 14(18), 4347. https://doi.org/10.3390/cancers14184347









