FAP-Specific Signalling Is an Independent Diagnostic Approach in ACC and Not a Surrogate Marker of MRI Sequences
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Radiochemistry
2.3. Image Acquisition
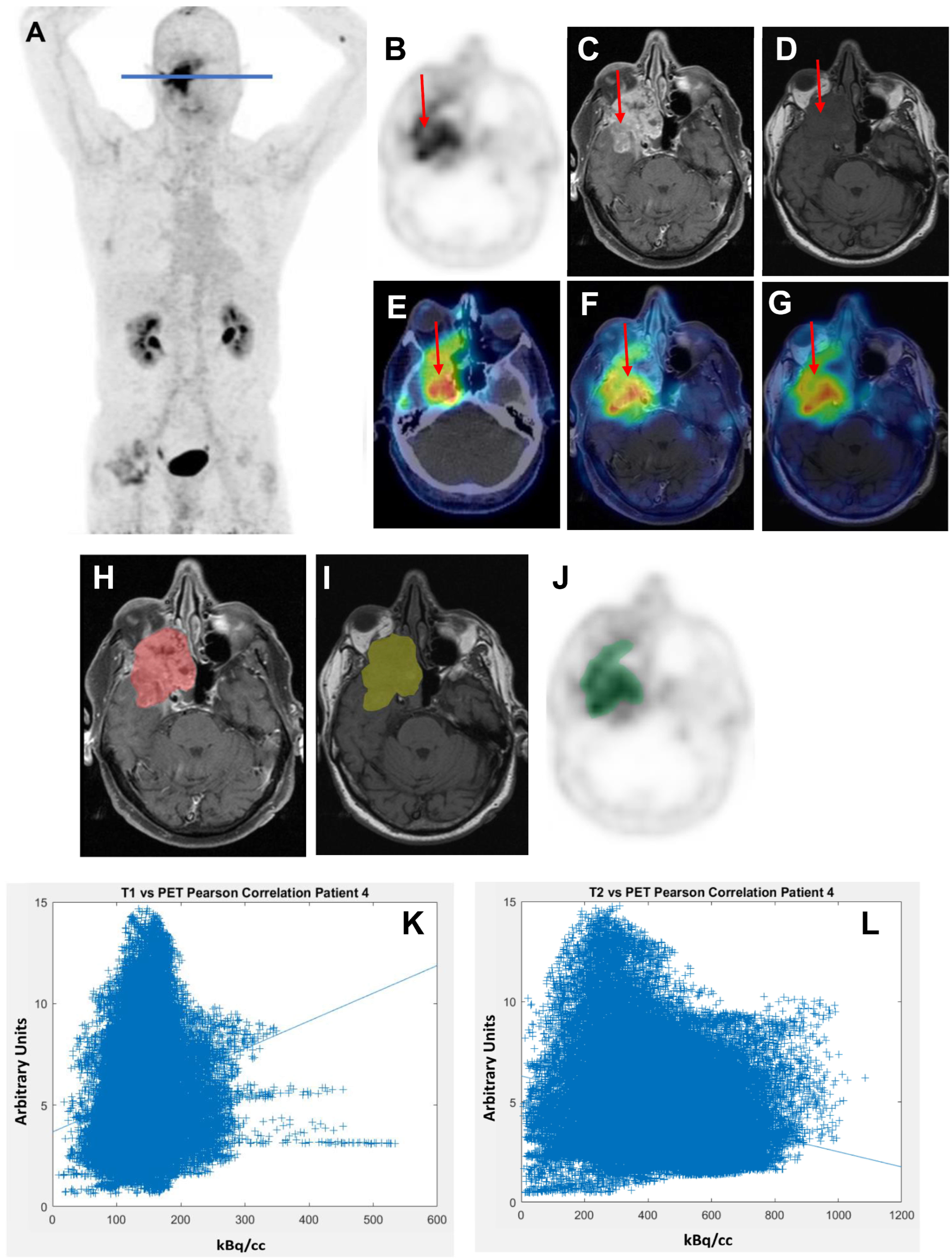
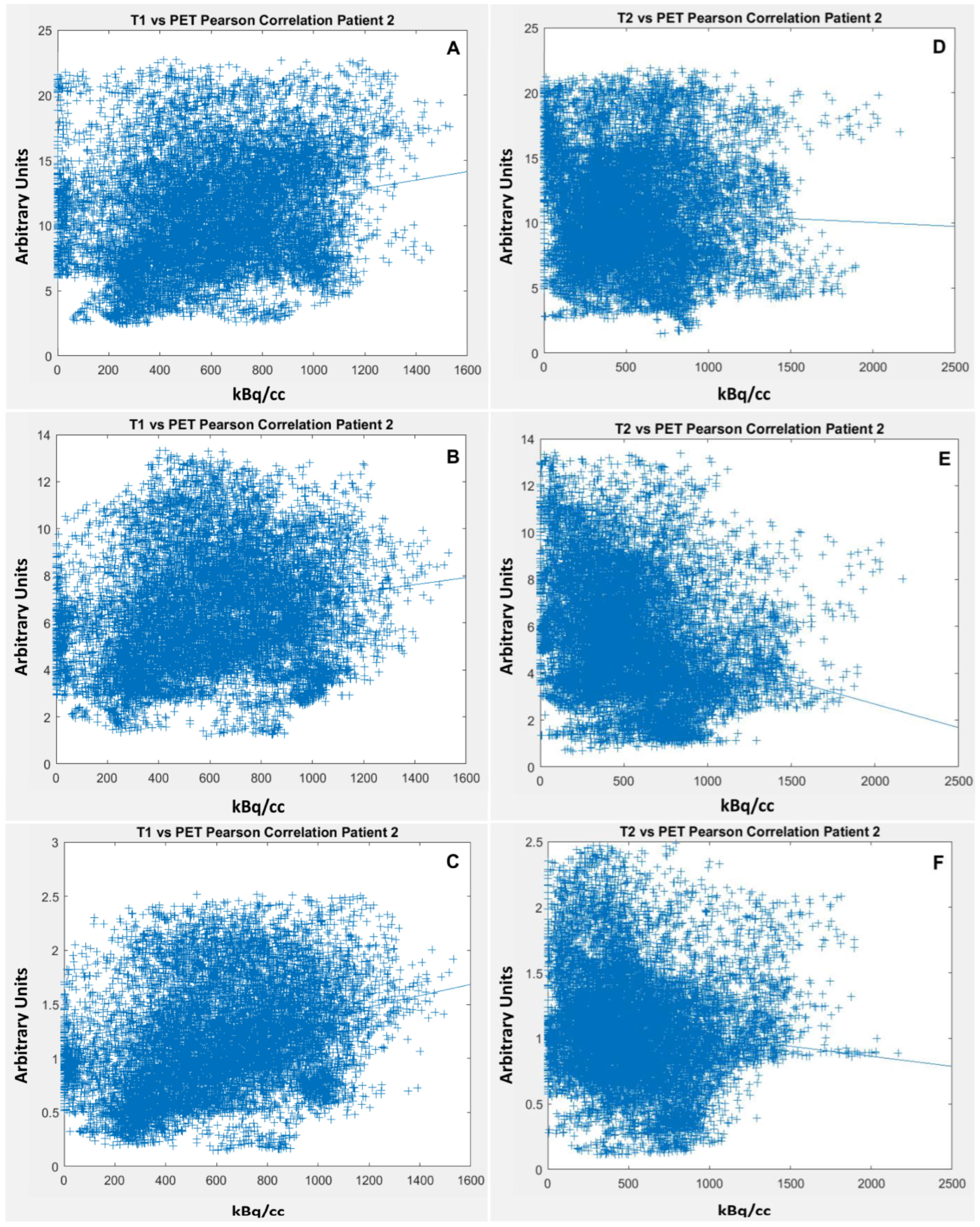
2.4. Co-Registration of 68Ga-FAPI PET/CT and MRI Scans
2.5. Statistics
3. Results
3.1. Description of the Results
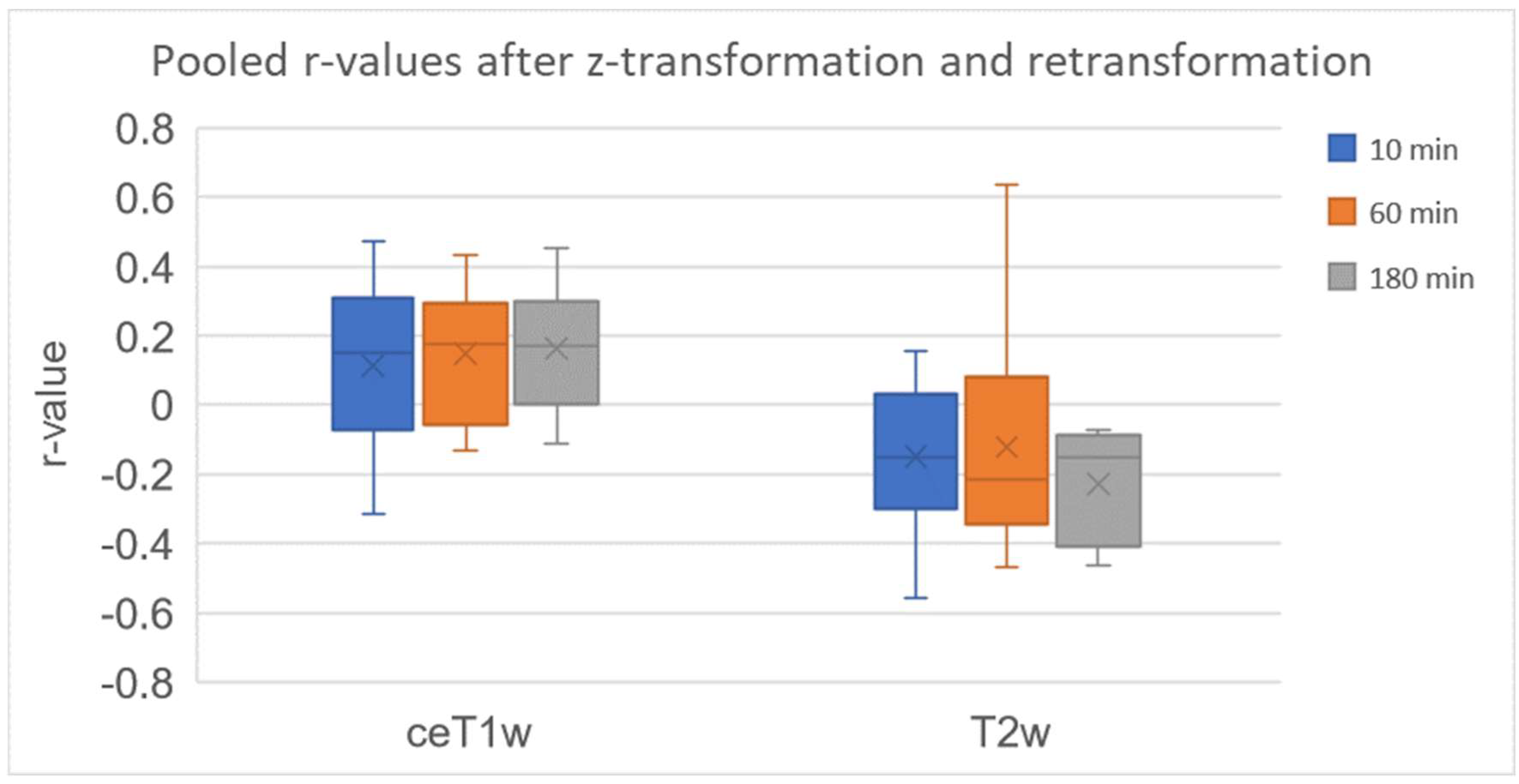
3.1.1. Very Weak Positive Correlation of 68Ga-FAPI PET and CeT1w Signalling in ACCs
3.1.2. Weak Negative Correlation of 68Ga-FAPI PET and T2w Signalling in ACCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Vander Poorten, V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef]
- Jang, S.; Patel, P.N.; Kimple, R.J.; McCulloch, T.M. Clinical Outcomes and Prognostic Factors of Adenoid Cystic Carcinoma of the Head and Neck. Anticancer Res. 2017, 37, 3045–3052. [Google Scholar] [PubMed]
- Castelnuovo, P.; Turri-Zanoni, M. Adenoid Cystic Carcinoma. Adv. Otorhinolaryngol. 2020, 84, 197–209. [Google Scholar] [PubMed]
- Moskaluk, C.A. Adenoid cystic carcinoma: Clinical and molecular features. Head Neck Pathol. 2013, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.M.; Chakraborty, S.; Moskaluk, C.A.; Joshi, P.J.; Thomas, C.Y. Adenoid cystic carcinoma: A review of recent advances, molecular targets, and clinical trials. Head Neck 2016, 38, 620–627. [Google Scholar] [CrossRef]
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am. J. Surg. 1974, 128, 512–520. [Google Scholar] [CrossRef]
- Szanto, P.A.; Luna, M.A.; Tortoledo, M.E.; White, R.A. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984, 54, 1062–1069. [Google Scholar] [CrossRef]
- Hanna, E.; Vural, E.; Prokopakis, E.; Carrau, R.; Snyderman, C.; Weissman, J. The Sensitivity and Specificity of High-Resolution Imaging in Evaluating Perineural Spread of Adenoid Cystic Carcinoma to the Skull Base. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 541–545. [Google Scholar] [CrossRef]
- Li, Y.; Hao, D.; Song, X.; Zhang, C. Computed tomography and magnetic resonance imaging of adenoid cystic carcinoma in the maxillary sinus: A retrospective study with radiologic-histopathologic correlations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 111–121. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.; Kong, J.; Zhang, F.; Fang, S.; Li, X.; Li, W.; Yang, X.; Luo, Y.; Lin, B.; et al. Carcinoma-Associated Fibroblasts Lead the Invasion of Salivary Gland Adenoid Cystic Carcinoma Cells by Creating an Invasive Track. PLoS ONE 2016, 11, e0150247. [Google Scholar]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Syed, M.; Liew, D.P.; Giesel, F.L.; Liermann, J.; Choyke, P.L.; Wefers, A.K.; Ritz, T.; Szymbara, M.; Schillings, L.; et al. 68Ga-FAPI-PET/CT improves diagnostic staging and radiotherapy planning of adenoid cystic carcinomas—Imaging analysis and histological validation. Radiother. Oncol. 2021, 160, 192–201. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Mier, W.; Hoffend, J.; Krämer, S.; Schuhmacher, J.; Hull, W.E.; Eisenhut, M.; Haberkorn, U. Conjugation of DOTA using isolated phenolic active esters: The labeling and biodistribution of albumin as blood pool marker. Bioconj. Chem. 2005, 16, 237–240. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Röhrich, M.; Floca, R.; Loi, L.; Adeberg, S.; Windisch, P.; Giesel, F.L.; Kratochwil, C.; Flechsig, P.; Rathke, H.; Lindner, T.; et al. FAP-specific PET signaling shows a moderately positive correlation with relative CBV and no correlation with ADC in 13 IDH wildtype glioblastomas. Eur. J. Radiol. 2020, 127, 109021. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-L.; Rosenthal, R.; Rubin, D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992, 111, 172–175. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Rohrich, M.; Loktev, A.; Wefers, A.K.; Altmann, A.; Paech, D.; Adeberg, S.; Windisch, P.; Hielscher, T.; Flechsig, P.; Floca, R.; et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Hattermann, K.; Hemion, C.; Jungbluth, A.A.; Held-Feindt, J. Expression and role of the cell surface protease seprase/fibroblast activation protein-alpha (FAP-alpha) in astroglial tumors. Biol. Chem. 2011, 392, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Kessler, L.; Hirmas, N.; Trajkovic-Arsic, M.; Hamacher, R.; Umutlu, L.; Nader, M.; Zarrad, F.; Weber, M.; Fendler, W.P. Equivalent tumor detection for early and late FAPI-46 PET acquisition. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3221–3227. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 2833–2843. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, Y.; Zheng, H.; Han, C.; Gu, J.; Sun, L.; Wu, H.; Wu, S.; Lin, Q.; Chen, H. Clinical utility of [68Ga]Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography for primary staging and recurrence detection in nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3606–3617. [Google Scholar] [CrossRef]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef]
- Dong, J.; Tian, L.; Li, S.; Mo, Y.; Liu, L.; Zhong, R. Differences in extension patterns between adenoid cystic carcinoma of the nasopharynx and nasopharyngeal carcinoma on MRI. Int. J. Clin. Exp. Pathol. 2015, 8, 15960–15968. [Google Scholar] [PubMed]
- Kato, H.; Kanematsu, M.; Makita, H.; Kato, K.; Hatakeyama, D.; Shibata, T.; Mizuta, K.; Aoki, M. CT and MR imaging findings of palatal tumors. Eur. J. Radiol. 2014, 83, e137–e146. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.M.; Mak, S.Y.; Bonington, S.C. Patterns of spread of head and neck adenoid cystic carcinoma. Clin. Radiol. 2015, 70, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Schaarschmidt, B.M.; Sauerwein, W.; Deuschl, C.; Arweiler-Harbeck, D.; Holtmann, L.; Stebner, V.; Umutlu, L.; Antoch, G.; Ruhlmann, V. 18F-FDG PET/MRI vs. MRI in patients with recurrent adenoid cystic carcinoma. Head Neck 2019, 41, 170–176. [Google Scholar]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef]
- Giesel, F.; Adeberg, S.; Syed, M.; Lindner, T.; Jimenez, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either 18F-AlF or Cold-kit 68Ga-labeling: Biodistribution, Radiation Dosimetry and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2020, 62, 201–207. [Google Scholar] [CrossRef]
- Glatting, F.M.; Hoppner, J.; Liew, D.P.; van Genabith, A.; Spektor, A.M.; Steinbach, L.; Hubert, A.; Kratochwil, C.; Giesel, F.L.; Dendl, K.; et al. Repetitive early FAPI-PET acquisition comparing FAPI-02, FAPI-46 and FAPI-74: Methodological and diagnostic implications for malignant, inflammatory and degenerative lesions. J. Nucl. Med. 2022, 63. [Google Scholar] [CrossRef]
| Patient Number | Age (Years) | Sex (M/F) | Location of Primary Tumour | Clinical Setting (and Duration of Disease) | Previous Therapy | Histological Subtype |
|---|---|---|---|---|---|---|
| 1 | 29 | F | Right parapharyngeal space | Primary | None | Mixed (tubular and cribriform) |
| 2 | 71 | F | Left maxillary sinus | Recurrence (5 months) | Resection | Cribriform |
| 3 | 56 | M | Left oropharynx | Recurrence (6 years) | Resection, neck dissection, re-resection | Not available |
| 4 | 71 | M | Right maxillary sinus | Primary | None | Tubular |
| 5 | 34 | F | Right nasopharynx | Primary | None | Not available |
| 6 | 66 | F | Left maxillary sinus | Recurrence (4 years) | Resection | Cribriform |
| 7 | 53 | F | Right hard palate | Recurrence (5 years) | Definitive radiotherapy | Mixed (tubular and cribriform) |
| 8 | 69 | F | Left parotid gland | Recurrence (28 years) | Resection, re-resections, resections of pulmonary metastases, Nivolumab | Cribriform |
| 9 | 63 | M | Floor of the mouth | Primary | None | Cribriform |
| 10 | 66 | F | Epi- and oropharynx, median | Primary | None | Tubular |
| 11 | 48 | M | Right parapharyngeal space | Primary | None | Cribriform |
| 12 | 68 | F | Epipharynx, left base of the skull | Primary | None | Cribriform |
| Patient Number | FAPI Tracer | Injected Activity (MBq) | SUVmax | SUVmean | ||||
|---|---|---|---|---|---|---|---|---|
| 10 Min | 60 Min | 180 Min | 10 Min | 60 Min | 180 Min | |||
| 1 | FAPI-2 | 258 | - | 16.8 | - | - | 7.73 | - |
| 2 | FAPI-74 | 269 | 6.15 | 6.24 | 3.84 | 3.81 | 2.58 | 2.32 |
| 3 | FAPI-74 | 221 | 10.2 | 8.15 | 4.27 | 4.63 | 3.37 | 2.75 |
| 4 | FAPI-74 | 177 | 12.0 | 11.2 | 6.71 | 7.31 | 4.07 | 3.97 |
| 5 | FAPI-46 | 282 | 14.0 | 12.8 | 7.95 | 6.99 | 4.65 | 4.36 |
| 6 | FAPI-46 | 285 | - | 17.6 | - | - | 8.05 | - |
| 7 | FAPI-46 | 220 | 9.17 | 7.94 | 7.85 | 4.63 | 4.55 | 3.93 |
| 8 | FAPI-2 | 268 | - | 4.26 | - | - | 2.92 | - |
| 9 | FAPI-46 | 211 | 9.64 | 10.7 | 13.3 | 4.59 | 5.53 | 4.92 |
| 10 | FAPI-46 | 269 | 12.2 | 13.1 | 12.2 | 8.08 | 6.52 | 5.53 |
| 11 | FAPI-46 | 268 | 10.8 | 10.7 | 13.4 | 6.63 | 5.35 | 4.08 |
| 12 | FAPI-46 | 244 | 12.1 | 14.4 | - | 4.99 | 5.83 | - |
| Patient Number | Number of Pixels | Correlation at 10 Min | p-Value | Correlation at 60 Min | p-Value | Correlation at 180 Min | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | 7892 | - | - | 0.172 | <0.001 | - | - |
| 2 | 12,118 | 0.226 | <0.001 | 0.181 | <0.001 | 0.319 | <0.001 |
| 3 | 77,987 | 0.470 | 0 | 0.312 | 0 | 0.093 | <0.001 |
| 4 | 124,187 | 0.150 | 0 | 0.173 | 0 | 0.175 | 0 |
| 5 | 37,870 | 0.038 | <0.001 | 0.246 | 0 | 0.243 | 0 |
| 6 | 177,586 | - | - | −0.080 | <0.001 | - | - |
| 7 | 151,081 | −0.156 | 0 | −0.130 | 0 | −0.112 | 0 |
| 8 | 2531 | - | - | 0.354 | <0.001 | - | - |
| 9 | 19,175 | −0.312 | 0 | −0.076 | <0.001 | −0.029 | <0.001 |
| 10 | 30,090 | 0.210 | <0.001 | 0.195 | <0.001 | 0.165 | <0.001 |
| 11 | 18,669 | 0.394 | 0 | 0.434 | 0 | 0.452 | 0 |
| 12 | 9654 | 0.010 | 0.351 | −0.006 | 0.533 | - | - |
| Patient Number | Number of Pixels | Correlation at 10 Min | p-Value | Correlation at 60 Min | p-Value | Correlation at 180 Min | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | 11,903 | - | - | 0.124 | <0.001 | - | - |
| 2 | 14,005 | −0.048 | <0.001 | −0.263 | <0.001 | −0.122 | <0.001 |
| 3 | 111,994 | 0.155 | 0 | 0.090 | <0.001 | −0.071 | <0.001 |
| 4 | 81,879 | −0.175 | 0 | −0.264 | 0 | −0.432 | 0 |
| 5 | 8098 | −0.401 | <0.001 | −0.424 | 0 | −0.392 | 0 |
| 6 | 47,534 | - | - | −0.351 | 0 | - | - |
| 7 | 160,768 | −0.072 | <0.001 | −0.110 | 0 | −0.133 | 0 |
| 8 | 458 | - | - | 0.563 | <0.001 | - | - |
| 9 | 18,432 | −0.508 | 0 | −0.435 | 0 | −0.364 | 0 |
| 10 | 13,520 | 0.112 | <0.001 | 0.051 | <0.001 | −0.074 | <0.001 |
| 11 | 12,597 | −0.150 | <0.001 | −0.230 | <0.001 | −0.166 | <0.001 |
| 12 | 12,586 | −0.172 | <0.001 | −0.193 | <0.001 | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, D.P.; Röhrich, M.; Loi, L.; Adeberg, S.; Syed, M.; Gutjahr, E.; Schlemmer, H.P.; Giesel, F.L.; Bendszus, M.; Haberkorn, U.; et al. FAP-Specific Signalling Is an Independent Diagnostic Approach in ACC and Not a Surrogate Marker of MRI Sequences. Cancers 2022, 14, 4253. https://doi.org/10.3390/cancers14174253
Liew DP, Röhrich M, Loi L, Adeberg S, Syed M, Gutjahr E, Schlemmer HP, Giesel FL, Bendszus M, Haberkorn U, et al. FAP-Specific Signalling Is an Independent Diagnostic Approach in ACC and Not a Surrogate Marker of MRI Sequences. Cancers. 2022; 14(17):4253. https://doi.org/10.3390/cancers14174253
Chicago/Turabian StyleLiew, Dawn P., Manuel Röhrich, Lisa Loi, Sebastian Adeberg, Mustafa Syed, Ewgenija Gutjahr, Heinz Peter Schlemmer, Frederik L. Giesel, Martin Bendszus, Uwe Haberkorn, and et al. 2022. "FAP-Specific Signalling Is an Independent Diagnostic Approach in ACC and Not a Surrogate Marker of MRI Sequences" Cancers 14, no. 17: 4253. https://doi.org/10.3390/cancers14174253
APA StyleLiew, D. P., Röhrich, M., Loi, L., Adeberg, S., Syed, M., Gutjahr, E., Schlemmer, H. P., Giesel, F. L., Bendszus, M., Haberkorn, U., & Paech, D. (2022). FAP-Specific Signalling Is an Independent Diagnostic Approach in ACC and Not a Surrogate Marker of MRI Sequences. Cancers, 14(17), 4253. https://doi.org/10.3390/cancers14174253








