Simplified Selection Criteria for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
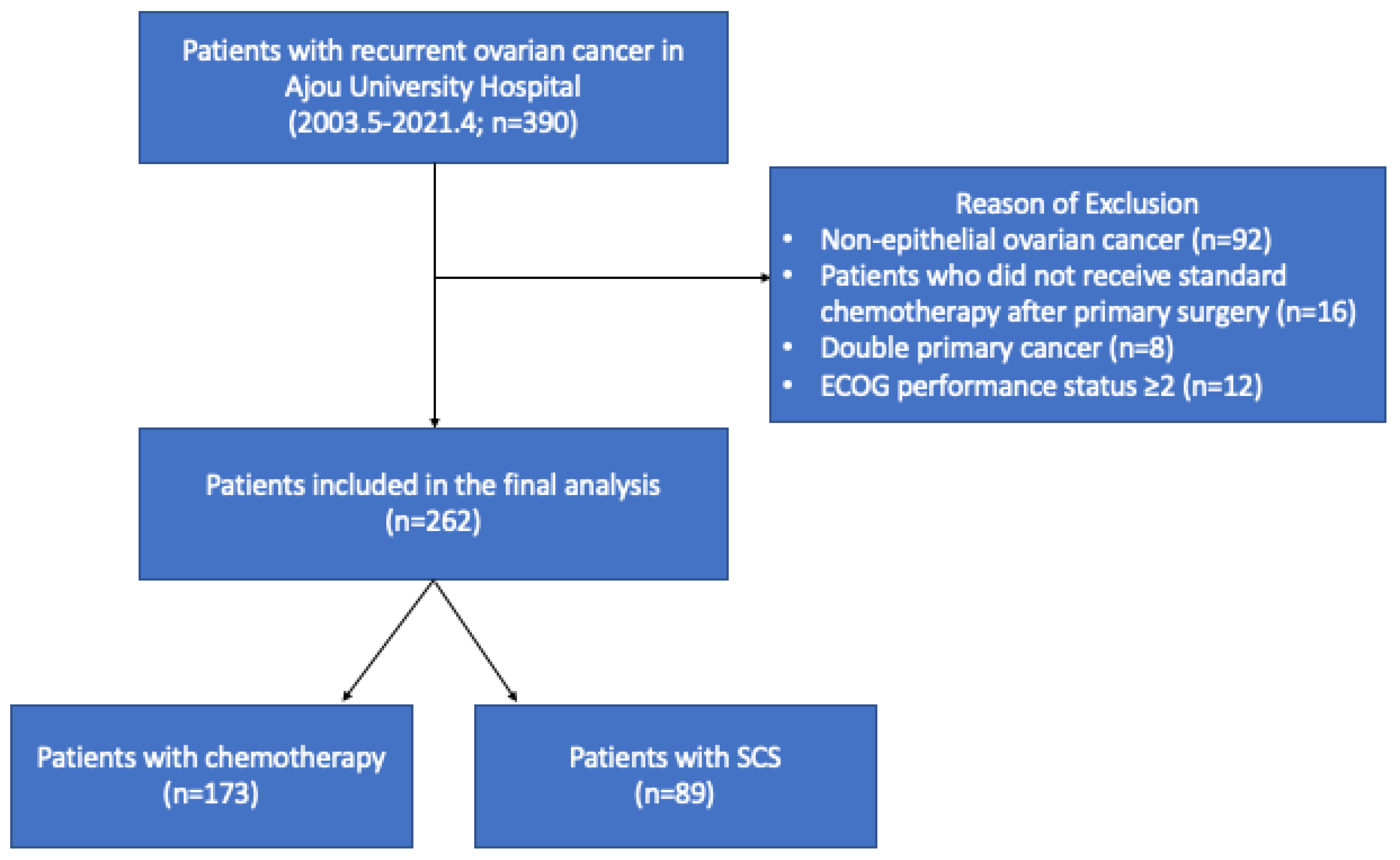
2. Materials and Methods
3. Results
3.1. Patients’ Characteristics
3.2. Comparative Analysis of the Treatment Groups
3.3. Predicting Factors for Complete Resection after SCS
3.4. Analysis with Known Selection Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K. Treatment for recurrent ovarian cancer-at first relapse. J. Oncol. 2010, 2010, 497429. [Google Scholar] [CrossRef] [PubMed]
- Al Rawahi, T.; Lopes, A.D.; Bristow, R.E.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, 2013, CD008765. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, R.; Kruitwagen, R.F.P.M.; IntHout, J.; Zusterzeel, P.L.M.; Van Gorp, T.; Massuger, L.F.A.G. Surgery for Recurrent Epithelial Ovarian Cancer in the Netherlands: A Population-Based Cohort Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2016, 26, 268–275. [Google Scholar] [CrossRef]
- Bristow, R.E.; Puri, I.; Chi, D.S. Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis. Gynecol. Oncol. 2009, 112, 265–274. [Google Scholar] [CrossRef]
- Marchetti, C.; Fagotti, A.; Tombolini, V.; Scambia, G.; De Felice, F. The Role of Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2020, 28, 3258–3263. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Bois, A.D.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Jensen, P.T.; Selle, F.; Guyon, F.; et al. Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: Final analysis of AGO DESKTOP III/ENGOT-ov20. J. Clin. Oncol. 2020, 38, 6000. [Google Scholar] [CrossRef]
- Zang, R.; Zhu, J.; Shi, T.; Liu, J.; Tu, D.; Yin, S.; Jiang, R.; Zhang, P.; Jia, H.; Luan, Y.; et al. A randomized phase III trial of secondary cytoreductive surgery in later recurrent ovarian cancer: SOC1/SGOG-OV2. J. Clin. Oncol. 2020, 38, 6001. [Google Scholar] [CrossRef]
- Ehmann, S.; Zivanovic, O.; Chi, D.S. Why was GOG-0213 a negative trial? J. Gynecol. Oncol. 2021, 32, e19. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; McCaughty, K.; Diaz, J.P.; Huh, J.; Schwabenbauer, S.; Hummer, A.J.; Venkatraman, E.S.; Aghajanian, C.; Sonoda, Y.; Abu-Rustum, N.R.; et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006, 106, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; du Bois, A.; Hahmann, M.; Hasenburg, A.; Burges, A.; Loibl, S.; Gropp, M.; Huober, J.; Fink, D.; Schröder, W.; et al. Surgery in recurrent ovarian cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 2006, 13, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.-J.; Chi, D.S.; Sehouli, J.; Tropé, C.G.; Jiang, R.; Ayhan, A.; Cormio, G.; Xing, Y.; Breitbach, G.-P.; Braicu, E.I.; et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: An evidence-based proposal for patient selection. Ann. Surg. Oncol. 2012, 19, 597–604. [Google Scholar] [CrossRef]
- Cowan, R.A.; Eriksson, A.G.Z.; Jaber, S.M.; Zhou, Q.; Iasonos, A.; Zivanovic, O.; Leitao, M.M., Jr.; Abu-Rustum, N.R.; Chi, D.S.; Gardner, G.J. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2017, 145, 230–235. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Dai, Z.; Deng, F.; Qu, J.; Ni, J. Secondary cytoreduction surgery improves prognosis in platinum-sensitive recurrent ovarian cancer. J. Exp. Clin. Cancer Res. 2013, 32, 61. [Google Scholar] [CrossRef]
- Margul, D.; Coleman, R.L.; Herzog, T.J. The current status of secondary cytoreduction in ovarian cancer: A systematic review. Clin. Adv. Hematol. Oncol. 2020, 18, 332–343. [Google Scholar]
- Harter, P.; Sehouli, J.; Reuss, A.; Hasenburg, A.; Scambia, G.; Cibula, D.; Mahner, S.; Vergote, I.; Reinthaller, A.; Burges, A.; et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: The Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int. J. Gynecol. Cancer 2011, 21, 289–295. [Google Scholar] [CrossRef]
- Fanfani, F.; Monterossi, G.; Fagotti, A.; Gallotta, V.; Costantini, B.; Vizzielli, G.; Petrillo, M.; Carbone, M.V.; Scambia, G. Positron emission tomography-laparoscopy based method in the prediction of complete cytoreduction in platinum-sensitive recurrent ovarian cancer. Ann. Surg. Oncol. 2015, 22, 649–654. [Google Scholar] [CrossRef]
- Bogani, G.; Rossetti, D.; Ditto, A.; Martinelli, F.; Chiappa, V.; Mosca, L.; Maggiore, U.L.R.; Ferla, S.; Lorusso, D.; Raspagliesi, F. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J. Gynecol. Oncol. 2018, 29, e66. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
| Age, years | 53 (20–80) |
| Initial FIGO stage | |
| stage I–II | 33 (12.6%) |
| stage III–IV | 229 (87.4%) |
| Type of primary surgery | |
| PDS | 189 (72.1%) |
| IDS | 73 (27.9%) |
| Residual disease status | |
| NGR | 131 (50.0%) |
| GR-1 | 71 (27.1%) |
| GR-B | 58 (22.1%) |
| Unknown | 2 (0.8%) |
| Histology | |
| Serous | 224 (85.5%) |
| Non-serous | 38 (14.5%) |
| BRCA mutation | |
| BRCA1 mutation | 12 (4.6%) |
| BRCA2 mutation | 4 (1.5%) |
| VUS | 15 (5.7%) |
| Normal | 66 (25.2%) |
| Unknown (not performed) | 165 (63.0%) |
| Characteristics of recurrence | |
| CA-125 (U/mL) | 69.3 (1.4–5770) |
| Limited carcinomatosis | 45 (17.2%) |
| Ascites | 30 (11.5%) |
| Extra-abdominal disease | 73 (27.8%) |
| Chest | 25 (9.5%) |
| Brain | 6 (2.3%) |
| Bone | 4 (1.5%) |
| Extra-abdominal LNs | 33 (12.6%) |
| Abdominal wall | 5 (1.9%) |
| Multiple lesions or diffuse carcinomatosis | 162 (61.8%) |
| Treatment for recurrent disease | |
| SCS | 89 (34.0%) |
| Chemotherapy | 173 (66.0%) |
| PFS, months | 15 (13.7–16.2) |
| OS, months | 53.0 (45.2–60.7) |
| Chemotherapy (n = 173) | SCS (n = 89) | p-Value | |
|---|---|---|---|
| Age, years | 55 (25–80) | 50 (20–78) | 0.001 |
| Initial FIGO stage | 0.001 | ||
| stage I–II | 12 (6.9%) | 21 (23.6%) | |
| stage III–IV | 161 (93.1%) | 68 (76.4%) | |
| Residual disease at primary surgery | 0.001 | ||
| NGR | 66 (38.2%) | 65 (73%) | |
| GR-1 | 54 (31.2%) | 17 (19.1%) | |
| GR-B | 52 (30.1%) | 6 (6.7%) | |
| Unknown | 1 (0.6%) | 1 (1.1%) | |
| Histology | 0.462 | ||
| Serous | 150 (86.7%) | 74 (83.1%) | |
| Non-serous | 23 (13.3%) | 15 (16.9%) | |
| BRCA mutation | 0.001 | ||
| BRCA1 mutation | 2 (1.2%) | 10 (11.2%) | |
| BRCA2 mutation | 1 (0.6%) | 3 (3.4%) | |
| VUS | 11 (6.4%) | 4 (4.5%) | |
| Wild type | 35 (20.3%) | 31 (34.8%) | |
| Unknown | 123 (71.5%) | 41 (46.1%) | |
| Characteristics of recurrence | |||
| Limited carcinomatosis | 11 (6.9%) | 34 (39.1%) | 0.001 |
| Ascites | 25 (14.5%) | 5 (5.6%) | 0.04 |
| Extra-abdominal disease | 59 (34.1%) | 23 (25.8%) | 0.172 |
| CA-125 (U/mL) | 114.7 (1.4–5770) | 39.1 (1.4–2998.5) | 0.108 |
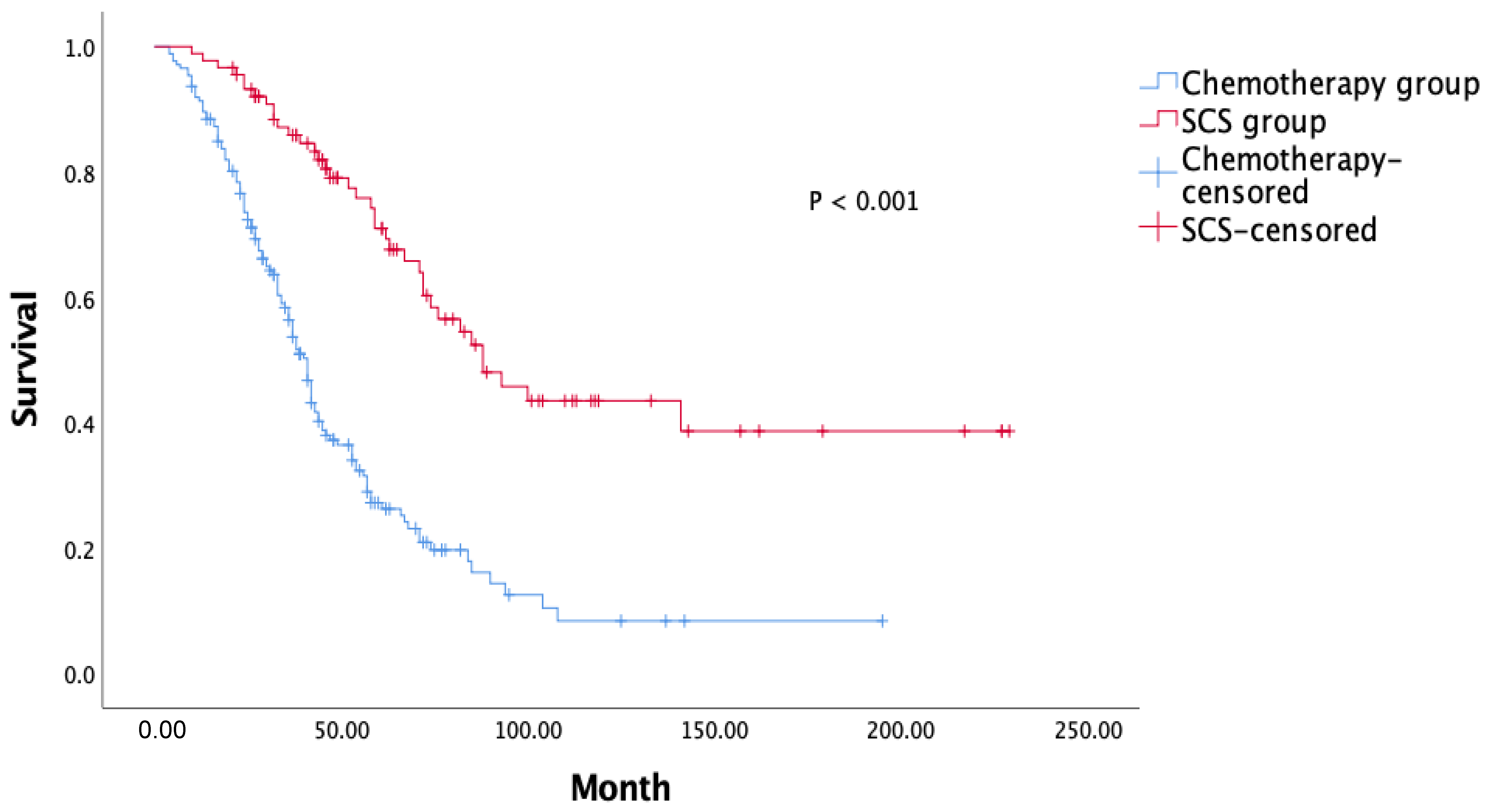
| PFS, months | 14 (12.6–15.3) | 19 (16.5–21.4) | 0.001 |
| OS, months | 41 (37.4–44.5) | 88 (64.2–111.7) | 0.001 |
| Adjusted HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.021 | 0.967–1.078 | 0.447 |
| FIGO stage (Stage I/II vs. III/IV) | 0.412 | 0.116–1.462 | 0.17 |
| Any residual disease at the time of primary surgery | 0.442 | 0.120–1.626 | 0.219 |
| PFS > 12 month | 0.559 | 0.168–1.843 | 0.337 |
| BRCA mutation | 0.325 | 0.036–2.952 | 0.318 |
| Limited regional recurrence | 0.259 | 0.069–0.968 | 0.045 |
| Ascites before SCS | 2.169 | 0.300–15.708 | 0.443 |
| Extra-abdominal disease | 0.675 | 0.184–2.477 | 0.554 |
| Inclusion Rate (n, %) | Complete Resection Rate (n, %) | |
|---|---|---|
| AGO criteria | 63 (70.8%) | 48 (76.2%) |
| MSKCC | 59 (66.3%) | 44 (74.6%) |
| Tian criteria (low risk) | 82 (92.1%) | 64 (78%) |
| Ajou criteria | ||
| PFS > 12 + limited regional recurrence | 66 (74.1%) | 52 (78.8%) |
| PFS > 6 + limited regional recurrence | 89 (100%) | 78 (87.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.-H.; Lee, J.; Yum, S.-H.; Kim, J.; Kong, T.-W.; Chang, S.-J.; Ryu, H.-S. Simplified Selection Criteria for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer. Cancers 2022, 14, 3987. https://doi.org/10.3390/cancers14163987
Son J-H, Lee J, Yum S-H, Kim J, Kong T-W, Chang S-J, Ryu H-S. Simplified Selection Criteria for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer. Cancers. 2022; 14(16):3987. https://doi.org/10.3390/cancers14163987
Chicago/Turabian StyleSon, Joo-Hyuk, Jimin Lee, Sun-Hyung Yum, Jeeyeon Kim, Tae-Wook Kong, Suk-Joon Chang, and Hee-Sug Ryu. 2022. "Simplified Selection Criteria for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer" Cancers 14, no. 16: 3987. https://doi.org/10.3390/cancers14163987
APA StyleSon, J.-H., Lee, J., Yum, S.-H., Kim, J., Kong, T.-W., Chang, S.-J., & Ryu, H.-S. (2022). Simplified Selection Criteria for Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer. Cancers, 14(16), 3987. https://doi.org/10.3390/cancers14163987






