Beyond N Staging in Breast Cancer: Importance of MRI and Ultrasound-based Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Anatomy of Axilla and Regional Lymph Nodes: The N-Stage
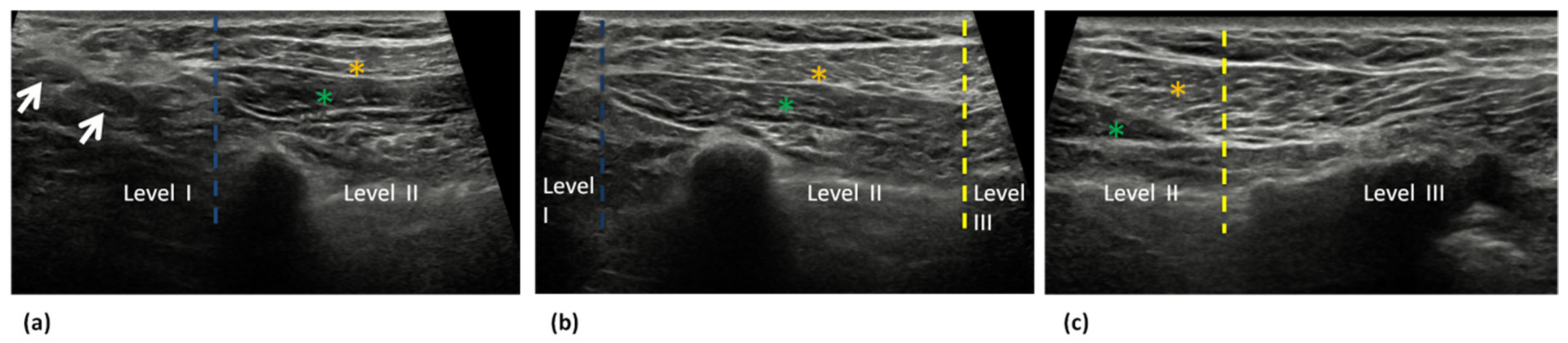
3. Ultrasound
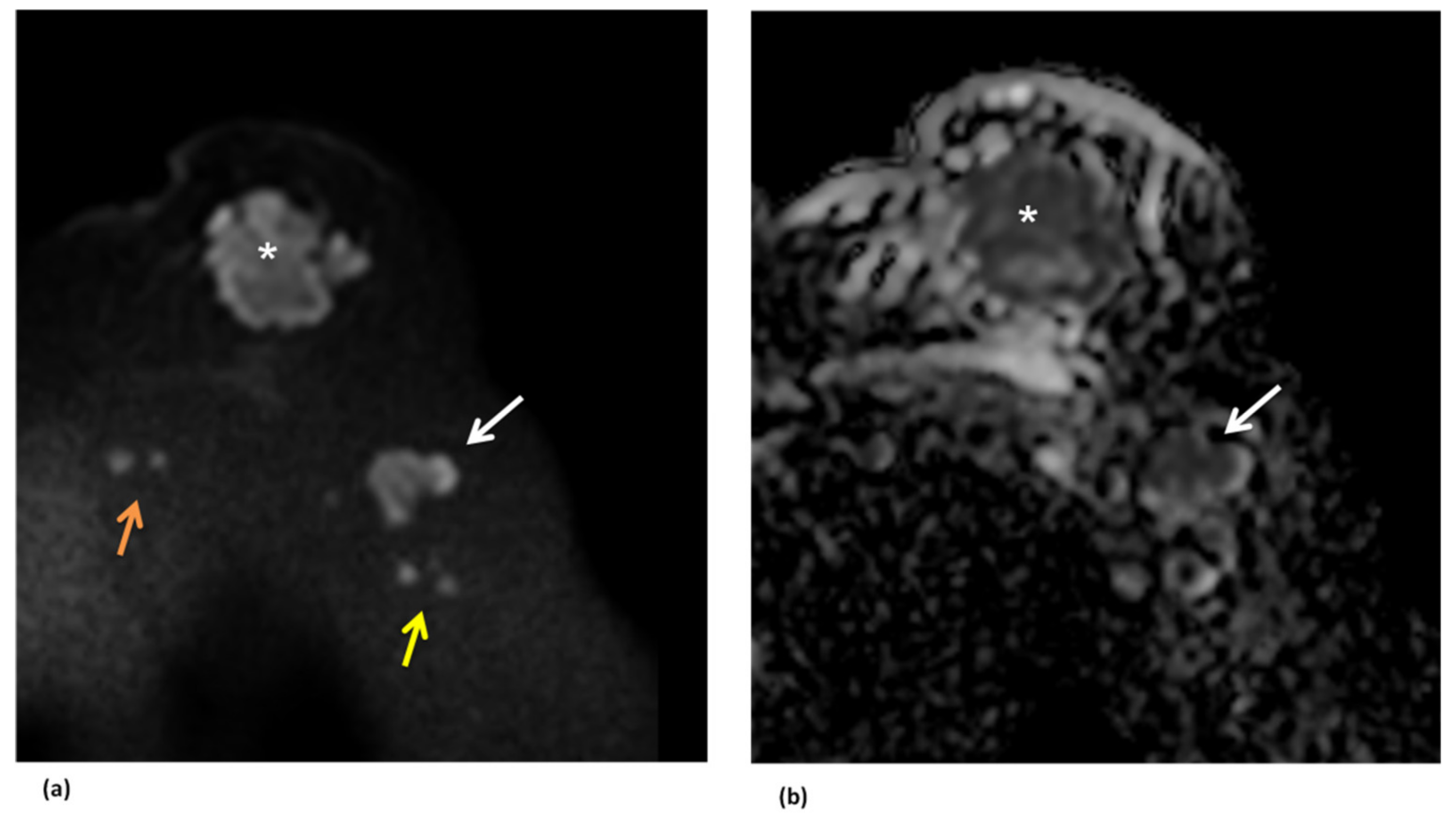
4. Magnetic Resonance Imaging (MRI)
5. US versus MRI
6. Future Perspectives
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Sivridis, E.; Giatromanolaki, A.; Galazios, G.; Koukourakis, M.I. Node-Related Factors and Survival in Node-Positive Breast Carcinomas. Breast Edinb. Scotl. 2006, 15, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Cupp, J.A.; Kuerer, H.M. Management of Axillary Disease. Surg. Oncol. Clin. N. Am. 2014, 23, 473–486. [Google Scholar] [CrossRef]
- Soares, E.W.S.; Nagai, H.M.; Bredt, L.C.; da Cunha, A.D.; Andrade, R.J.; Soares, G.V.S. Morbidity after Conventional Dissection of Axillary Lymph Nodes in Breast Cancer Patients. World J. Surg. Oncol. 2014, 12, 67. [Google Scholar] [CrossRef]
- Belmonte, R.; Garin, O.; Segura, M.; Pont, A.; Escalada, F.; Ferrer, M. Quality-of-Life Impact of Sentinel Lymph Node Biopsy versus Axillary Lymph Node Dissection in Breast Cancer Patients. Value Health 2012, 15, 907–915. [Google Scholar] [CrossRef]
- Sacre, R.A. Clinical Evaluation of Axillar Lymph Nodes Compared to Surgical and Pathological Findings. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 1986, 12, 169–173. [Google Scholar]
- National Comprehansive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 12 April 2021).
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs. No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary Dissection vs. No Axillary Dissection in Women with Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef]
- Brackstone, M.; Baldassarre, F.G.; Perera, F.E.; Cil, T.; Chavez Mac Gregor, M.; Dayes, I.S.; Engel, J.; Horton, J.K.; King, T.A.; Kornecki, A.; et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J. Clin. Oncol. 2021, 39, 3056–3082. [Google Scholar] [CrossRef]
- Chung, H.L.; Le-Petross, H.T.; Leung, J.W.T. Imaging Updates to Breast Cancer Lymph Node Management. RadioGraphics 2021, 41, 1283–1299. [Google Scholar] [CrossRef]
- Pilewskie, M.; Jochelson, M.; Gooch, J.C.; Patil, S.; Stempel, M.; Morrow, M. Is Preoperative Axillary Imaging Beneficial in Identifying Clinically Node-Negative Patients Requiring Axillary Lymph Node Dissection? J. Am. Coll. Surg. 2016, 222, 138–145. [Google Scholar] [CrossRef]
- Ecanow, J.S.; Abe, H.; Newstead, G.M.; Ecanow, D.B.; Jeske, J.M. Axillary Staging of Breast Cancer: What the Radiologist Should Know. RadioGraphics 2013, 33, 1589–1612. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Hortobagyi, G.N.; Rouzier, R.; Kuerer, H.; Sneige, N.; Buzdar, A.U.; Kau, S.W.; Fornage, B.; Sahin, A.; Broglio, K.; et al. Outcome after Pathologic Complete Eradication of Cytologically Proven Breast Cancer Axillary Node Metastases Following Primary Chemotherapy. J. Clin. Oncol. 2005, 23, 9304–9311. [Google Scholar] [CrossRef]
- Reig, B.; Heacock, L.; Lewin, A.; Cho, N.; Moy, L. Role of MRI to Assess Response to Neoadjuvant Therapy for Breast Cancer. J. Magn. Reson. Imaging 2020, 52. [Google Scholar] [CrossRef]
- Lanetz, P.J.; Moy, L.; Baron, P.; Diflorio, R.M.; Green, E.D.; Heller, S.; Holbrook, A.I.; Lee, S.-J.; Lewin, A.; Lourenco, A.P.; et al. ACR Appropriateness Criteria ® Monitoring Response to Neoadjuvant Systemic Therapy for Breast Cancer. J. Am. Coll. Radiol. 2017, 14, S462–S475. [Google Scholar] [CrossRef]
- Dixon, J.M.; Dobie, V.; Chetty, U. The Importance of Interpectoral Nodes in Breast Cancer. Eur. J. Cancer 1993, 29, 334–336. [Google Scholar] [CrossRef]
- Banjar, F.K.; Wilson, A.M. Anatomy, Head and Neck, Supraclavicular Lymph Node. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Manca, G.; Volterrani, D.; Mazzarri, S.; Duce, V.; Svirydenka, A.; Giuliano, A.; Mariani, G. Sentinel Lymph Node Mapping in Breast Cancer: A Critical Reappraisal of the Internal Mammary Chain Issue. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 114–126. [Google Scholar]
- Willard-Mack, C.L. Normal Structure, Function, and Histology of Lymph Nodes. Toxicol. Pathol. 2006, 34, 409–424. [Google Scholar] [CrossRef]
- Luciani, A.; Itti, E.; Rahmouni, A.; Meignan, M.; Clement, O. Lymph Node Imaging: Basic Principles. Eur. J. Radiol. 2006, 58, 338–344. [Google Scholar] [CrossRef]
- Dialani, V.; James, D.F.; Slanetz, P.J. A Practical Approach to Imaging the Axilla. Insights Imaging 2015, 6, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, F.; de Margerie Mellon, C.; Bricout, M.; Cauderlier, E.; Chapelier, M.; Albiter, M.; Bourrier, P.; Espié, M.; de Kerviler, E.; de Bazelaire, C. Diagnostic Strategy for the Assessment of Axillary Lymph Node Status in Breast Cancer. Diagn. Interv. Imaging 2015, 96, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Leung, J.W.T.; Moy, L.; Ha, S.M.; Moon, W.K. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020, 295, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Altintoprak, K.M.; Yirgin, I.K.; Atasoy, M.M.; Celik, L. Diagnostic Accuracy of Metastatic Axillary Lymph Nodes in Breast MRI. SpringerPlus 2016, 5, 735. [Google Scholar] [CrossRef]
- Baltzer, P.A.T.; Dietzel, M.; Burmeister, H.P.; Zoubi, R.; Gajda, M.; Camara, O.; Kaiser, W.A. Application of MR Mammography beyond Local Staging: Is There a Potential to Accurately Assess Axillary Lymph Nodes? Evaluation of an Extended Protocol in an Initial Prospective Study. Am. J. Roentgenol. 2011, 196, W641–W647. [Google Scholar] [CrossRef]
- De Cataldo, C.; Bruno, F.; Palumbo, P.; Di Sibio, A.; Arrigoni, F.; Clemente, A.; Bafile, A.; Gravina, G.L.; Cap-pabianca, S.; Barile, A.; et al. Apparent Diffusion Coefficient Magnetic Resonance Imaging (ADC-MRI) in the Axillary Breast Cancer Lymph Node Metastasis Detection: A Narrative Review. Gland Surg. 2020, 9, 2225–2234. [Google Scholar] [CrossRef]
- Fardanesh, R.; Thakur, S.B.; Sevilimedu, V.; Horvat, J.V.; Gullo, R.L.; Reiner, J.S.; Eskreis-Winkler, S.; Thakur, N.; Pinker, K. Differentiation Between Benign and Metastatic Breast Lymph Nodes Using Apparent Diffusion Coefficients. Front. Oncol. 2022, 12, 795265. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, H.; Wang, C.; Chen, X.; Wang, M.; Zhou, P.; Ren, J. Diagnostic Performance of T2-Weighted Imaging and Intravoxel Incoherent Motion Diffusion-Weighted MRI for Predicting Metastatic Axillary Lymph Nodes in T1 and T2 Stage Breast Cancer. Acta Radiol. 2021, 63, 447–457. [Google Scholar] [CrossRef]
- Ahn, H.S.; Jang, M.; Kim, S.M.; La Yun, B.; Lee, S.H. Usefulness of Preoperative Breast Magnetic Resonance Imaging with a Dedicated Axillary Sequence for the Detection of Axillary Lymph Node Metastasis in Patients with Early Ductal Breast Cancer. Radiol. Med. 2019, 124, 1220–1228. [Google Scholar] [CrossRef]
- Samiei, S.; Smidt, M.L.; Vanwetswinkel, S.; Engelen, S.M.E.; Schipper, R.-J.; Lobbes, M.B.I.; van Nijnatten, T.J.A. Diagnostic Performance of Standard Breast MRI Compared to Dedicated Axillary MRI for Assessment of Node-Negative and Node-Positive Breast Cancer. Eur. Radiol. 2020, 30, 4212–4222. [Google Scholar] [CrossRef]
- Ha, S.M.; Chae, E.Y.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Kim, H.H. Diagnostic Performance of Standard Breast MR Imaging Compared to Dedicated Axillary MR Imaging in the Evaluation of Axillary Lymph Node. BMC Med. Imaging 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Heller, S.L. Abbreviated and Ultrafast Breast MRI in Clinical Practice. RadioGraphics 2020, 40, 1507–1527. [Google Scholar] [CrossRef]
- Memarsadeghi, M.; Riedl, C.C.; Kaneider, A.; Galid, A.; Rudas, M.; Matzek, W.; Helbich, T.H. Axillary Lymph Node Metastases in Patients with Breast Carcinomas: Assessment with Nonenhanced versus USPIO-Enhanced MR Im-aging. Radiology 2006, 241, 367–377. [Google Scholar] [CrossRef]
- Marino, M.A.; Avendano, D.; Zapata, P.; Riedl, C.C.; Pinker, K. Lymph Node Imaging in Patients with Primary Breast Cancer: Concurrent Diagnostic Tools. Oncologist 2020, 25, e231–e242. [Google Scholar] [CrossRef]
- Kim, W.H.; Kim, H.J.; Lee, S.M.; Cho, S.H.; Shin, K.M.; Lee, S.Y.; Lim, J.K. Prediction of High Nodal Burden with Ultrasound and Magnetic Resonance Imaging in Clinically Node-Negative Breast Cancer Patients. Cancer Imaging 2019, 19, 4. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, M.J.; Moon, H.J.; Kim, E.-K.; Yoon, J.H. False Negative Results of Preoperative Axillary Ul-trasound in Patients with Invasive Breast Cancer: Correlations with Clinicopathologic Findings. Ultrasound Med. Biol. 2012, 38, 1881–1886. [Google Scholar] [CrossRef]
- Alvarez, S.; Añorbe, E.; Alcorta, P.; López, F.; Alonso, I.; Cortés, J. Role of Sonography in the Diagnosis of Axillary Lymph Node Metastases in Breast Cancer: A Systematic Review. Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef]
- Balasubramanian, I.; Fleming, C.A.; Corrigan, M.A.; Redmond, H.P.; Kerin, M.J.; Lowery, A.J. Meta-Analysis of the Diagnostic Accuracy of Ultrasound-Guided Fine-Needle Aspiration and Core Needle Biopsy in Diagnosing Axillary Lymph Node Metastasis. Br. J. Surg. 2018, 105, 1244–1253. [Google Scholar] [CrossRef]
- Chayakulkheeree, J.; Pungrassami, D.; Prueksadee, J. Performance of Breast Magnetic Resonance Imaging in Axillary Nodal Staging in Newly Diagnosed Breast Cancer Patients. Pol. J. Radiol. 2019, 84, e413–e418. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.H. Breast Magnetic Resonance Imaging for Assessment of Internal Mammary Lymph Node Status in Breast Cancer. J. Breast Cancer 2016, 19, 191–198. [Google Scholar] [CrossRef]
- Liang, X.; Yu, J.; Wen, B.; Xie, J.; Cai, Q.; Yang, Q. MRI and FDG-PET/CT Based Assessment of Axillary Lymph Node Metastasis in Early Breast Cancer: A Meta-Analysis. Clin. Radiol. 2017, 72, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Schipper, R.-J.; Paiman, M.-L.; Beets-Tan, R.G.H.; Nelemans, P.J.; de Vries, B.; Heuts, E.M.; van de Vijver, K.K.; Keymeulen, K.B.; Brans, B.; Smidt, M.L.; et al. Diagnostic Performance of Dedicated Axillary T2- and Diffu-sion-Weighted MR Imaging for Nodal Staging in Breast Cancer. Radiology 2015, 275, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaranelo, A.M.; Eiada, R.; Jacks, L.M.; Kulkarni, S.R.; Crystal, P. Accuracy of Unenhanced MR Imaging in the Detection of Axillary Lymph Node Metastasis: Study of Reproducibility and Reliability. Radiology 2012, 262, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Kramer, G.; Belghazi, K.; Duvivier, K.; van den Tol, P.; Schreurs, H. Can We Identify or Exclude Extensive Axillary Nodal Involvement in Breast Cancer Patients Preoperatively? J. Oncol. 2019, 2019, 8404035. [Google Scholar] [CrossRef]
- Jiang, Y.; Edwards, A.V.; Newstead, G.M. Artificial Intelligence Applied to Breast MRI for Improved Diagnosis. Radiology 2021, 298, 38–46. [Google Scholar] [CrossRef]
- Calabrese, A.; Santucci, D.; Landi, R.; Beomonte Zobel, B.; Faiella, E.; de Felice, C. Radiomics MRI for Lymph Node Status Prediction in Breast Cancer Patients: The State of Art. J. Cancer Res. Clin. Oncol. 2021, 147, 1587–1597. [Google Scholar] [CrossRef]
- Cui, X.; Wang, N.; Zhao, Y.; Chen, S.; Li, S.; Xu, M.; Chai, R. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer Using Radiomics Features of DCE-MRI. Sci. Rep. 2019, 9, 2240. [Google Scholar] [CrossRef]
- Chai, R.; Ma, H.; Xu, M.; Arefan, D.; Cui, X.; Liu, Y.; Zhang, L.; Wu, S.; Xu, K. Differentiating Axillary Lymph Node Metastasis in Invasive Breast Cancer Patients: A Comparison of Radiomic Signatures from Multiparametric Breast MR Sequences. J. Magn. Reson. Imaging 2019, 50, 1125–1132. [Google Scholar] [CrossRef]
- Liu, J.; Sun, D.; Chen, L.; Fang, Z.; Song, W.; Guo, D.; Ni, T.; Liu, C.; Feng, L.; Xia, Y.; et al. Radiomics Analysis of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Prediction of Sentinel Lymph Node Me-tastasis in Breast Cancer. Front. Oncol. 2019, 9, 980. [Google Scholar] [CrossRef]
- Shan, Y.; Xu, W.; Wang, R.; Wang, W.; Pang, P.; Shen, Q. A Nomogram Combined Radiomics and Kinetic Curve Pattern as Imaging Biomarker for Detecting Metastatic Axillary Lymph Node in Invasive Breast Cancer. Front. Oncol. 2020, 10, 1463. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Q.; Yang, W.; Lu, Z.; Deng, C.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Preoperative Prediction of Sentinel Lymph Node Metastasis in Breast Cancer Based on Radiomics of T2-Weighted Fat-Suppression and Diffusion-Weighted MRI. Eur. Radiol. 2018, 28, 582–591. [Google Scholar] [CrossRef]
- Han, L.; Zhu, Y.; Liu, Z.; Yu, T.; He, C.; Jiang, W.; Kan, Y.; Dong, D.; Tian, J.; Luo, Y. Radiomic Nomogram for Pre-diction of Axillary Lymph Node Metastasis in Breast Cancer. Eur. Radiol. 2019, 29, 3820–3829. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, Y.; Xie, C.; Hu, Q.; Ouyang, J.; Chen, Y.; Gu, Y.; Li, A.; Lu, N.; He, Z.; et al. Development and Validation of a Preoperative Magnetic Resonance Imaging Radiomics-Based Signature to Predict Axillary Lymph Node Metastasis and Disease-Free Survival in Patients With Early-Stage Breast Cancer. JAMA Netw. Open 2020, 3, e2028086. [Google Scholar] [CrossRef]
- Tan, H.; Gan, F.; Wu, Y.; Zhou, J.; Tian, J.; Lin, Y.; Wang, M. Preoperative Prediction of Axillary Lymph Node Me-tastasis in Breast Carcinoma Using Radiomics Features Based on the Fat-Suppressed T2 Sequence. Acad. Radiol. 2020, 27, 1217–1225. [Google Scholar] [CrossRef]
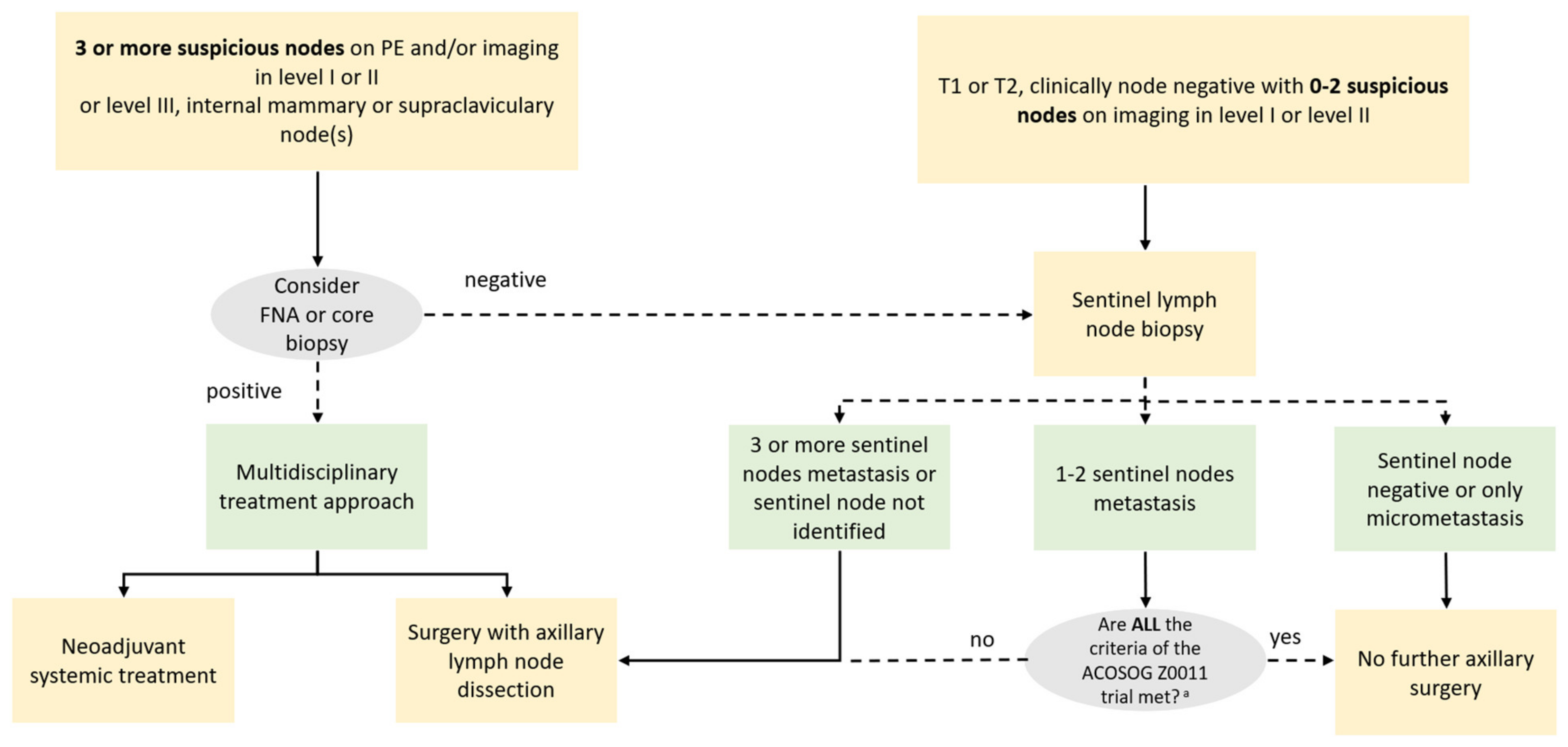




| American College of Surgeons Oncology Group (ACOSOG) Criteria—Z0011 Trial |
|---|
| ■ T1 or T2 tumor |
| ■ Clinically negative nodes |
| ■ 1 or 2 positive nodes on sentinel lymph node biopsy |
| ■ Planned breast conserving surgery |
| ■ Planned whole-breast radiation therapy |
| ■ No neoadjuvant chemotherapy planned |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, V.; Mazzotta, G.; Pignatelli, V.; Bufi, E.; D’Angelo, A.; Conti, M.; Panico, C.; Fiorentino, V.; Pierconti, F.; Kilburn-Toppin, F.; et al. Beyond N Staging in Breast Cancer: Importance of MRI and Ultrasound-based Imaging. Cancers 2022, 14, 4270. https://doi.org/10.3390/cancers14174270
Di Paola V, Mazzotta G, Pignatelli V, Bufi E, D’Angelo A, Conti M, Panico C, Fiorentino V, Pierconti F, Kilburn-Toppin F, et al. Beyond N Staging in Breast Cancer: Importance of MRI and Ultrasound-based Imaging. Cancers. 2022; 14(17):4270. https://doi.org/10.3390/cancers14174270
Chicago/Turabian StyleDi Paola, Valerio, Giorgio Mazzotta, Vincenza Pignatelli, Enida Bufi, Anna D’Angelo, Marco Conti, Camilla Panico, Vincenzo Fiorentino, Francesco Pierconti, Fleur Kilburn-Toppin, and et al. 2022. "Beyond N Staging in Breast Cancer: Importance of MRI and Ultrasound-based Imaging" Cancers 14, no. 17: 4270. https://doi.org/10.3390/cancers14174270
APA StyleDi Paola, V., Mazzotta, G., Pignatelli, V., Bufi, E., D’Angelo, A., Conti, M., Panico, C., Fiorentino, V., Pierconti, F., Kilburn-Toppin, F., Belli, P., & Manfredi, R. (2022). Beyond N Staging in Breast Cancer: Importance of MRI and Ultrasound-based Imaging. Cancers, 14(17), 4270. https://doi.org/10.3390/cancers14174270







