Theranostic Applications of an Ultra-Sensitive T1 and T2 Magnetic Resonance Contrast Agent Based on Cobalt Ferrite Spinel Nanoparticles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MCFS
2.2. Preparation of MCFS Liposomes
2.3. Cell Biocompatibility of MCFS Nanoparticles
2.4. In Vitro and In Vivo MR Imaging
2.5. Acute Toxicity Study
2.6. Animal Models
2.7. Treatment Study
2.8. Statistical Analysis
3. Results
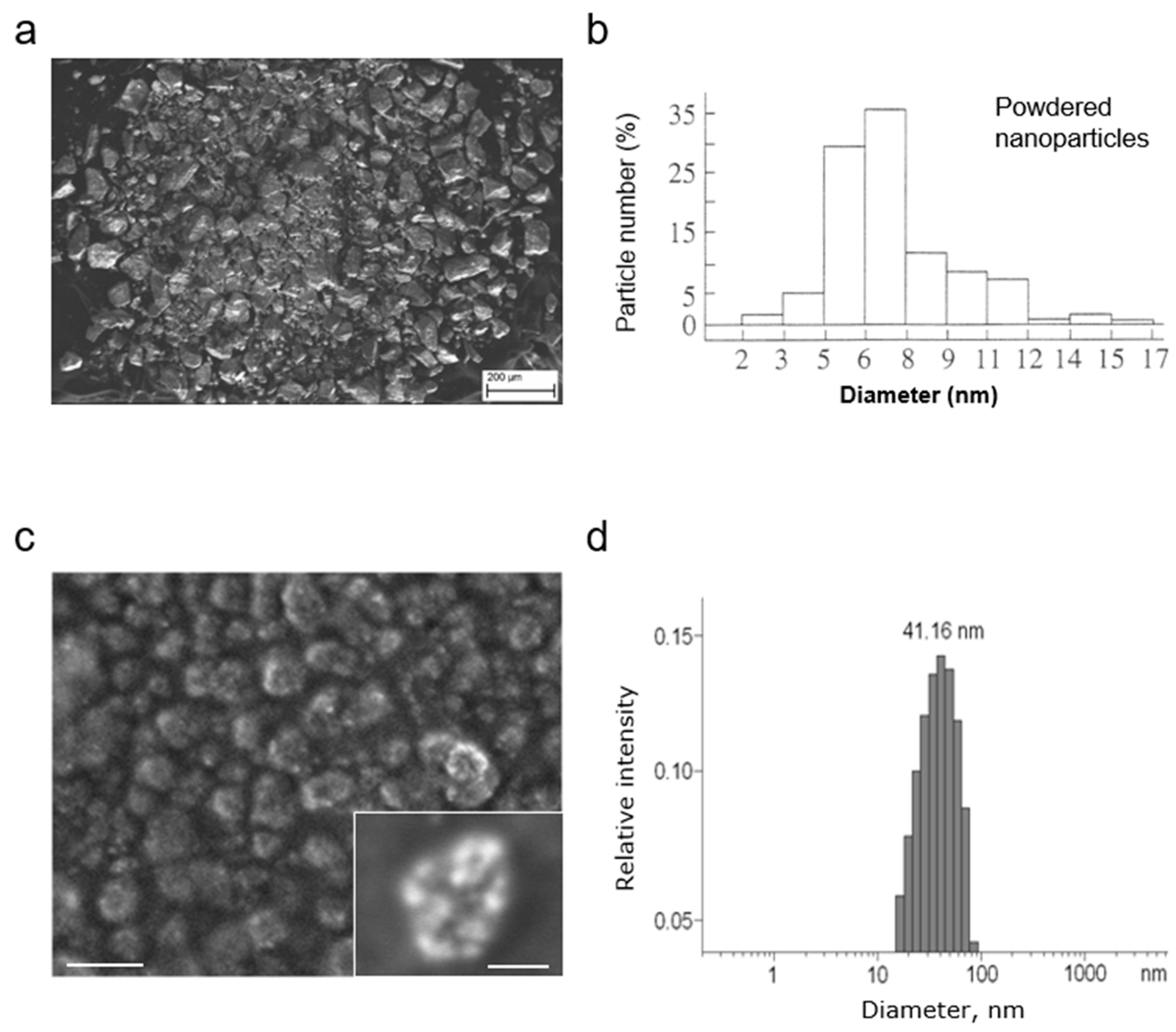
3.1. Development and Characterization of MCFS Nanoparticles
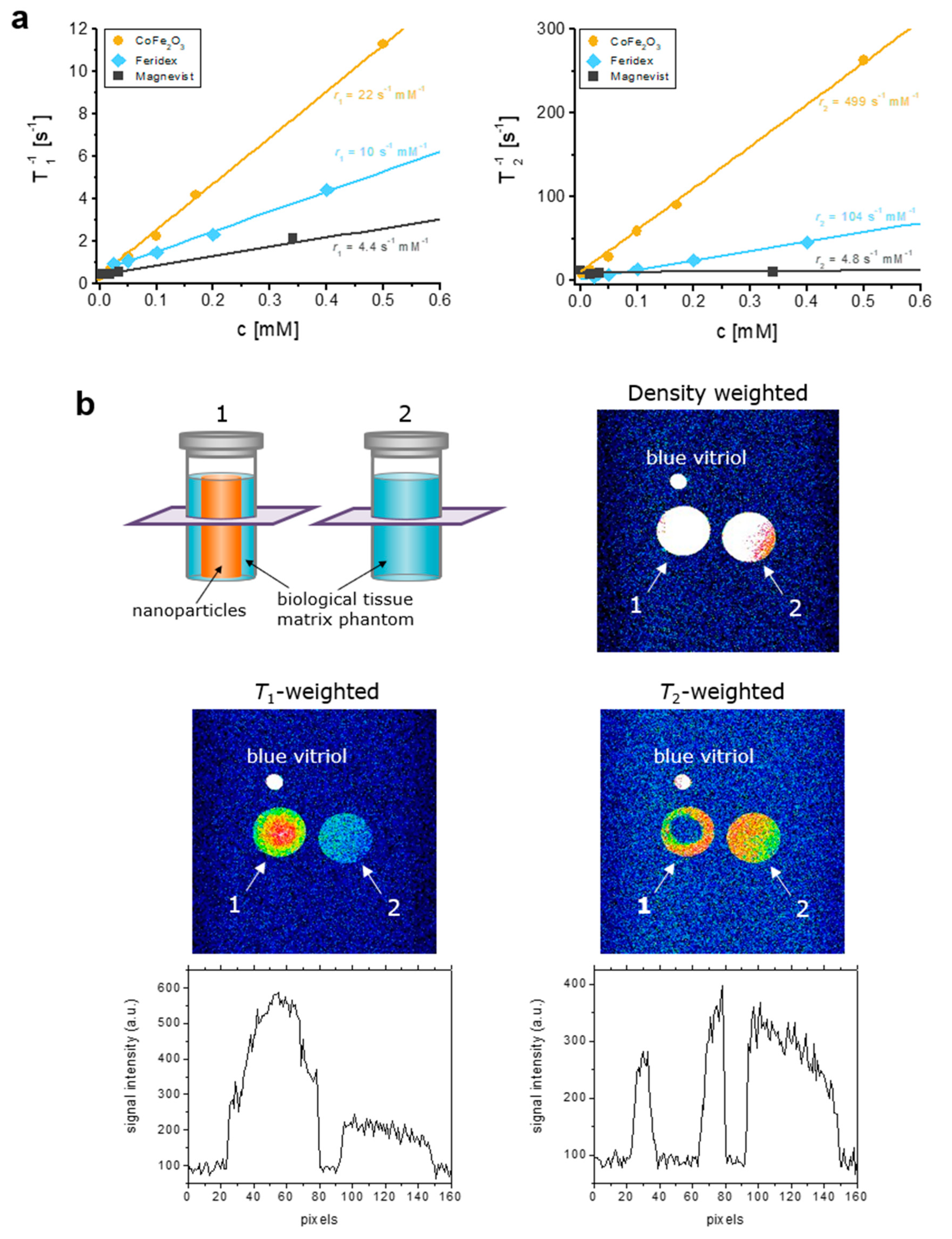
3.2. MR Contrast Properties of MCFS Nanoparticles In Vitro
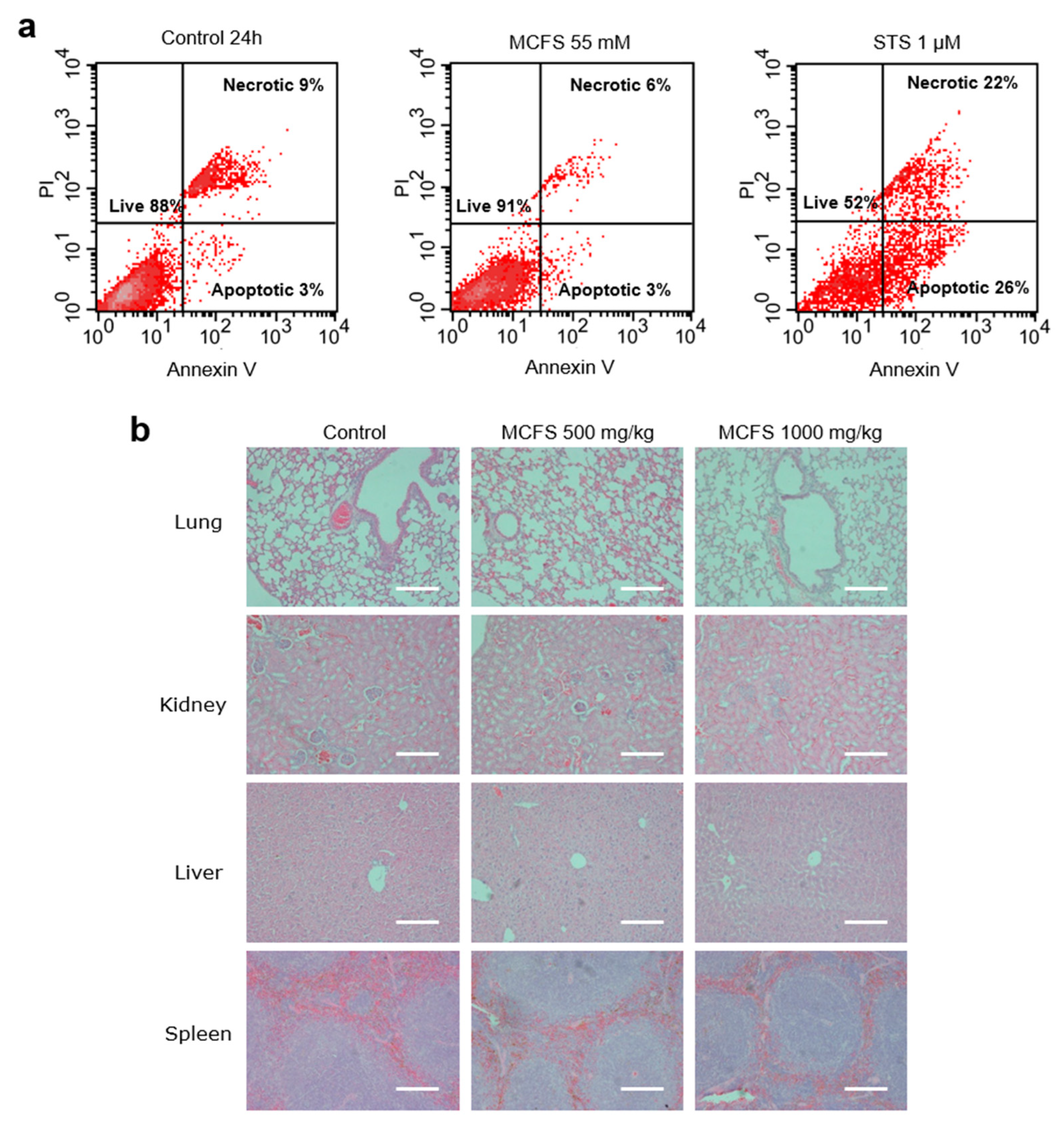
3.3. Safety and Toxicity of MCFS Nanoparticles
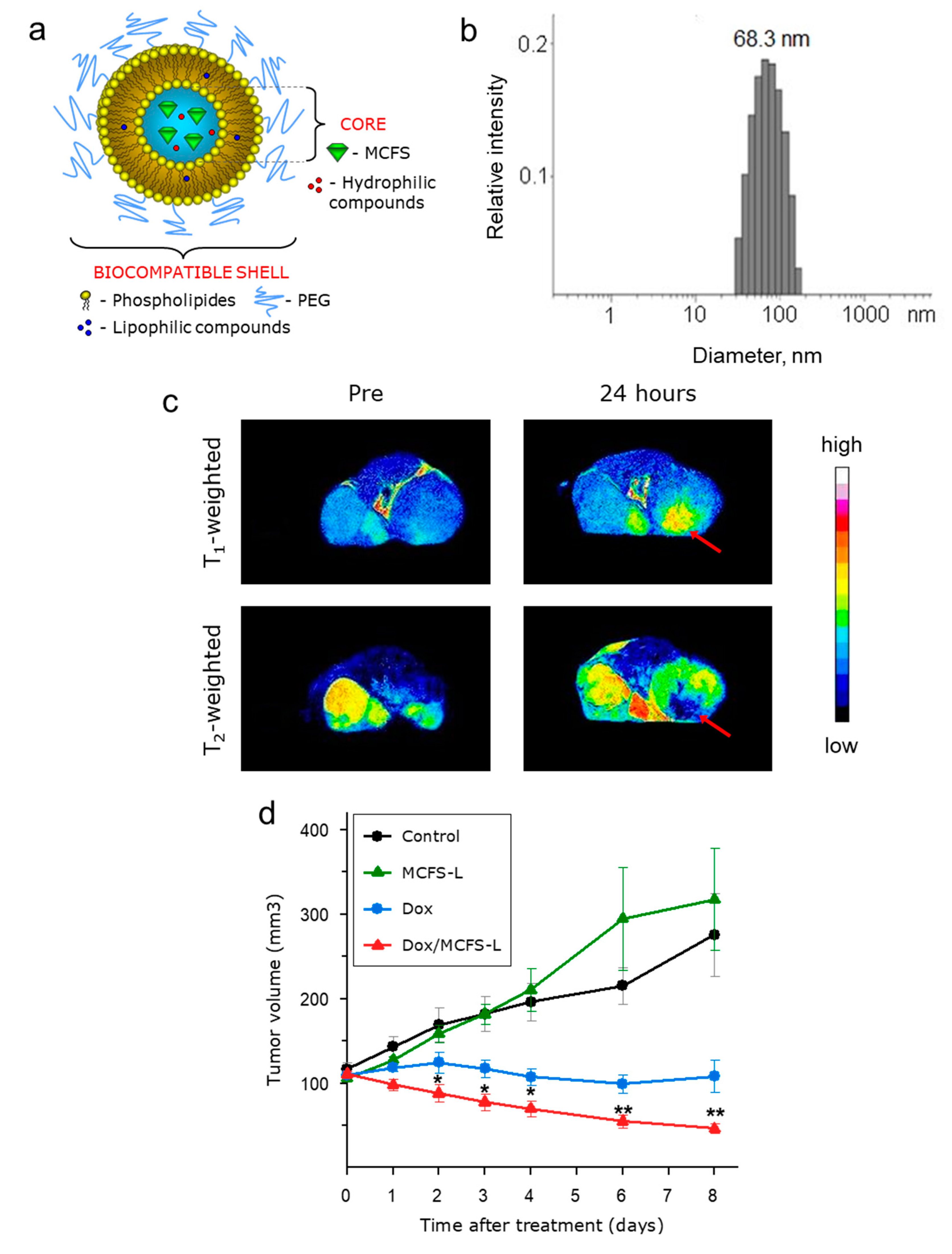
3.4. MCFS Nanoparticles as an MRI-Visible Drug Delivery System In Vivo
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latorre, M.; Rinaldi, C. Applications of magnetic nanoparticles in medicine: Magnetic fluid hyperthermia. Puerto Rico Health Sci. J. 2009, 28, 227–238. [Google Scholar]
- Meyers, J.D.; Doane, T.; Burda, C.; Basilion, J.P. Nanoparticles for imaging and treating brain cancer. Nanomedicine 2013, 8, 123–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.; Wu, H.; Li, J.; Xie, J.; Zang, F.; Ma, M.; Gu, N.; Zhang, Y. Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater. 2019, 96, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.D.M.; Roslin, T.; Tikhonov, G.; Meyke, E.; Lo, C.; Gurarie, E.; Abadonova, M.; Abduraimov, O.; Adrianova, O.; Akimova, T.; et al. Differences in spatial versus temporal reaction norms for spring and autumn phenological events. Proc. Natl. Acad. Sci. USA 2020, 117, 31249–31258. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef]
- Su, C.H.; Sheu, H.S.; Lin, C.Y.; Huang, C.C.; Lo, Y.W.; Pu, Y.C.; Weng, J.C.; Shieh, D.B.; Chen, J.H.; Yeh, C.S. Nanoshell magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2007, 129, 2139–2146. [Google Scholar] [CrossRef]
- Naiden, E.; Zhuravlev, V.; Itin, V.; Terekhova, O.; Magaeva, A.; Ivanov, Y. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys. Solid State 2008, 50, 894–900. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roma-Rodrigues, C.; Pombo, I.; Raposo, L.; Pedrosa, P.; Fernandes, A.R.; Baptista, P.V. Nanotheranostics Targeting the Tumor Microenvironment. Front. Bioeng. Biotechnol. 2019, 7, 197. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Dou, W.; Jansen, J.A.; Walboomers, X.F. Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-substitutes. Mol. Imaging Biol. 2019, 21, 1003–1019. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese Oxide Nanoparticles As MRI Contrast Agents In Tumor Multimodal Imaging And Therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Heydari Sheikh Hossein, H.; Jabbari, I.; Zarepour, A.; Zarrabi, A.; Ashrafizadeh, M.; Taherian, A.; Makvandi, P. Functionalization of Magnetic Nanoparticles by Folate as Potential MRI Contrast Agent for Breast Cancer Diagnostics. Molecules 2020, 25, 4053. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.A.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. New Insight about Biocompatibility and Biodegradability of Iron Oxide Magnetic Nanoparticles: Stereological and In Vivo MRI Monitor. Sci. Rep. 2019, 9, 7173. [Google Scholar] [CrossRef] [Green Version]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Hu, H. Recent Advances of Bioresponsive Nano-Sized Contrast Agents for Ultra-High-Field Magnetic Resonance Imaging. Front. Chem. 2020, 8, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspani, S.; Magalhaes, R.; Araujo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Khu, N.H.; Yeh, C.S. The characteristics of sub 10 nm manganese oxide T1 contrast agents of different nanostructured morphologies. Biomaterials 2010, 31, 4073–4078. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.H.; Kim, S.T.; Kim, S.H.; Kim, S.W.; et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. Engl. 2007, 46, 5397–5401. [Google Scholar] [CrossRef]
- Du, C.; Wang, J.; Liu, X.; Li, H.; Geng, D.; Yu, L.; Chen, Y.; Zhang, J. Construction of Pepstatin A-Conjugated ultrasmall SPIONs for targeted positive MR imaging of epilepsy-overexpressed P-glycoprotein. Biomaterials 2020, 230, 119581. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Liu, J.; Sun, J.; Hu, X.; Zhao, M.; Liu, J.; Bai, R.; Kim, D.; Sun, X.; et al. Dynamically Reversible Iron Oxide Nanoparticle Assemblies for Targeted Amplification of T1-Weighted Magnetic Resonance Imaging of Tumors. Nano Lett. 2019, 19, 4213–4220. [Google Scholar] [CrossRef]
- Hutten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Hachmann, W.; Heinzmann, U.; Wojczykowski, K.; Jutzi, P.; Saikaly, W.; Thomas, G. New magnetic nanoparticles for biotechnology. J. Biotechnol. 2004, 112, 47–63. [Google Scholar] [CrossRef]
- Seo, W.S.; Lee, J.H.; Sun, X.M.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, Y.; Hu, Z.; Mei, X.; Uvdal, K. Magneto-fluorescent nanoparticles with high-intensity NIR emission, T1- and T2-weighted MR for multimodal specific tumor imaging. J. Mater. Chem. B 2015, 3, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qian, K.; Mei, X. A theranostic nanoplatform: Magneto-gold@fluorescence polymer nanoparticles for tumor targeting T1&T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale 2018, 10, 10467–10478. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, P.T. Principles of Nuclear Magnetic Resonance Microscopy; Oxford Univ. Press: New York, NY, USA, 1991. [Google Scholar]
- Kim, S.H.; Choi, B.I.; Lee, J.Y.; Kim, S.J.; So, Y.H.; Eun, H.W.; Lee, J.M.; Han, J.K. Diagnostic accuracy of multi-/single-detector row CT and contrast-enhanced MRI in the detection of hepatocellular carcinomas meeting the milan criteria before liver transplantation. Intervirology 2008, 51 (Suppl. S1), 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Flask, C.; Weinberg, B.; Shuai, X.; Pagel, M.D.; Farrell, D.; Duerk, J.; Gao, J.M. Magnetite-loaded polymeric micelles as ultrasensitive magnetic-resonance probes. Adv. Mater. 2005, 17, 1949–1951. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Lee, J.; Huh, Y.; Jun, Y.; Seo, J.W.; Jang, J.T.; Song, H.T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Medcine 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Bell-McGuinn, K.; Garfall, A.; Bogyo, M.; Hanahan, D.; Joyce, J.A. Inhibition of cysteine cathepsin protease activity enhances chemotherapy regimens by decreasing tumor growth and invasiveness in a mouse model of multistage cancer. Cancer Res. 2007, 67, 7378–7385. [Google Scholar] [CrossRef] [Green Version]
- Knauth, M.; Egelhof, T.; Roth, S.U.; Wirtz, C.R.; Sartor, K. Monocrystalline iron oxide nanoparticles: Possible solution to the problem of surgically induced intracranial contrast enhancement in intraoperative MR imaging. AJNR Am. J. Neuroradiol. 2001, 22, 99–102. [Google Scholar]
- Fortin-Ripoche, J.P.; Martina, M.S.; Gazeau, F.; Ménager, C.; Wilhelm, C.; Bacri, J.C.; Lesieur, S.; Clément, O. Magnetic targeting of magnetoliposomes to solid tumors with MR imaging monitoring in mice: Feasibility. Radiology 2005, 239, 415–424. [Google Scholar] [CrossRef]
- Martina, M.S.; Fortin, J.P.; Menager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, O.; Papazoglou, A.; Krüger, A.; Brodoefel, H.; Korovin, M.; Deussing, J.; Augustin, N.; Nielsen, B.S.; Almholt, K.; Bogyo, M. Tumor cell–derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006, 66, 5242–5250. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.F.; Tang, P.L.; Qian, Z.M.; Ashraf, M. Effects by doxorubicin on the myocardium are mediated by oxygen free radicals. Life Sci. 2001, 68, 889–901. [Google Scholar] [CrossRef]
- Bertinchant, J.P.; Polge, A.; Juan, J.M.; Oliva-Lauraire, M.C.; Giuliani, I.; Marty-Double, C.; Burdy, J.Y.; Fabbro-Peray, P.; Laprade, M.; Bali, J.P.; et al. Evaluation of cardiac troponin I and T levels as markers of myocardial damage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clin. Chim. Acta 2003, 329, 39–51. [Google Scholar] [CrossRef]
- Stollfuss, J.C.; Becker, K.; Sendler, A.; Seidl, S.; Settles, M.; Auer, F.; Beer, A.; Rummeny, E.J.; Woertler, K. Rectal carcinoma: High-spatial-resolution MR imaging and T2 quantification in rectal cancer specimens. Radiology 2006, 241, 132–141. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Klimpel, D.; Schaschke, N.; Mikac, U.; Vizovisek, M.; Fonovic, M.; Turk, V.; Turk, B.; Vasiljeva, O. Selective targeting of tumor and stromal cells by a nanocarrier system displaying lipidated cathepsin B inhibitor. Angew. Chem. 2014, 53, 10077–10081. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharm. 2019, 55, 50–61. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Jiang, K.; Zhang, Y.; Zhan, C.; Ying, M.; Zhang, M.; Lu, L.; Wang, R.; Wang, S.; et al. Liposomes with cyclic RGD peptide motif triggers acute immune response in mice. J. Control Release 2019, 293, 201–214. [Google Scholar] [CrossRef]
- Wang, X.; Meng, N.; Wang, S.; Zhang, Y.; Lu, L.; Wang, R.; Ruan, H.; Jiang, K.; Wang, H.; Ran, D.; et al. Non-immunogenic, low-toxicity and effective glioma targeting MTI-31 liposomes. J. Control Release 2019, 316, 381–392. [Google Scholar] [CrossRef]
- Lu, R.; Zhou, L.; Yue, Q.; Liu, Q.; Cai, X.; Xiao, W.; Hai, L.; Guo, L.; Wu, Y. Liposomes modified with double-branched biotin: A novel and effective way to promote breast cancer targeting. Bioorg. Med. Chem. 2019, 27, 3115–3127. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sinica B 2019, 9, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer. Pharmaceutics 2020, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Rodrigues, A.R.O.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Castanheira, E.M.S.; Coutinho, P.J.G. Stealth Magnetoliposomes Based on Calcium-Substituted Magnesium Ferrite Nanoparticles for Curcumin Transport and Release. Int. J. Mol. Sci. 2020, 21, 3641. [Google Scholar] [CrossRef]
- Guy, C.T.; Cardiff, R.D.; Muller, W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A trans-genic mouse model for metastatic disease. Mol. Cell Biol. 1992, 12, 954–961. [Google Scholar]
- Mikhaylov, G.; Vasiljeva, O. Promising approaches in using magnetic nanoparticles in oncology. Biol. Chem. 2011, 392, 955–960. [Google Scholar] [CrossRef]
- Elhasany, K.A.; Khattab, S.N.; Bekhit, A.A.; Ragab, D.M.; Abdulkader, M.A.; Zaky, A.; Helmy, M.W.; Ashour, H.M.A.; Teleb, M.; Haiba, N.S.; et al. Combination of magnetic targeting with synergistic inhibition of NF-kappaB and glutathione via micellar drug nanomedicine enhances its anti-tumor efficacy. Eur. J. Pharm. Biopharm. 2020, 155, 162–176. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Lowa, N.; Wiekhorst, F.; Bleul, R. Albumin-Coated Single-Core Iron Oxide Nanoparticles for Enhanced Molecular Magnetic Imaging (MRI/MPI). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef]
| Treatment Groups, Dose | Renal Function | Cardiac Function | Liver Function | Pancreatic Function | ||||
|---|---|---|---|---|---|---|---|---|
| Creatinine µmol/L | Urea Nitrogen mmol/L | Creatine Kinase (MB) U/L | Lactate Dehydrogenase U/L | Alkaline Phosphatase U/L | Alanine Transaminase U/L | Aspartate Transaminase U/L | α-Amylase U/L | |
| Control, Males | 19 ± 3.8 | 10 ± 0.3 | 229.8 ± 61 | 1129 ± 295 | 99.7 ± 16.4 | 43.7 ± 4.8 | 96 ± 11.7 | 2281 ± 268 |
| Control, Females | 46 ± 7.5 | 13.3 ± 0.3 | 242.2 ± 133 | 960.2 ± 213 | 113.7 ± 8.2 | 59.5 ± 4 | 87.5 ± 23.1 | 2620 ± 373 |
| 500 mg/kg, Males | 16 ± 2.1 | 10 ± 0.8 | 185.2 ± 111 | 711.7 ± 62 | 97.2 ± 10.8 | 43.2 ± 3.94 | 66.5 ± 7.9 | 2976 ± 248 |
| 500 mg/kg, Females | 37.7 ± 1.1 | 12 ± 0.6 | 72.7 ± 6.1 | 586 ± 21.8 | 104 ± 7.5 | 68.7 ± 7.3 | 58.2 ± 3.7 | 3157 ± 11 |
| 1000 mg/kg, Males | 19.5 ± 1.5 | 8.8 ± 1 | 239.7 ± 93 | 1191 ± 269 | 82 ± 10 | 60.5 ± 22.8 | 84.5 ± 26.6 | 2685 ± 117 |
| 1000 mg/kg, Females | 51.7 ± 8.3 | 12.1 ± 0.2 | 95 ± 19.7 | 706.7 ± 63 | 78.2 ± 6.5 | 52 ± 8.3 | 63.5 ± 5.1 | 2986 ± 236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhaylov, G.; Mikac, U.; Butinar, M.; Turk, V.; Turk, B.; Psakhie, S.; Vasiljeva, O. Theranostic Applications of an Ultra-Sensitive T1 and T2 Magnetic Resonance Contrast Agent Based on Cobalt Ferrite Spinel Nanoparticles. Cancers 2022, 14, 4026. https://doi.org/10.3390/cancers14164026
Mikhaylov G, Mikac U, Butinar M, Turk V, Turk B, Psakhie S, Vasiljeva O. Theranostic Applications of an Ultra-Sensitive T1 and T2 Magnetic Resonance Contrast Agent Based on Cobalt Ferrite Spinel Nanoparticles. Cancers. 2022; 14(16):4026. https://doi.org/10.3390/cancers14164026
Chicago/Turabian StyleMikhaylov, Georgy, Urska Mikac, Miha Butinar, Vito Turk, Boris Turk, Sergey Psakhie, and Olga Vasiljeva. 2022. "Theranostic Applications of an Ultra-Sensitive T1 and T2 Magnetic Resonance Contrast Agent Based on Cobalt Ferrite Spinel Nanoparticles" Cancers 14, no. 16: 4026. https://doi.org/10.3390/cancers14164026
APA StyleMikhaylov, G., Mikac, U., Butinar, M., Turk, V., Turk, B., Psakhie, S., & Vasiljeva, O. (2022). Theranostic Applications of an Ultra-Sensitive T1 and T2 Magnetic Resonance Contrast Agent Based on Cobalt Ferrite Spinel Nanoparticles. Cancers, 14(16), 4026. https://doi.org/10.3390/cancers14164026








