The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
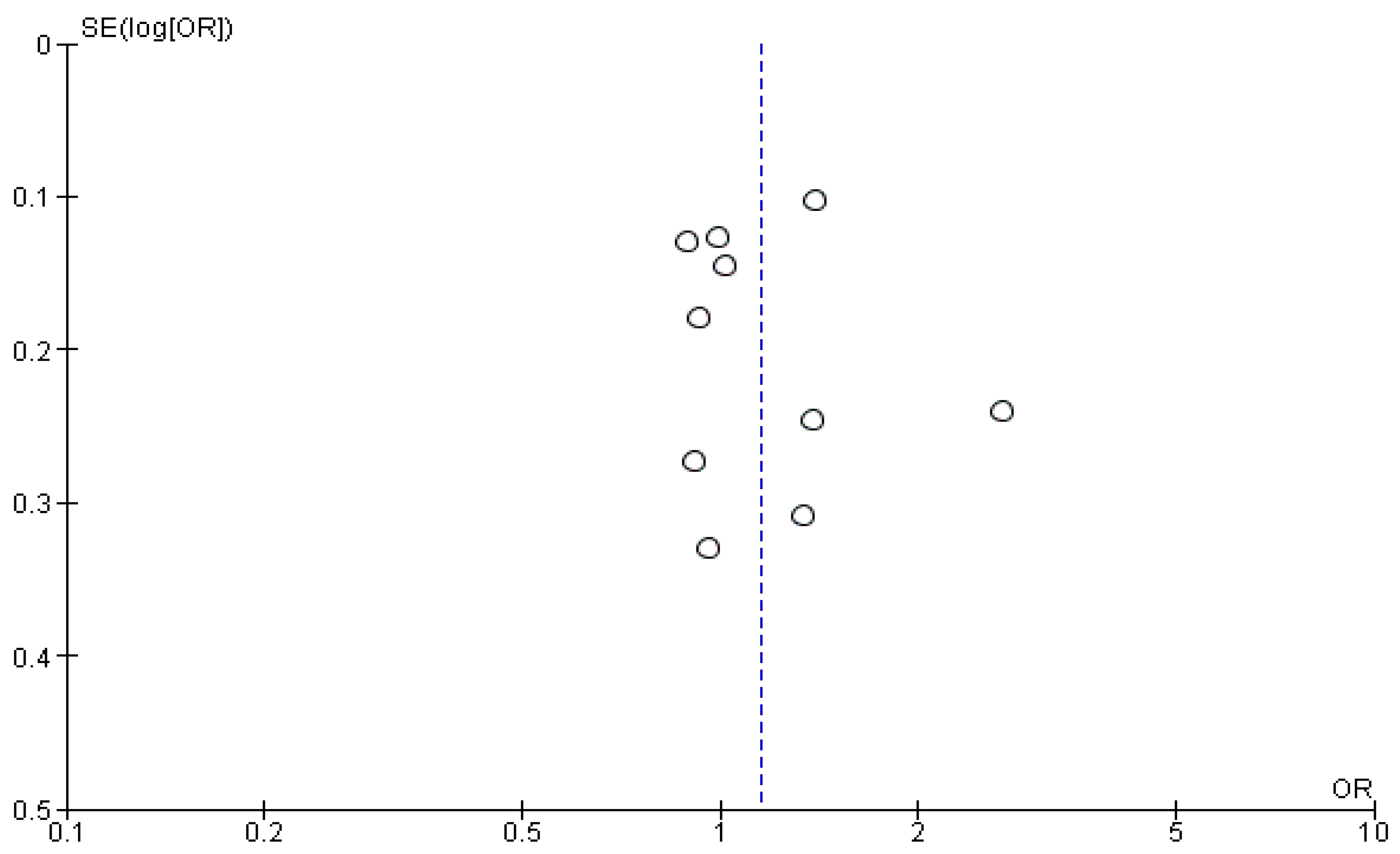
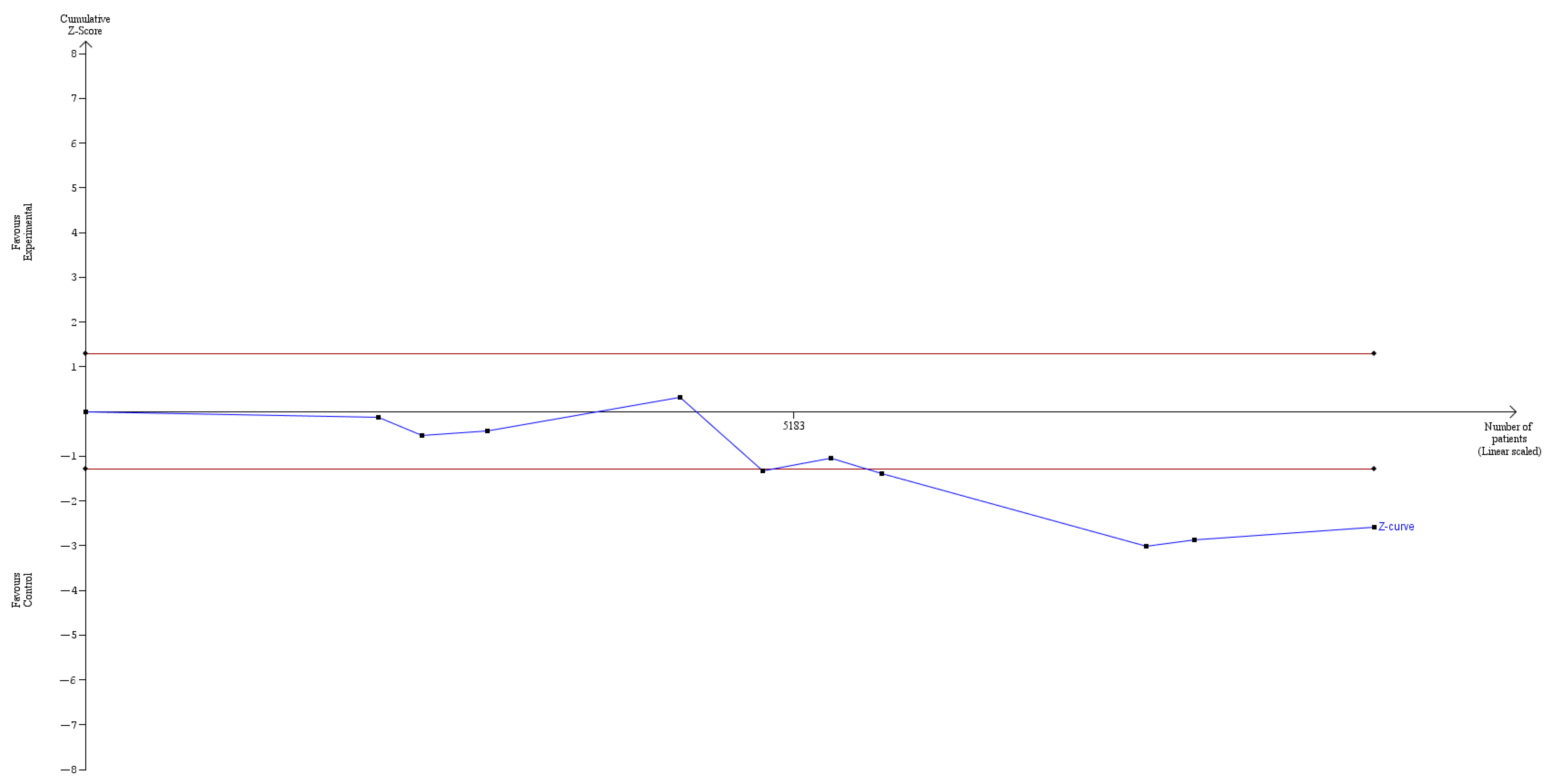
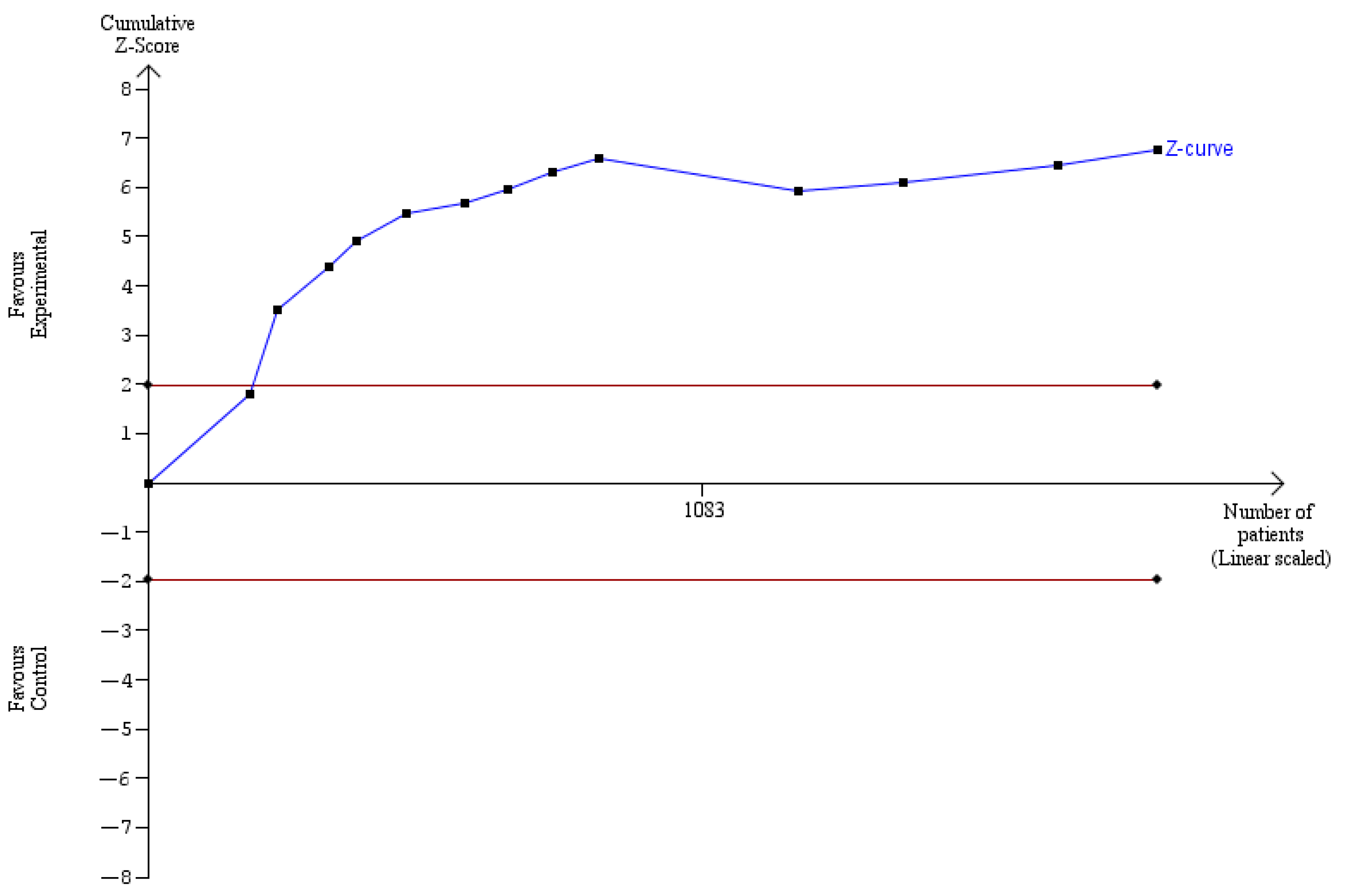
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Z.; Xu, M.; Peng, H. The role of carbon nanoparticle in lymph node detection and parathyroid gland protection during thyroidectomy for non-anaplastic thyroid carcinoma—A meta-analysis. PLoS ONE 2020, 15, e0223627. [Google Scholar] [CrossRef] [PubMed]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Prete, A.; De Souza, P.B.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Shukla, N.; Osazuwa-Peters, N.; Megwalu, U.C. Association between Age and Nodal Metastasis in Papillary Thyroid Carcinoma. Otolaryngol. Head Neck Surg. 2021, 165, 43–49. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.; Newbold, K.; Papotti, M.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Moo, T.-A.S.; Fahey, T.J. Lymph Node Dissection in Papillary Thyroid Carcinoma. Semin. Nucl. Med. 2011, 41, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Tan, J.; Tan, Q.; Xu, L.; He, T.; Lv, Q. Application of Carbon Nanoparticles in Tracing Lymph Nodes and Locating Tumors in Colorectal Cancer: A Concise Review. Int. J. Nanomed. 2020, 15, 9671–9681. [Google Scholar] [CrossRef]
- Lisik, K.; Krokosz, A. Application of Carbon Nanoparticles in Oncology and Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, M.; Zhu, G.; Ma, M.; Du, H.; Long, Y. Lymph node mapping with carbon nanoparticles and the risk factors of lymph node metastasis in gastric cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Q.; Chen, G.; Lu, J.; Zeng, Y.; Chen, X.; Yan, J. Sentinel Lymph Node Detection Using Carbon Nanoparticles in Patients with Early Breast Cancer. PLoS ONE 2015, 10, e0135714. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Li, M.; Hu, J.; Chen, Z.; Yu, J.; Dong, Y.; Sun, C.; Han, J. Somatic mitochondrial DNA D—loop mutations in meningioma discovered: A preliminary data A comprehensive overview of mitochondrial DNA 4977-bp. J. Cancer Res. Ther. 2018, 14, 1525–1534. [Google Scholar] [PubMed]
- Liang, S.; Wang, Z.; Chen, J.; Yang, X.; Liang, X.; Sun, X.; Li, X.; Zhou, R.; Li, Y.; Wang, J. Carbon nanoparticles combined with indocyanine green for sentinel lymph node detection in endometrial carcinoma. J. Surg. Oncol. 2021, 124, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, D.; Lv, J.Y.; Yu, D.; Xin, S.J. Application of carbon nanoparticles in lymph node dissection and parathyroid protection during thyroid cancer surgeries: A systematic review and meta-analysis. Onco. Targets Ther. 2017, 10, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, Y.P.; Li, J.G.; Chen, G. Clinical feasibility of imaging with indocyanine green combined with carbon nanoparticles for sentinel lymph node identification in papillary thyroid microcarcinoma. Medicine 2019, 98, e16935. [Google Scholar] [CrossRef]
- Liu, J.; Xu, C.; Wang, R.; Han, P.; Zhao, Q.; Li, H.; Bai, Y.; Liu, L.; Zhang, S.; Yao, X. Do carbon nanoparticles really improve thyroid cancer surgery? A retrospective analysis of real-world data. World J. Surg. Oncol. 2020, 18, 84. [Google Scholar] [CrossRef]
- He, J.; Zhang, C.; Zhang, Z.; Xia, F. Evaluation of the clinical value of carbon nanoparticles in endoscopic thyroidectomy and prophylactic central neck dissection through total mammary areolas approach for thyroid cancer. World J. Surg. Oncol. 2021, 19, 320. [Google Scholar] [CrossRef]
- Liu, X.; Chang, S.; Jiang, X.; Huang, P.; Yuan, Z. Identifying Parathyroid Glands With Carbon Nanoparticle Suspension Does Not Help Protect Parathyroid Function in Thyroid Surgery. Surg. Innov. 2016, 23, 381–389. [Google Scholar] [CrossRef]
- Xue, S.; Ren, P.; Wang, P.; Chen, G. Short and Long-Term Potential Role of Carbon Nanoparticles in Total Thyroidectomy with Central Lymph Node Dissection. Sci. Rep. 2018, 8, 11936. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, E.; Giannakodimos, A.; Athanasiadis, D.I.; Chrysikos, D.; Paschou, S.A.; Schizas, D.; Patelis, N.; Papasilekas, T.; Themistoklis, K.; Spartalis, M.; et al. The Potential Role of Carbon Nanoparticles in Lymph Node Tracing, Recurrent Laryngeal Nerve Identification and Parathyroid Preservation During Thyroid Surgery: A Systematic Review. Curr. Pharm. Des. 2020, 27, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Yu, J.; Fan, Y.-X.; Lu, X.-B. Carbon nanoparticle lymph node tracer improves the outcomes of surgical treatment in papillary thyroid cancer. Cancer Biomark. 2018, 23, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Gu, J. The application of carbon nanoparticles in the lymph node biopsy of cN0 papillary thyroid carcinoma: A randomized controlled clinical trial. Asian J. Surg. 2017, 40, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Luo, D.; Diao, F.; Huang, M.; Huang, K.; Peng, X.; Lin, S.; Li, H. A Carbon Nanoparticle Lymphatic Tracer Protected Parathyroid Glands During Radical Thyroidectomy for Papillary Thyroid Non-Microcarcinoma. Surg. Innov. 2017, 24, 29–34. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, L.; Xu, G.; Song, Y.; Li, G.; Zhang, N. Potential role of carbon nanoparticles in protection of parathyroid glands in patients with papillary thyroid cancer. Medicine 2016, 95, e5002. [Google Scholar] [CrossRef]
- Shi, C.; Tian, B.; Li, S.; Shi, T.; Qin, H.; Liu, S. Enhanced identification and functional protective role of carbon nanoparticles on parathyroid in thyroid cancer surgery: A retrospective Chinese population study. Medicine 2016, 95, e5148. [Google Scholar] [CrossRef]
- Wang, B.; Du, Z.-P.; Qiu, N.-C.; Liu, M.-E.; Liu, S.; Jiang, D.-Z.; Zhang, W.; Qiu, M. Application of carbon nanoparticles accelerates the rapid recovery of parathyroid function during thyroid carcinoma surgery with central lymph node dissection: A retrospective cohort study. Int. J. Surg. 2016, 36, 164–169. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, N.; Zhang, W.; Shan, C.; Jiang, Z.; Liu, S.; Qiu, M. The role of carbon nanoparticles in identifying lymph nodes and preserving parathyroid in total endoscopic surgery of thyroid carcinoma. Surg. Endosc. 2015, 29, 2914–2920. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Nie, X.; Wang, W.; Shang, J. Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. Int. J. Clin. Exp. Med. 2015, 8, 9640–9648. [Google Scholar]
- Gao, B.; Tian, W.; Jiang, Y.; Zhang, S.; Guo, L.; Zhao, J.; Zhang, G.; Hao, S.; Xu, Y.; Luo, D. Application of carbon nanoparticles for parathyroid protection in reoperation of thyroid diseases. Int. J. Clin. Exp. Med. 2015, 8, 22254–22261. [Google Scholar]
- Hao, R.; Chen, J.; Zhao, L.; Liu, C.; Wang, O.; Huang, G.; Zhang, X.; Zhao, J. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur. J. Surg. Oncol. EJSO 2012, 38, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Wang, Z.; Pan, C.; Wang, Y.; Lin, Z.; Pan, Z.; Yu, J. Preliminary Study on the Clinical Significance and Methods of Using Carbon Nanoparticles in Endoscopic Papillary Thyroid Cancer Surgery. Contrast Media Mol. Imaging 2021, 2021, 6652315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, G.; Lin, Y.; Zhang, G.; Gao, J. The advantages of carbon nanoparticles in level VII lymph node dissection in patients with papillary thyroid cancer. Gland Surg. 2021, 10, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xia, F.; Zhang, Z.; Cong, R.; Li, X. Preoperative application of carbon nanoparticles in bilateral axillo-breast approach robotic thyroidectomy for papillary thyroid cancer. Gland Surg. 2021, 10, 3188–3199. [Google Scholar] [CrossRef]
- Li, T.; Ma, Z.; Lu, C.; Mu, R.; Wang, H.; Luo, Y.; Lv, J.; Hou, Z.; Zhang, Q.; Cheng, X.; et al. Application of carbon nanoparticles combined with intraoperative neuromonitoring in papillary thyroid microcarcinoma surgery. Am. J. Otolaryngol. 2020, 42, 102790. [Google Scholar] [CrossRef]
- Min, L.; Lang, B.H.H.; Chen, W.; Ai, Q.; Jiang, J.; Huang, Z.H. Utility of Activated Carbon Nanoparticle (CNP) During total Thyroidectomy for Clinically Nodal Positive Papillary Thyroid Carcinoma (PTC). World J. Surg. 2020, 44, 356–362. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhang, D.B.; Zhang, W.F.; Wang, X. Application of Nanocarbon in Breast Approach Endoscopic Thyroidectomy Thyroid Cancer Surgery. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 547–552. [Google Scholar] [CrossRef]
- Xu, Z.; Meng, Y.; Song, J.; Wang, Y.; Yao, X. The role of carbon nanoparticles in guiding central neck dissection and protecting the parathyroid in transoral vestibular endoscopic thyroidectomy for thyroid cancer. Videosurgery Other Miniinvasive Tech. 2020, 14, 455–461. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, Y.; Dionigi, G.; Hu, Y.; Zhang, J.; Wang, T.; Xue, G.; Sun, H. A Randomized Comparison of Carbon Nanoparticles in Endoscopic Lymph Node Dissection Via the Bilateral Areola Approach for Papillary Thyroid Cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2020, 30, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Seifert, P.; Freesmeyer, M. Preoperative diagnostics in differentiated thyroid carcinoma. Nuklearmedizin 2017, 56, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Bakkar, S. Surgical management of papillary thyroid carcinoma: An overview. Updat. Surg. 2017, 69, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.T.; Doherty, G. Central Neck Dissection for Papillary Thyroid Cancer. Cancer Control 2011, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Su, A.-P.; Wang, B.; Gong, Y.-P.; Wu, W.-S.; Gong, R.-X.; Li, Z.-H.; Zhu, J.-Q. Carbon nanoparticles facilitate lymph nodes dissection and parathyroid glands identification in reoperation of papillary thyroid cancer. Medicine 2017, 96, e8380. [Google Scholar] [CrossRef]
- Chao, T.C.; Jeng, L.; Bin, L.J.D.; Chen, M.F. Reoperative thyroid surgery. World J. Surg. 1997, 21, 644–647. [Google Scholar] [CrossRef]
- Koimtzis, G.D.; Stefanopoulos, L.; Giannoulis, K.; Papavramidis, T.S. What are the real rates of temporary hypoparathyroidism following thyroidectomy? It is a matter of definition: A systematic review. Endocrine 2021, 73, 1–7. [Google Scholar] [CrossRef]
- Sadowski, S.M.; Fortuny, J.V.; Triponez, F. A reappraisal of vascular anatomy of the parathyroid gland based on fluorescence techniques. Gland Surg. 2017, 6, S30–S37. [Google Scholar] [CrossRef] [Green Version]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and Biocompatibility of Carbon Nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599. [Google Scholar] [CrossRef]
- Hillary, S.L.; Guillermet, S.; Brown, N.J.; Balasubramanian, S.P. Use of methylene blue and near-infrared fluorescence in thyroid and parathyroid surgery. Langenbecks Arch. Surg. 2018, 403, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Spartalis, E.; Ntokos, G.; Georgiou, K.; Zografos, G.; Tsourouflis, G.; Dimitroulis, D.; Nikiteas, N.I. Intraoperative Indocyanine Green (ICG) Angiography for the Identification of the Parathyroid Glands: Current Evidence and Future Perspectives. In Vivo 2019, 34, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.E. Methylene Blue for Rapid Identification of the Parathyroids. BMJ 1971, 3, 680–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Tian, W.; Xu, Z.; Jiang, K.; Sun, H.; Wang, P.; Huang, T.; Guo, Z.; Zhang, H.; Liu, S.; et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann. Transl. Med. 2015, 3, 230. [Google Scholar] [PubMed]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.-G.; Zhang, S.-Z.; Chen, G. Comparison of Indocyanine Green and Carbon Nanoparticles in Endoscopic Techniques for Central Lymph Nodes Dissection in Patients with Papillary Thyroid Cancer. Surg. Endosc. 2020, 34, 5354–5359. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02074221/full (accessed on 1 June 2022). [CrossRef]
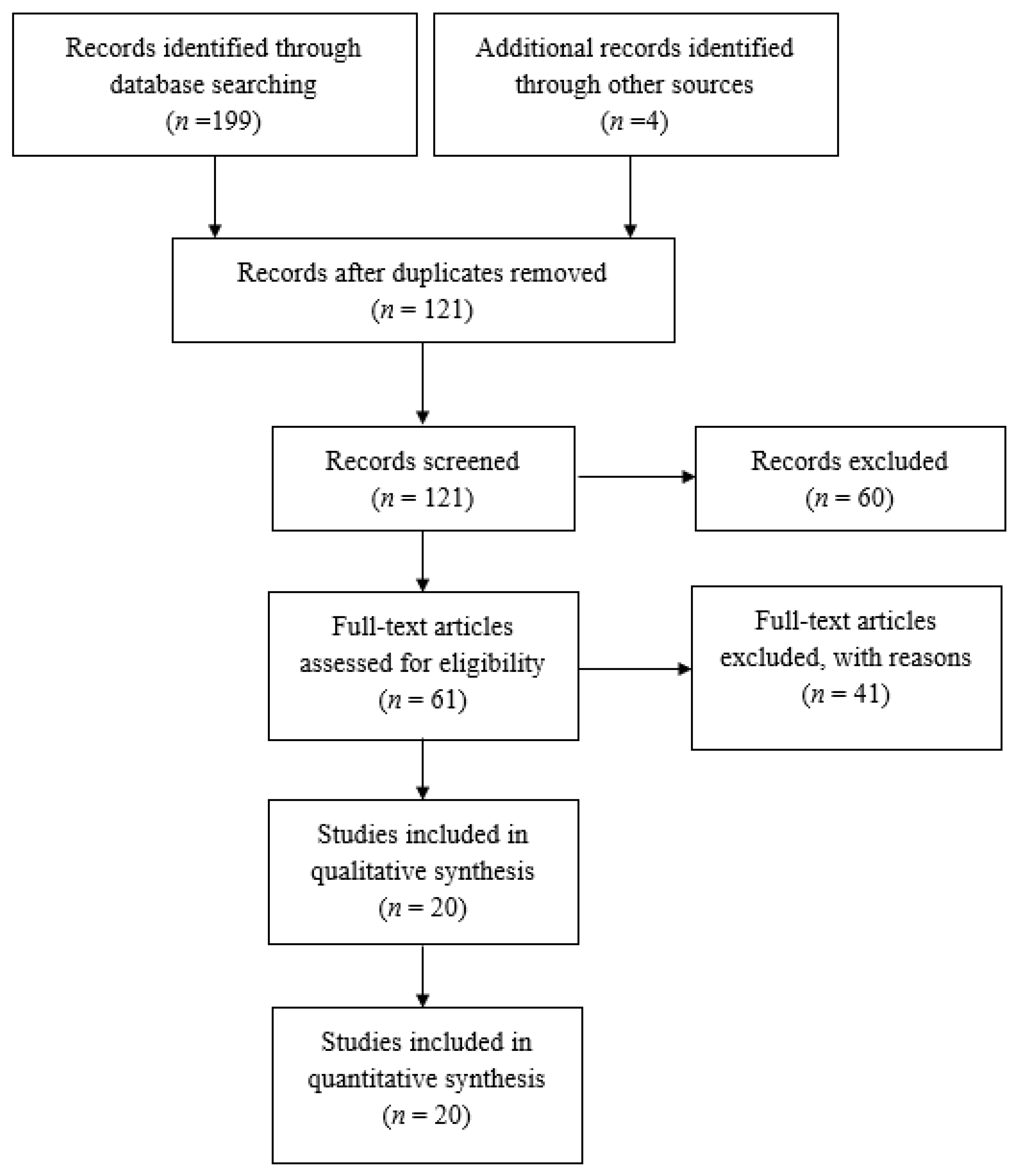
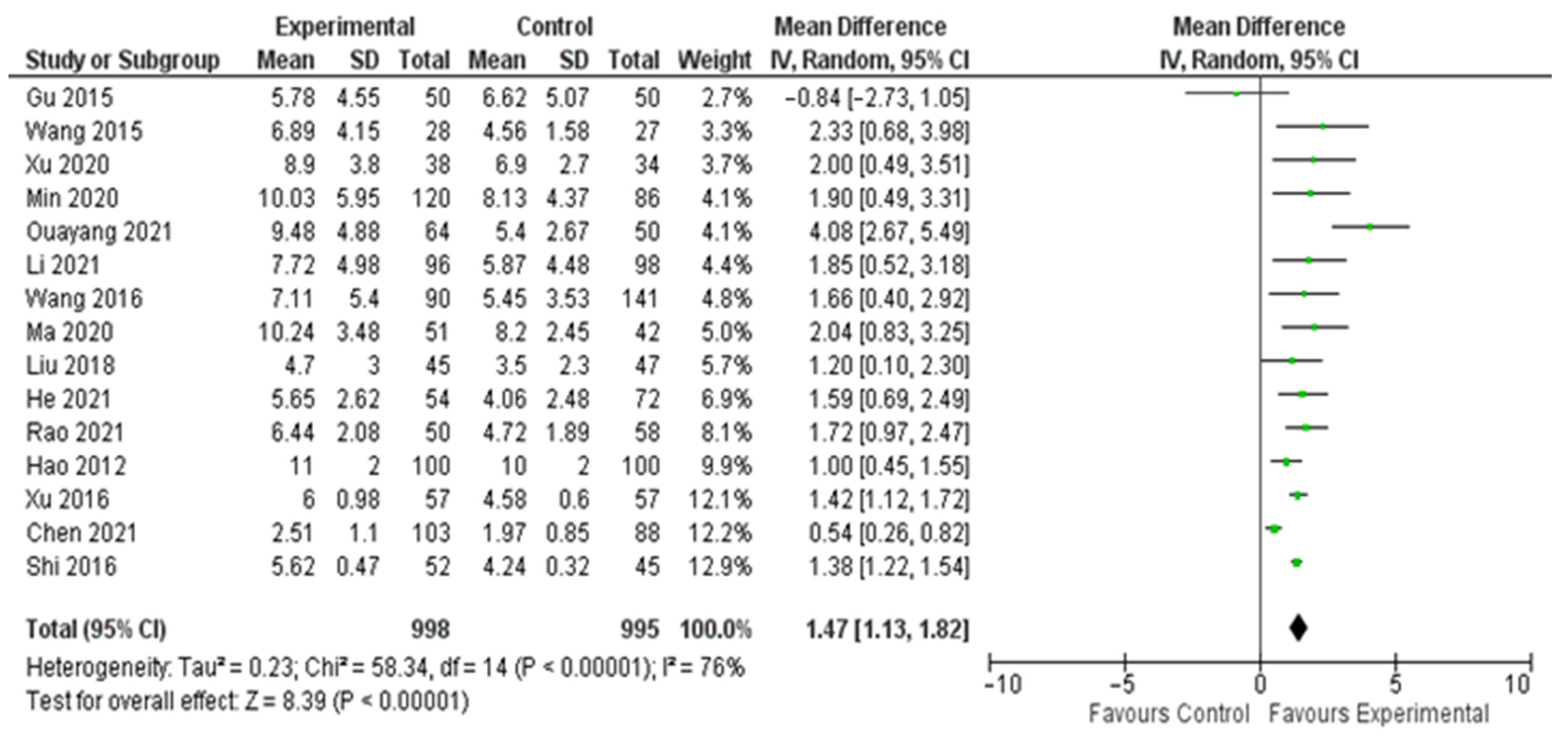
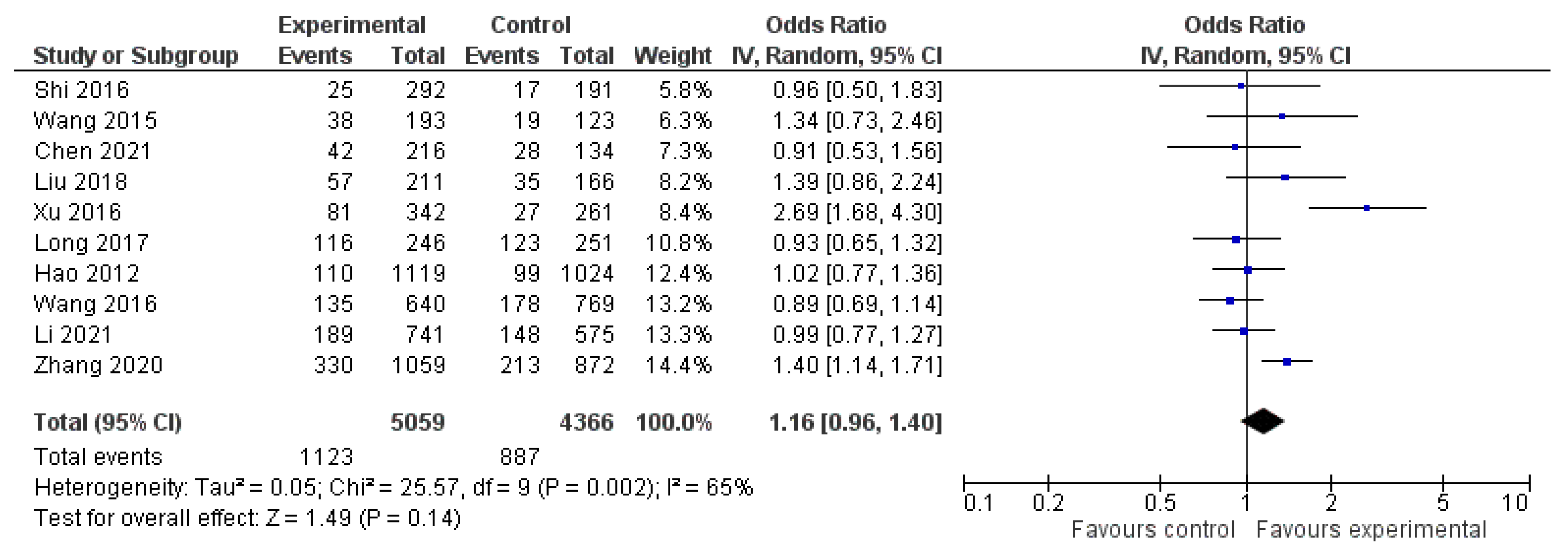

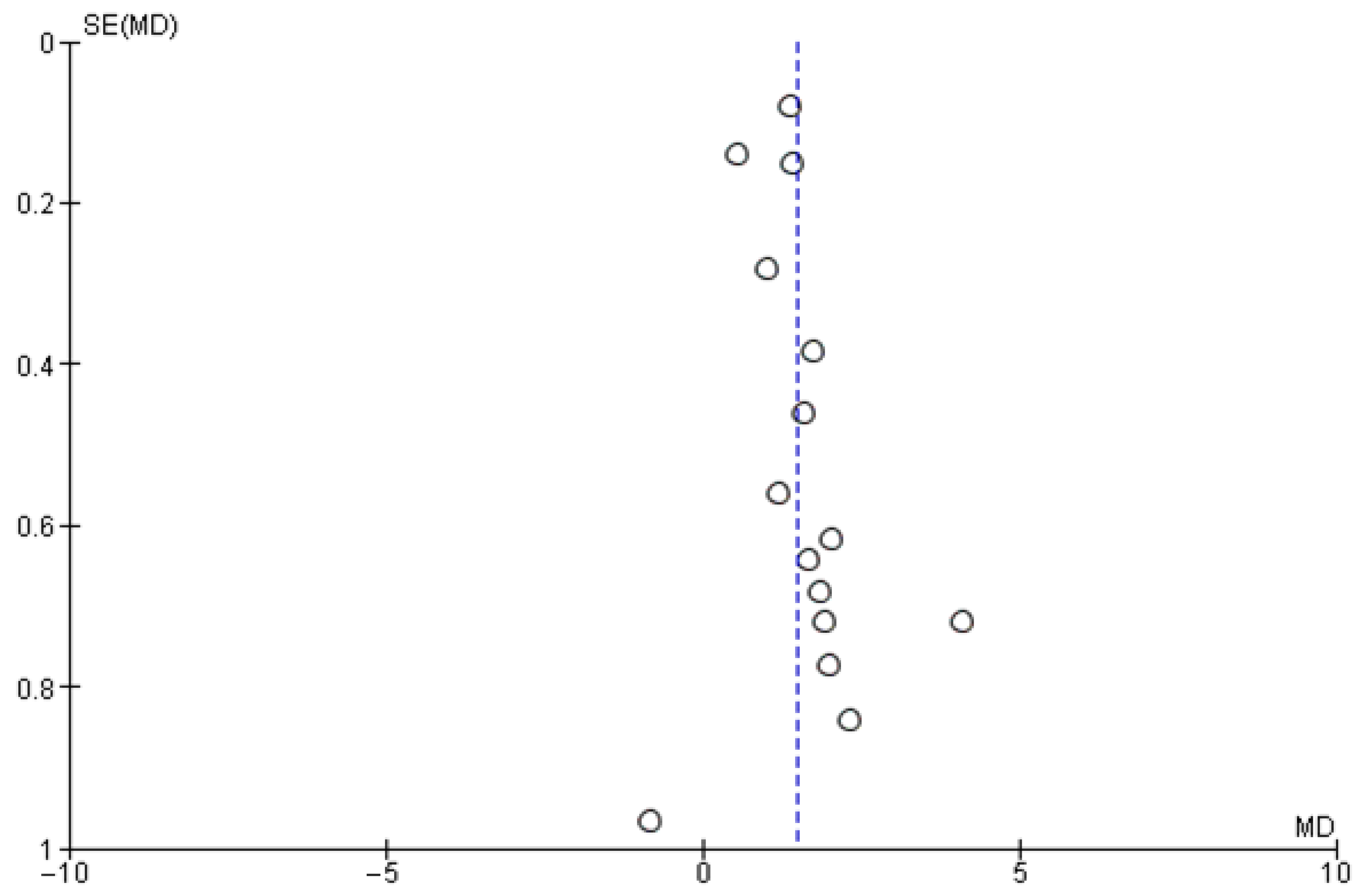

| Study | Study Period | Experimental Group (n, Age) | Control Group (n, Age) | Sex (Male/Female) | Study Design |
|---|---|---|---|---|---|
| Liu, et al. [19] | November 2017 to October 2018 | 334, 44.0 ± 11.7 | 52, 46.6 ± 13.0 | 98/288 | Retrospective |
| He, et al. [20] | January 2018 to December 2020 | 54, 34.7 ± 9.8 | 72, 35.0 ± 9.1 | 4/122 | Retrospective |
| Rao, et al. [24] | January 2015 to April 2019 | 50, 46.8 ± 11.9 | 58, 44.0 ± 10.2 | 25/83 | Randomized, control trial |
| Chen, et al. [25] | September 2019 to December 2020 | 103, 48.57 ± 13.01 | 88, 45.49 ± 13.25 | 46/145 | Randomized, control trial |
| Ouyang, et al. [26] | March 2020 to March 2021 | 64, 34.72 ± 8.79 | 50, 37.24 ± 9.63 | 17/97 | Retrospective |
| Li, et al. [27] | February 2017 to April 2019 | 96, 42.54 ± 12.49 | 98, 43.86 ± 12.35 | 37/157 | Retrospective |
| Min, et al. [28] | 2017 to 2018 | 120, 40.05 ± 12.37 | 86, 39.63 ± 11.22 | 44/162 | Retrospective |
| Ma, et al. [29] | June 2014 to June 2019 | 51, 31.8 ± 7.3 | 42, 30.2 ± 9.2 | 13/80 | Randomized, control trial |
| Xu, et al. [30] | January 2017 to January 2019 | 38, 30.5 ± 7.0 | 34, 32.6 ± 7.2 | 6/66 | Retrospective |
| Zhang, et al. [31] | February 2016 to June 2018 | 152, 33.5 ± 10.02 | 150, 34.1 ± 10.13 | 22/280 | Randomized, control trial |
| Liu, et al. [32] | February 2013 to May 2015 | 45, 46.17 ± 10.20 | 47, 45.39 ± 12.03 | 29/63 | Prospective |
| Xu, et al. [33] | September 2013 to August 2014 | 57, 45.37 ± 10.71 | 57, 42.68 ± 14.43 | 9/105 | Randomized control trial |
| Long, et al. [34] | January 2012 to May 2013 | 49, 44.5 ± 9.6 | 54, 43.8 ± 10.3 | 20/68 | Randomized, control trial |
| Yu, et al. [35] | August 2012 to June 2013 | 41, 41.6 ± 17.1 | 41, 41.7 ± 18.9 | 19/63 | Randomized, control trial |
| Shi, et al. [36] | January 2014 to February 2015 | 52, 45.2 ± 5.8 | 45, 42 ± 4.3 | 12/85 | Not mentioned |
| Wang, et al. [37] | January 2013 to January 2014 | 90, 44.36 ± 11.48 | 141, 44.09 ± 12.41 | 62/169 | Prospective |
| Wang, et al. [38] | March 2013 to March 2014 | 28, 30.25 ± 6.04 | 27, 29.44 ± 6.27 | 3/52 | Randomized, control trial |
| Gu, et al. [39] | June 2012 and August 2014 | 50, 46.98 ± 9.027 | 50, 46.98 ± 9.027 | 16/84 | Randomized, control trial |
| Gao, et al. [40] | January 2012 to December 2014 | 27, 49.4 ± 2.5 | 27, 52.5 ± 1.8 | 4/50 | Randomized control trial |
| Hao, et al. [41] | January 2008 to December 2009 | 100, 41 | 100, 44 | 25/175 | Retrospective |
| Study | Lymph Nodes Harvested (Experimental Group) | Lymph Nodes Harvested (Control Group) | Metastatic/Total Lymph Nodes Harvested (Experimental Group) | Metastatic/Total Lymph Nodes Harvested (Control Group) | Parathyroid Glands Removed (Experimental Group) | Parathyroid Glands Removed (Control Group) |
|---|---|---|---|---|---|---|
| Liu, et al. [19] | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 44/334 | 7/52 |
| He, et al. [20] | 5.65 ± 2.62 | 4.06 ± 2.48 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Rao, et al. [24] | 6.44 ± 2.08 | 4.72 ± 1.89 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Chen, et al. [25] | 2.51 ± 1.10 | 1.97 ± 0.85 | 42/216 | 28/134 | Not mentioned | Not mentioned |
| Ouyang, et al. [26] | 9.48 ± 4.88 | 5.40 ± 2.67 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Li, et al. [27] | 7.72 ± 4.98 | 5.87 ± 4.48 | 189/741 | 148/575 | 3/96 | 10/98 |
| Min, et al. [28] | 10.03 ± 5.95 | 8.13 ± 4.37 | Not mentioned | Not mentioned | 16/120 | 20/86 |
| Ma, et al. [29] | 10.24 ± 3.48 | 8.20 ± 2.45 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Xu, et al. [30] | 8.9 ± 3.8 | 6.9 ± 2.7 | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Zhang, et al. [31] | Not mentioned | Not mentioned | 330/1059 | 213/872 | 2/152 | 9/150 |
| Liu, et al. [32] | 4.7 ± 3.0 | 3.5 ± 2.3 | 57/211 | 35/166 | 3/45 | 10/47 |
| Xu, et al. [33] | 6.00 ± 0.98 | 4.58 ± 0.60 | 81/342 | 27/261 | 2/57 | 7/57 |
| Long, et al. [34] | Not mentioned | Not mentioned | 116/246 | 123/251 | 3/42 | 11/46 |
| Yu, et al. [35] | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 3/41 | 9/41 |
| Shi, et al. [36] | 5.62 ± 0.47 | 4.24 ± 0.32 | 25/292 | 17/191 | 1/52 | 7/45 |
| Wang, et al. [37] | 7.11 ± 5.4 | 5.45 ± 3.53 | 135/640 | 178/769 | Not mentioned | Not mentioned |
| Wang, et al. [38] | 6.89 ± 4.15 | 4.56 ± 1.58 | 38/193 | 19/123 | 0/28 | 5/27 |
| Gu, et al. [39] | 5.78 ± 4.55 | 6.62 ± 5.07 | Not mentioned | Not mentioned | 3/50 | 13/50 |
| Gao, et al. [40] | Not mentioned | Not mentioned | Not mentioned | Not mentioned | 1/27 | 10/27 |
| Hao, et al. [41] | 11 ± 2 | 10 ± 2 | 110/1119 | 99/1024 | 0/100 | 4/100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koimtzis, G.; Stefanopoulos, L.; Alexandrou, V.; Tteralli, N.; Brooker, V.; Alawad, A.A.; Carrington-Windo, E.; Karakasis, N.; Geropoulos, G.; Papavramidis, T. The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4016. https://doi.org/10.3390/cancers14164016
Koimtzis G, Stefanopoulos L, Alexandrou V, Tteralli N, Brooker V, Alawad AA, Carrington-Windo E, Karakasis N, Geropoulos G, Papavramidis T. The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(16):4016. https://doi.org/10.3390/cancers14164016
Chicago/Turabian StyleKoimtzis, Georgios, Leandros Stefanopoulos, Vyron Alexandrou, Nikos Tteralli, Verity Brooker, Awad Ali Alawad, Eliot Carrington-Windo, Nikolaos Karakasis, Georgios Geropoulos, and Theodosios Papavramidis. 2022. "The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 16: 4016. https://doi.org/10.3390/cancers14164016
APA StyleKoimtzis, G., Stefanopoulos, L., Alexandrou, V., Tteralli, N., Brooker, V., Alawad, A. A., Carrington-Windo, E., Karakasis, N., Geropoulos, G., & Papavramidis, T. (2022). The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(16), 4016. https://doi.org/10.3390/cancers14164016






