Neurotransmitters: Potential Targets in Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
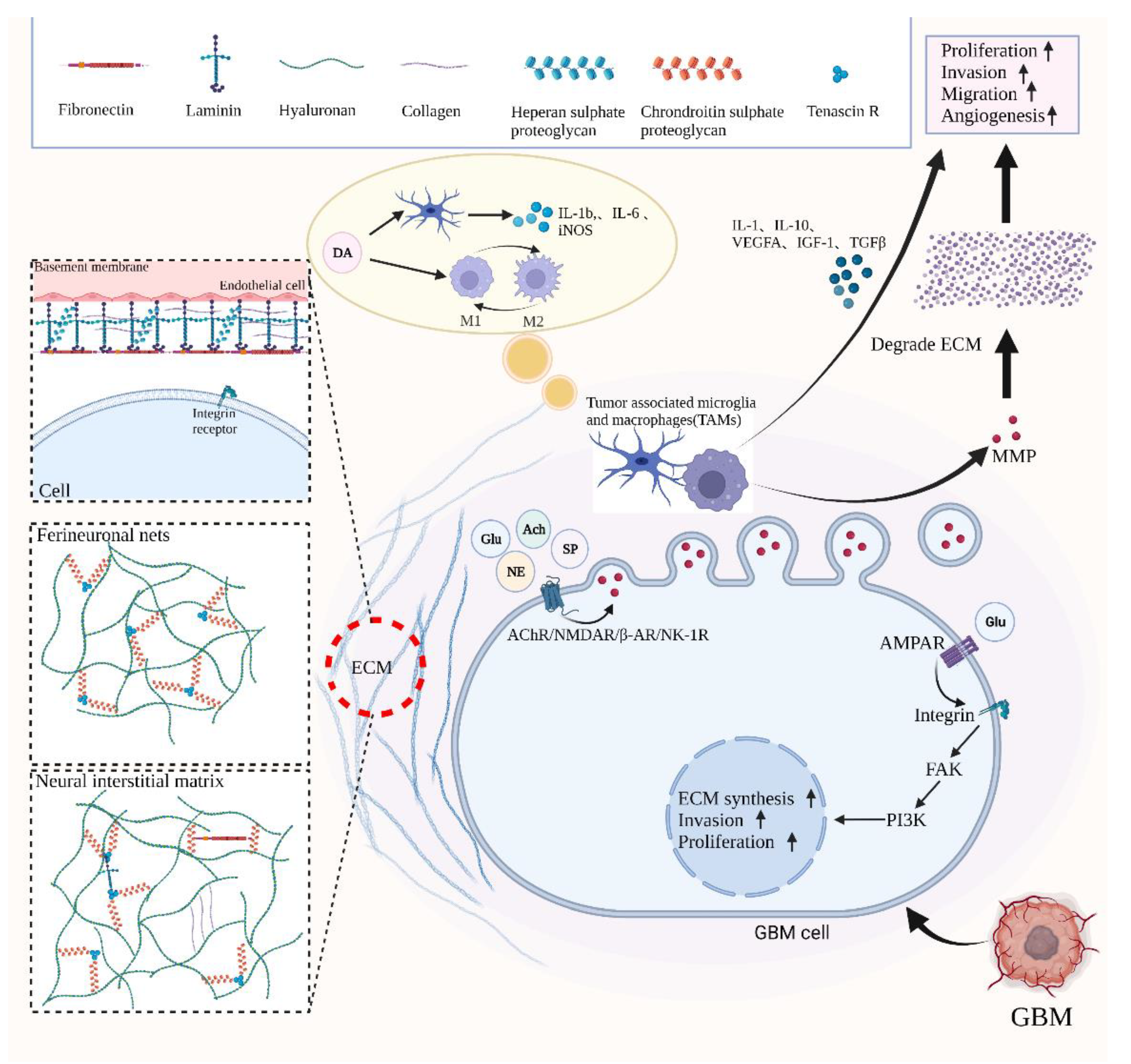
2. Neurotransmitters Function as Necessary Components of Neural Microenvironment and GBM Microenvironment
3. Neurotransmitters and Their Activation on Intracellular Signaling of GBM
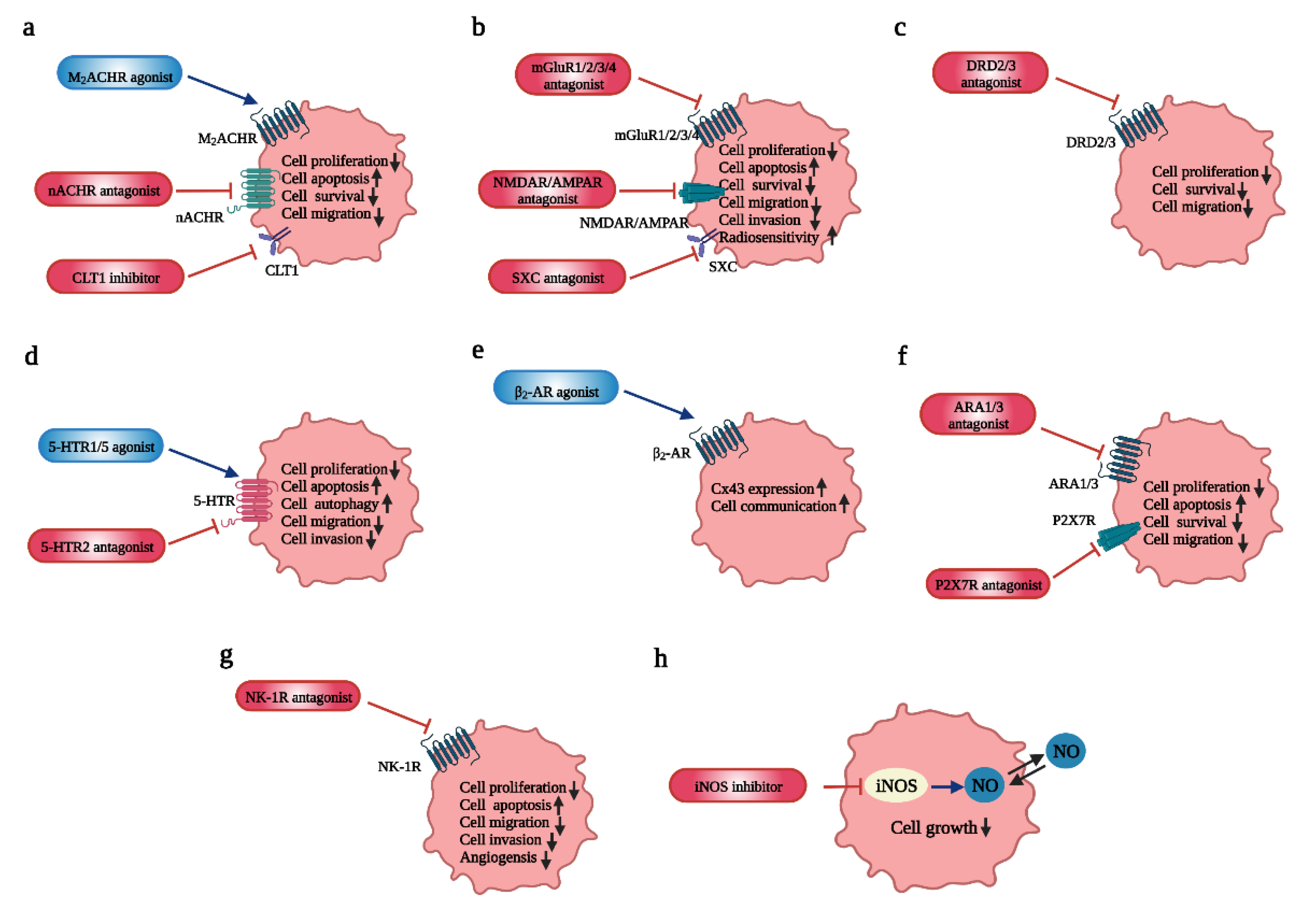
4. The Influence of Neurotransmitters on GBM and Potential Therapeutic Targets
| Drugs | Targets | Approved by FDA? | Effects | Type of Study | References |
|---|---|---|---|---|---|
| Basic Studies | |||||
| Arecaidine propargyl ester (APE) | M2 mAChR agonist | NO |
| in vitro (GBM line U251MG and GBM line U87MG, GSC line GB7 and GSC line GB8) | [134,135] |
| Iper-8-naphthalimide(N-8-Iper) | M2 mAChR agonist | NO |
| in vitro (GSC line GB7); in vitro (GSC line GB7 and GSC line G166) | [31,136] |
| Iper-6-phthalimide(P-6-Iper) | M2 mAChR agonist | NO | no appreciable effects on GSCs growth | in vitro (GSC line GB7) | [31] |
| Atracurium Besylate | nAChR antagonist | YES |
| in vitro (GSC line HSR-GBM1, GSC line HSR040622 and GSC line HSR040821) | [32] |
| StN-2(MG624) | α7 and α9 nAChR antagonist | NO |
| in vitro (GBM cell line U87) | [137] |
| StN-4 | α7 and α9/10 nAChR antagonist (silent agonist) | NO |
| in vitro (human U87MG and GBM5 cells) | [138] |
| StN-8 | α7 and α9/10 nAChR antagonist | NO |
| in vitro (human U87MG and GBM5 cells) | [138] |
| 3-(2,4-dimethoxybenzylidene) anabaseine (GTS-21) | α7 nAChR antagonist | NO | inhibit cell proliferation in a dose- and time-dependent manner in an α7-nAChR-dependent/α7-nAChR-independent manner | in vitro (GBM cell line A172, U87 and G28, and patient-derived glioblastoma cells) | [35] |
| Kynurenic acid (KYNA) | α7 nAChR and all iGluR antagonist | NO |
| in vitro (GBM cell line T98G) | [139] |
| Amb4269951 | Choline Transporter-Like Protein 1(CTL1) inhibitor | NO |
| in vitro (GBM cell line U251MG) and in vivo (mice xenograft models) | [126] |
| Dizocilpine (MK801) | NMDAR (N-methyl-D-aspartate receptor) antagonist | NO |
| in vitro (GBM cell line LN18, GBM cell line U251MG and patient-derived glioblastoma cells) | [140] |
| Memantine | NMDAR antagonist | YES | induce cytotoxicity of GBM in a dose-dependent fashion | in vitro (human GBM cell line T98G and U87MG) | [141] |
| MP1-MP2 | NMDAR antagonist | NO | induce significant cell death by apoptosis | in vitro (human GBM cell line U87MG) | [142] |
| Ifenprodil | NMDAR GluN2B antagonist | YES | more potent than MK801 in inhibiting cell migration and survival, as well as in the sensitivity to radiation | in vitro (cell lines) | [40] |
| Perampanel (PER) | AMPAR antagonist | YES |
| in vitro (GBM cell line U87, U138 and A172; GBM cell line HROG02, HROG05, HROG15 and HROG24) | [143,144] |
| GYKI 52466 | AMPAR GluR1 antagonist | NO | diminish cell death induced by cancer in peritumor brain | in vitro (rat F98 glioma-implanted brain slice cultures) | [145] |
| Fluoxetine | AMPAR GluR1 antagonist | YES |
| in vitro (GBM cell line U87 and Hs683 and rat glioma cell line C6) and in vivo (experimental animal models) | [41] |
| Riluzole | mGluR1 antagonist | YES |
| in vitro (GBM cell line U87, patient-derived glioblastoma cells) and in vivo (U87 cell xenograft model) | [127,146] |
| BAY36-7620 | mGluR1 antagonist | NO |
| in vitro (GBM cell line U87) and in vivo (U87 cell xenograft model) | [127] |
| CPCCOEt | mGluR1 antagonist | NO |
| in vitro (GBM cell line A172 and U87) | [147] |
| JNJ16259685 | mGluR1 antagonist | NO |
| in vitro (GBM cell line A172 and U87) | [147] |
| (2S)-α-ethylglutamate (EGlu) | mGluR2/3 antagonist | NO | cell proliferation inhibition in a time-dependent fashion | in vitro (GBM cell line U87) | [119] |
| MTPG | mGluR2/3 antagonist | NO | cell proliferation inhibition in a time-dependent fashion | in vitro (GBM cell line U87) | [119] |
| LY341495 | mGluR2/3 antagonist | NO |
| in vitro (GBM cell line U87) | [119] |
| Sulfasalazine (SSZ) | xc−cystine/glutamate transporter antagonist | YES |
| in vitro (rat glioma cell F98, human glioma cell U251, primary astrocytes and neurons, and rat organotypic brain slices) | [148] |
| VU0155041 | mGluR4 antagonist | NO |
| in vitro (GBM cell line LN229) | [46] |
| Quetiapine | DRD2 antagonist | YES |
| in vitro (patient-derived HK-157, HK-308, HK-374, and HK-382 GBM lines) and in vivo (the GL261 orthotopic mouse models of GBM, and HK-374 patient-derived orthotopic xenografts) | [149] |
| Perphenazine | DRD2/3 and 5-HT antagonist | NO |
| in vivo | [150] |
| Valerenic acid | HTR5A agonist | NO |
| in vitro (U251 MG human glioblastoma cell lines) | [60] |
| Brexpiprazole | DRD2 and 5-HT1A agonist, 5HT2A antagonist | YES | increase the sensitivity of GSCs to osimertinib through the downregulation of survivin expression | in vitro (GSCs:A172GS, GS-Y01, GS-NCC01, and GS-Y) | [151,152] |
| Clenbuterol hydrochloride | β2-AR agonist | NO | upregulate Cx43 expression that promotes cell communication | in vitro (OECs) | [153] |
| AZ10606120 | P2X7R antagonist | NO |
| in vitro (U251 MG cells) | [154,155] |
| Paris saponin H (PSH) | ARA1 and ARA3 inhibitor | NO |
| in vitro (U251 cells) | [156] |
| 1400W, S-MIU | iNOS inhibitor | NO | inhibit the cell growth of astrocytes and U87 cell line with EGFRvIII expression | in vivo (U87 cell line, rat model) | [157,158] |
| Clinical Studies | |||||
| Memantine | NMDAR antagonist | YES | in safe combinations with TMZ in newly diagnosed GBM with a 21-month median survival and a 2-year survival rate of 43% | Phase I, randomized, (newly diagnosed, n = 85) | [159] |
| Talampanel (LY300164) | AMPAR antagonist | NO |
| Phase II, single-agent (recurrent, n = 22) Phase II, single-arm (newly diagnosed, n = 72) Phase II, randomized, (newly diagnosed, n = 365) | [160,161,162] |
| ONC201 | DRD2 antagonist | NO |
| in vitro (U87MG, U251MG, U373MG, Hs683, SF-295, A172 and LN-18 cell lines) and in vivo (patient-derived GBM cell lines and orthotopic xenograft mouse models) Phase II, non-Randomized, (recurrent, n = 140) | [163,164,165] |
| Chlorpromazine | DRD2 antagonist | YES |
| in vitro (cell lines T98G, U-251 MG and U-87 MG) Phase II, recruiting, (newly diagnosed, n = 41) | [166] |
| Escitalopram oxalate | SSRI (selective serotonin reuptake inhibitors) | YES |
| in vitro (U87MG cells GBM8401 cells) Phase II, Randomized, (newly diagnosed, n = 100) | [167] |
4.1. Acetylcholine (ACh): Regulates GBM Proliferation, Survival and Invasion
4.2. Glutamate (Glu): Modulates GBM Growth and Progression, as Well as Increases Radiosensitivity
4.3. Gamma-Aminobutyric Acid (GABA): Inhibits GBM Growth
4.4. Dopamine: Inhibits GBM Invasiveness and Migration
4.5. Serotonin (5-HT): Involved in Cell Apoptosis, Autophagy and Chemotherapy Reinforcement
4.6. Norepinephrine/Noradrenaline (NE/NA): Inhibits GBM Invasiveness and Migration, as Well as Modulates Tumorigenesis
4.7. Purinergic: Involved in Tumorigenesis, GBM Invasion, Migration
4.8. Neuropeptide
4.8.1. Neuropeptide Y (NPY): Stimulates Tumor Growth and Progression
4.8.2. Substance P (SP): Induces GBM Proliferation, Invasiveness and Migration
4.9. NO: Involved in GBM Proliferation and Migration and Cell Apoptosis
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R.; et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv. Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wardak, Z.; Choe, K.S. Molecular pathways and potential therapeutic targets in glioblastoma multiforme. Expert Rev. Antican. 2013, 13, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Linz, U. Commentary on Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet Oncol. 2009;10:459–466). Cancer 2010, 116, 1844–1846. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Radin, D.P.; Tsirka, S.E. Interactions between Tumor Cells, Neurons, and Microglia in the Glioma Microenvironment. Int. J. Mol. Sci. 2020, 21, 8476. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Johung, T.; Monje, M. Neuronal activity in the glioma microenvironment. Curr. Opin. Neurobiol. 2017, 47, 156–161. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Monje, M.; Borniger, J.C.; D’Silva, N.J.; Deneen, B.; Dirks, P.B.; Fattahi, F.; Frenette, P.S.; Garzia, L.; Gutmann, D.H.; Hanahan, D.; et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell 2020, 181, 219–222. [Google Scholar] [CrossRef]
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Jung, E.; Alfonso, J.; Osswald, M.; Monyer, H.; Wick, W.; Winkler, F. Emerging intersections between neuroscience and glioma biology. Nat. Neurosci. 2019, 22, 1951–1960. [Google Scholar] [CrossRef]
- Cristofaro, I.; Alessandrini, F.; Spinello, Z.; Guerriero, C.; Fiore, M.; Caffarelli, E.; Laneve, P.; Dini, L.; Conti, L.; Tata, A.M. Cross Interaction between M2 Muscarinic Receptor and Notch1/EGFR Pathway in Human Glioblastoma Cancer Stem Cells: Effects on Cell Cycle Progression and Survival. Cells 2020, 9, 657. [Google Scholar] [CrossRef]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef]
- Guan, X.; Hasan, M.N.; Maniar, S.; Jia, W.; Sun, D. Reactive Astrocytes in Glioblastoma Multiforme. Mol. Neurobiol. 2018, 55, 6927–6938. [Google Scholar] [CrossRef]
- Wegrzyn, D.; Freund, N.; Faissner, A.; Juckel, G. Poly I:C Activated Microglia Disrupt Perineuronal Nets and Modulate Synaptic Balance in Primary Hippocampal Neurons in vitro. Front. Synaptic Neurosci. 2021, 13, 637549. [Google Scholar] [CrossRef]
- Geribaldi-Doldan, N.; Hervas-Corpion, I.; Gomez-Oliva, R.; Dominguez-Garcia, S.; Ruiz, F.A.; Iglesias-Lozano, I.; Carrascal, L.; Pardillo-Diaz, R.; Gil-Salu, J.L.; Nunez-Abades, P.; et al. Targeting Protein Kinase C in Glioblastoma Treatment. Biomedicines 2021, 9, 381. [Google Scholar] [CrossRef]
- Zarco, N.; Norton, E.; Quinones-Hinojosa, A.; Guerrero-Cazares, H. Overlapping migratory mechanisms between neural progenitor cells and brain tumor stem cells. Cell Mol. Life Sci. 2019, 76, 3553–3570. [Google Scholar] [CrossRef]
- Di Liberto, V.; Borroto-Escuela, D.O.; Frinchi, M.; Verdi, V.; Fuxe, K.; Belluardo, N.; Mudò, G. Existence of muscarinic acetylcholine receptor (mAChR) and fibroblast growth factor receptor (FGFR) heteroreceptor complexes and their enhancement of neurite outgrowth in neural hippocampal cultures. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 235–245. [Google Scholar] [CrossRef]
- Ramos Costa, A.P.; Levone, B.R.; Gururajan, A.; Moloney, G.; Hoeller, A.A.; Lino-de-Oliveira, C.; Dinan, T.G.; O’Leary, O.F.; Monteiro de Lima, T.C.; Cryan, J.F. Enduring effects of muscarinic receptor activation on adult hippocampal neurogenesis, microRNA expression and behaviour. Behav. Brain Res. 2019, 362, 188–198. [Google Scholar] [CrossRef]
- Takahashi, T. Multiple Roles for Cholinergic Signaling from the Perspective of Stem Cell Function. Int. J. Mol. Sci. 2021, 22, 666. [Google Scholar] [CrossRef]
- Jansson, L.C.; Åkerman, K.E. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural Transm. 2014, 121, 819–836. [Google Scholar] [CrossRef]
- Malva, J.O.; Xapelli, S.; Baptista, S.; Valero, J.; Agasse, F.; Ferreira, R.; Silva, A.P. Multifaces of neuropeptide Y in the brain--neuroprotection, neurogenesis and neuroinflammation. Neuropeptides 2012, 46, 299–308. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Haydar, T.F.; Bordey, A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005, 8, 1179–1187. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef]
- Thompson, E.G.; Sontheimer, H. Acetylcholine Receptor Activation as a Modulator of Glioblastoma Invasion. Cells 2019, 8, 1203. [Google Scholar] [CrossRef]
- Ferretti, M.; Fabbiano, C.; Di Bari, M.; Ponti, D.; Calogero, A.; Tata, A.M. M2 muscarinic receptors inhibit cell proliferation in human glioblastoma cell lines. Life Sci. 2012, 91, 1134–1137. [Google Scholar] [CrossRef]
- Ferretti, M.; Fabbiano, C.; Di Bari, M.; Conte, C.; Castigli, E.; Sciaccaluga, M.; Ponti, D.; Ruggieri, P.; Raco, A.; Ricordy, R.; et al. M2 receptor activation inhibits cell cycle progression and survival in human glioblastoma cells. J. Cell. Mol. Med. 2013, 17, 552–566. [Google Scholar] [CrossRef]
- Alessandrini, F.; Cristofaro, I.; Di Bari, M.; Zasso, J.; Conti, L.; Tata, A.M. The activation of M2 muscarinic receptor inhibits cell growth and survival in human glioblastoma cancer stem cells. Int. Immunopharmacol. 2015, 29, 105–109. [Google Scholar] [CrossRef]
- Cristofaro, I.; Spinello, Z.; Matera, C.; Fiore, M.; Conti, L.; De Amici, M.; Dallanoce, C.; Tata, A.M. Activation of M2 muscarinic acetylcholine receptors by a hybrid agonist enhances cytotoxic effects in GB7 glioblastoma cancer stem cells. Neurochem. Int. 2018, 118, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Spina, R.; Voss, D.M.; Asnaghi, L.; Sloan, A.; Bar, E.E. Atracurium Besylate and other neuromuscular blocking agents promote astroglial differentiation and deplete glioblastoma stem cells. Oncotarget 2016, 7, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Jameson, M.J.; Broaddus, W.C.; Lin, P.S.; Chung, T.D. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2013, 30, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Pucci, S.; Fasoli, F.; Moretti, M.; Benfante, R.; Di Lascio, S.; Viani, P.; Daga, A.; Gordon, T.J.; McIntosh, M.; Zoli, M.; et al. Choline and nicotine increase glioblastoma cell proliferation by binding and activating alpha 7-and alpha 9-containing nicotinic receptors. Pharmacol. Res. 2021, 163, 12. [Google Scholar] [CrossRef]
- Kolodziej, M.A.; Gott, H.; Kopischke, B.; Bender, M.K.F.; Weigand, M.A.; Di Fazio, P.; Schwarm, F.P.; Uhle, F. Antiproliferative effect of GTS-21 in glioblastoma cells. Oncol. Lett. 2021, 22, 759. [Google Scholar] [CrossRef]
- Piovesana, R.; Salazar Intriago, M.S.; Dini, L.; Tata, A.M. Cholinergic Modulation of Neuroinflammation: Focus on α7 Nicotinic Receptor. Int. J. Mol. Sci. 2021, 22, 4912. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Prickett, T.D.; Samuels, Y. Molecular pathways: Dysregulated glutamatergic signaling pathways in cancer. Clin. Cancer Res. 2012, 18, 4240–4246. [Google Scholar] [CrossRef]
- Yohay, K.; Tyler, B.; Weaver, K.D.; Pardo, A.C.; Gincel, D.; Blakeley, J.; Brem, H.; Rothstein, J.D. Efficacy of local polymer-based and systemic delivery of the anti-glutamatergic agents riluzole and memantine in rat glioma models. J. Neurosurg. 2014, 120, 854–863. [Google Scholar] [CrossRef]
- Müller-Längle, A.; Lutz, H.; Hehlgans, S.; Rödel, F.; Rau, K.; Laube, B. NMDA Receptor-Mediated Signaling Pathways Enhance Radiation Resistance, Survival and Migration in Glioblastoma Cells-A Potential Target for Adjuvant Radiotherapy. Cancers 2019, 11, 503. [Google Scholar] [CrossRef]
- Liu, K.H.; Yang, S.T.; Lin, Y.K.; Lin, J.W.; Lee, Y.H.; Wang, J.Y.; Hu, C.J.; Lin, E.Y.; Chen, S.M.; Then, C.K.; et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget 2015, 6, 5088–5101. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Lauro, C.; Quaglio, D.; Ghirga, F.; Botta, B.; Trettel, F.; Limatola, C. Neuro-Signals from Gut Microbiota: Perspectives for Brain Glioma. Cancers 2021, 13, 2810. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Korber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Mohtashami, E.; Mousavi, S.H.; Soukhtanloo, M.; Vahedi, M.M.; Hosseini, A.; Afshari, A.R.; Sahebkar, A. Deciphering the Role of Glutamate Signaling in Glioblastoma Multiforme: Current Therapeutic Modalities and Future Directions. Curr. Pharm. Des. 2020, 26, 4777–4788. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Zheng, X.Y.; Luan, Y.; Liu, Y.F.; Li, X.X.; Liu, C.X.; Lu, H.X.; Chen, X.L.; Liu, Y. Activity of Metabotropic Glutamate Receptor 4 Suppresses Proliferation and Promotes Apoptosis With Inhibition of Gli-1 in Human Glioblastoma Cells. Front. Neurosci. 2018, 12, 16. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Aditi Devi, N.; Hurmath Fathima, K.; Dalavaikodihalli Nanjaiah, N. Activation of NMDA receptor of glutamate influences MMP-2 activity and proliferation of glioma cells. Neurol. Sci. 2014, 35, 823–829. [Google Scholar] [CrossRef]
- Piao, Y.; Lu, L.; de Groot, J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol. 2009, 11, 260–273. [Google Scholar] [CrossRef]
- Corsi, L.; Mescola, A.; Alessandrini, A. Glutamate Receptors and Glioblastoma Multiforme: An Old ‘‘Route’’ for New Perspectives. Int. J. Mol. Sci. 2019, 20, 1796. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Allaire, A.; Diamandis, P.; Bisaillon, M.; Scott, M.S.; Richer, M. A machine learning analysis of a “normal-like” IDH-WT diffuse glioma transcriptomic subgroup associated with prolonged survival reveals novel immune and neurotransmitter-related actionable targets. BMC Med. 2020, 18, 280. [Google Scholar] [CrossRef]
- Catavero, C.; Bao, H.; Song, J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2018, 371, 33–46. [Google Scholar] [CrossRef]
- Smits, A.; Jin, Z.; Elsir, T.; Pedder, H.; Nistér, M.; Alafuzoff, I.; Dimberg, A.; Edqvist, P.H.; Pontén, F.; Aronica, E.; et al. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS ONE 2012, 7, e37041. [Google Scholar] [CrossRef]
- Babateen, O.; Jin, Z.; Bhandage, A.; Korol, S.V.; Westermark, B.; Forsberg Nilsson, K.; Uhrbom, L.; Smits, A.; Birnir, B. Etomidate, propofol and diazepam potentiate GABA-evoked GABAA currents in a cell line derived from human glioblastoma. Eur. J. Pharmacol. 2015, 748, 101–107. [Google Scholar] [CrossRef]
- D’Urso, P.I.; D’Urso, O.F.; Storelli, C.; Mallardo, M.; Gianfreda, C.D.; Montinaro, A.; Cimmino, A.; Pietro, C.; Marsigliante, S. miR-155 is up-regulated in primary and secondary glioblastoma and promotes tumour growth by inhibiting GABA receptors. Int. J. Oncol. 2012, 41, 228–234. [Google Scholar]
- Herbin, M.; Simonis, C.; Revéret, L.; Hackert, R.; Libourel, P.A.; Eugène, D.; Diaz, J.; de Waele, C.; Vidal, P.P. Dopamine Modulates Motor Control in a Specific Plane Related to Support. PLoS ONE 2016, 11, e0155058. [Google Scholar] [CrossRef]
- Yoest, K.E.; Cummings, J.A.; Becker, J.B. Estradiol, dopamine and motivation. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 83–89. [Google Scholar] [CrossRef]
- Lan, Y.L.; Wang, X.; Xing, J.S.; Lou, J.C.; Ma, X.C.; Zhang, B. The potential roles of dopamine in malignant glioma. Acta Neurol. Belg. 2017, 117, 613–621. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Lu, C.; Shahzad, M.M.K.; Pena, G.N.A.; Allen, J.K.; Stone, R.L.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Farley, D.; et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin. Cancer Res. 2011, 17, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M.; Wallace, J.A.; Wilkie, M.B.; DiNome, A.; Krebs, H. Roles for serotonin in neurogenesis. Monogr. Neural Sci. 1983, 9, 3–10. [Google Scholar]
- Lu, Q.; Ding, Y.; Li, Y.; Lu, Q. 5-HT receptor agonist Valerenic Acid enhances the innate immunity signal and suppresses glioblastoma cell growth and invasion. Int. J. Biol. Sci. 2020, 16, 2104–2115. [Google Scholar] [CrossRef]
- Weselek, G.; Keiner, S.; Fauser, M.; Wagenführ, L.; Müller, J.; Kaltschmidt, B.; Brandt, M.D.; Gerlach, M.; Redecker, C.; Hermann, A.; et al. Norepinephrine is a negative regulator of the adult periventricular neural stem cell niche. Stem Cells 2020, 38, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shan, W.; Zuo, Z. Norepinephrine inhibits migration and invasion of human glioblastoma cell cultures possibly via MMP-11 inhibition. Brain Res. 2021, 1756, 147280. [Google Scholar] [CrossRef] [PubMed]
- Braadland, P.R.; Ramberg, H.; Grytli, H.H.; Tasken, K.A. beta-Adrenergic Receptor Signaling in Prostate Cancer. Front. Oncol 2014, 4, 375. [Google Scholar] [PubMed]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef]
- Debom, G.N.; Rubenich, D.S.; Braganhol, E. Adenosinergic Signaling as a Key Modulator of the Glioma Microenvironment and Reactive Astrocytes. Front. Neurosci. 2021, 15, 648476. [Google Scholar] [CrossRef]
- Silva, A.P.; Pinheiro, P.S.; Carvalho, A.P.; Carvalho, C.M.; Jakobsen, B.; Zimmer, J.; Malva, J.O. Activation of neuropeptide Y receptors is neuroprotective against excitotoxicity in organotypic hippocampal slice cultures. FASEB J. 2003, 17, 1118–1120. [Google Scholar] [CrossRef]
- Abualsaud, N.; Caprio, L.; Galli, S.; Krawczyk, E.; Alamri, L.; Zhu, S.; Gallicano, G.I.; Kitlinska, J. Neuropeptide Y/Y5 Receptor Pathway Stimulates Neuroblastoma Cell Motility Through RhoA Activation. Front. Cell Dev. Biol. 2020, 8, 627090. [Google Scholar] [CrossRef]
- Körner, M.; Reubi, J.C. Neuropeptide Y receptors in primary human brain tumors: Overexpression in high-grade tumors. J. Neuropathol. Exp. Neurol. 2008, 67, 741–749. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Ye, J.; Lu, D.; Chen, Z.; Xiang, A.P.; Jiang, M.H. Substance P enhances endogenous neurogenesis to improve functional recovery after spinal cord injury. Int. J. Biochem. Cell Biol. 2017, 89, 110–119. [Google Scholar] [CrossRef]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid-substance p. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef]
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792. [Google Scholar] [CrossRef]
- Afshari, A.R.; Motamed-Sanaye, A.; Sabri, H.; Soltani, A.; Karkon-Shayan, S.; Radvar, S.; Javid, H.; Mollazadeh, H.; Sathyapalan, T.; Sahebkar, A. Neurokinin-1 receptor (NK-1R) antagonists: Potential targets in the treatment of glioblastoma multiforme. Curr. Med. Chem. 2021, 28, 4877–4892. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, X.F.; Yuan, G.Q.; Hu, H.; Song, X.Y.; Li, J.Y.; Miao, X.K.; Zhou, T.X.; Yang, W.L.; Zhang, X.W.; et al. β-Arrestin 1 has an essential role in neurokinin-1 receptor-mediated glioblastoma cell proliferation and G(2)/M phase transition. J. Biol. Chem. 2017, 292, 8933–8947. [Google Scholar] [CrossRef]
- Munoz, M.F.; Arguelles, S.; Rosso, M.; Medina, R.; Covenas, R.; Ayala, A.; Munoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. Biomed. Res. Int. 2022, 2022, 6291504. [Google Scholar] [CrossRef]
- Mou, L.; Kang, Y.; Zhou, Y.; Zeng, Q.; Song, H.; Wang, R. Neurokinin-1 receptor directly mediates glioma cell migration by up-regulation of matrix metalloproteinase-2 (MMP-2) and membrane type 1-matrix metalloproteinase (MT1-MMP). J. Biol. Chem. 2013, 288, 306–318. [Google Scholar] [CrossRef]
- Contestabile, A. Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog. Neurobiol. 2008, 84, 317–328. [Google Scholar] [CrossRef]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef]
- Cahill, K.E.; Morshed, R.A.; Yamini, B. Nuclear factor-κB in glioblastoma: Insights into regulators and targeted therapy. Neuro Oncol. 2016, 18, 329–339. [Google Scholar] [CrossRef]
- Yakovlev, V.A. Role of nitric oxide in the radiation-induced bystander effect. Redox Biol. 2015, 6, 396–400. [Google Scholar] [CrossRef]
- Tran, A.N.; Boyd, N.H.; Walker, K.; Hjelmeland, A.B. NOS Expression and NO Function in Glioma and Implications for Patient Therapies. Antioxid. Redox Signal. 2017, 26, 986–999. [Google Scholar] [CrossRef]
- Badn, W.; Siesjö, P. The dual role of nitric oxide in glioma. Curr. Pharm. Des. 2010, 16, 428–430. [Google Scholar] [CrossRef]
- Shinoda, J.; Whittle, I.R. Nitric oxide and glioma: A target for novel therapy? Br. J. Neurosurg. 2001, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Asl, A.; Bonni, A. iNOS: A potential therapeutic target for malignant glioma. Curr. Mol. Med. 2013, 13, 1241–1249. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Yao, M.; Li, S.; Wu, X.; Diao, S.; Zhang, G.; He, H.; Bian, L.; Lu, Y. Cellular origin of glioblastoma and its implication in precision therapy. Cell. Mol. Immunol. 2018, 15, 737–739. [Google Scholar] [CrossRef]
- Crivii, C.B.; Bosca, A.B.; Melincovici, C.S.; Constantin, A.M.; Marginean, M.; Dronca, E.; Sufletel, R.; Gonciar, D.; Bungardean, M.; Sovrea, A. Glioblastoma Microenvironment and Cellular Interactions. Cancers 2022, 14, 1092. [Google Scholar] [CrossRef]
- Schonberg, D.L.; Lubelski, D.; Miller, T.E.; Rich, J.N. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol. Asp. Med. 2014, 39, 82–101. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro Oncol. 2021, 24, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Wu, M.; Johnson, K.; Kim, H.; Celebre, A.; Shahzad, U.; Graham, M.S.; Kessler, J.A.; Chuang, J.H.; Karamchandani, J.; et al. BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma. Sci. Rep. 2019, 9, 14569. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Wang, Y.; Xiao, S.; Mai, R.; Zhu, Z.; Cao, Y. Glioblastoma stem cell (GSC)-derived PD-L1-containing exosomes activates AMPK/ULK1 pathway mediated autophagy to increase temozolomide-resistance in glioblastoma. Cell Biosci. 2021, 11, 63. [Google Scholar] [CrossRef]
- Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Ciriello, G.; Cerami, E.; Sander, C.; Schultz, N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 2012, 22, 398–406. [Google Scholar] [CrossRef]
- Rutkowska, A.; Stoczynska-Fidelus, E.; Janik, K.; Wlodarczyk, A.; Rieske, P. EGFR(vIII): An Oncogene with Ambiguous Role. J. Oncol. 2019, 2019, 1092587. [Google Scholar] [CrossRef]
- Byrne, K.F.; Pal, A.; Curtin, J.F.; Stephens, J.C.; Kinsella, G.K. G-protein-coupled receptors as therapeutic targets for glioblastoma. Drug Discov. Today 2021, 26, 2858–2870. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Kim, H.; Han, K.S. Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca2+ Signaling, and Glutamate. Front. Cell. Neurosci. 2021, 15, 663092. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef]
- Tilak, M.; Holborn, J.; New, L.A.; Lalonde, J.; Jones, N. Receptor Tyrosine Kinase Signaling and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2021, 22, 1831. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Day, E.K.; Sosale, N.G.; Xiao, A.; Zhong, Q.; Purow, B.; Lazzara, M.J. Glioblastoma Cell Resistance to EGFR and MET Inhibition Can Be Overcome via Blockade of FGFR-SPRY2 Bypass Signaling. Cell Rep. 2020, 30, 3383–3396.e7. [Google Scholar] [CrossRef]
- Guo, G.; Narayan, R.N.; Horton, L.; Patel, T.R.; Habib, A.A. The Role of EGFR-Met Interactions in the Pathogenesis of Glioblastoma and Resistance to Treatment. Curr. Cancer Drug Targets 2017, 17, 297–302. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Smith, J.S.; Tachibana, I.; Passe, S.M.; Huntley, B.K.; Borell, T.J.; Iturria, N.; O’Fallon, J.R.; Schaefer, P.L.; Scheithauer, B.W.; James, C.D.; et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J. Natl. Cancer Inst. 2001, 93, 1246–1256. [Google Scholar] [CrossRef]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Duerr, E.M.; Rollbrocker, B.; Hayashi, Y.; Peters, N.; Meyer-Puttlitz, B.; Louis, D.N.; Schramm, J.; Wiestler, O.D.; Parsons, R.; Eng, C.; et al. PTEN mutations in gliomas and glioneuronal tumors. Oncogene 1998, 16, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Bartels, G.; Gehlhaar, C.; Holtkamp, N.; von Deimling, A. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol. 2005, 109, 639–642. [Google Scholar] [CrossRef]
- Qin, A.; Musket, A.; Musich, P.R.; Schweitzer, J.B.; Xie, Q. Receptor tyrosine kinases as druggable targets in glioblastoma: Do signaling pathways matter? Neuro-Oncol. Adv. 2021, 3, vdab133. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Tee, A.R. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Morrow, S.; Rahman Kawakibi, A.; Zhou, L.; Ralff, M.; Ray, J.; Jhaveri, A.; Ferrarini, I.; Lee, Y.; Parker, C.; et al. ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia 2020, 22, 725–744. [Google Scholar] [CrossRef]
- Arcella, A.; Carpinelli, G.; Battaglia, G.; D’Onofrio, M.; Santoro, F.; Ngomba, R.T.; Bruno, V.; Casolini, P.; Giangaspero, F.; Nicoletti, F. Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro Oncol. 2005, 7, 236–245. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Gray, G.K.; McFarland, B.C.; Nozell, S.E.; Benveniste, E.N. NF-κB and STAT3 in glioblastoma: Therapeutic targets coming of age. Expert Rev. Neurother. 2014, 14, 1293–1306. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB(p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Godwin, P.; Baird, A.M.; Heavey, S.; Barr, M.P.; O’Byrne, K.J.; Gately, K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front. Oncol. 2013, 3, 120. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef]
- Watanabe, S.; Nishijima, N.; Hirai, K.; Shibata, K.; Hase, A.; Yamanaka, T.; Inazu, M. Anticancer Activity of Amb4269951, a Choline Transporter-Like Protein 1 Inhibitor, in Human Glioma Cells. Pharmaceuticals 2020, 13, 104. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.R.; Li, H.Y.; Zhao, Z.J.; Liao, Y.W.; Wang, X.Y.; Su, J.; Sang, S.S.; Liu, Q. Anti-cancer effect of metabotropic glutamate receptor 1 inhibition in human glioma U87 cells: Involvement of PI3K/Akt/mTOR pathway. Cell. Physiol. Biochem. 2015, 35, 419–432. [Google Scholar] [CrossRef]
- He, J.J.; Zhang, W.H.; Liu, S.L.; Chen, Y.F.; Liao, C.X.; Shen, Q.Q.; Hu, P. Activation of β-adrenergic receptor promotes cellular proliferation in human glioblastoma. Oncol. Lett. 2017, 14, 3846–3852. [Google Scholar] [CrossRef][Green Version]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Uyar, R. Glioblastoma microenvironment: The stromal interactions. Pathol. Res. Pract. 2022, 232, 153813. [Google Scholar] [CrossRef]
- Ramaiah, M.J.; Kumar, K.R. mTOR-Rictor-EGFR axis in oncogenesis and diagnosis of glioblastoma multiforme. Mol. Biol. Rep. 2021, 48, 4813–4835. [Google Scholar] [CrossRef]
- Varalda, M.; Antona, A.; Bettio, V.; Roy, K.; Vachamaram, A.; Yellenki, V.; Massarotti, A.; Baldanzi, G.; Capello, D. Psychotropic Drugs Show Anticancer Activity by Disrupting Mitochondrial and Lysosomal Function. Front. Oncol. 2020, 10, 562196. [Google Scholar] [CrossRef]
- Di Bari, M.; Tombolillo, V.; Alessandrini, F.; Guerriero, C.; Fiore, M.; Asteriti, I.A.; Castigli, E.; Sciaccaluga, M.; Guarguaglini, G.; Degrassi, F.; et al. M2 Muscarinic Receptor Activation Impairs Mitotic Progression and Bipolar Mitotic Spindle Formation in Human Glioblastoma Cell Lines. Cells 2021, 10, 1727. [Google Scholar] [CrossRef]
- Cristofaro, I.; Limongi, C.; Piscopo, P.; Crestini, A.; Guerriero, C.; Fiore, M.; Conti, L.; Confaloni, A.; Tata, A.M. M2 Receptor Activation Counteracts the Glioblastoma Cancer Stem Cell Response to Hypoxia Condition. Int. J. Mol. Sci. 2020, 21, 1700. [Google Scholar] [CrossRef]
- Guerriero, C.; Matera, C.; Del Bufalo, D.; De Amici, M.; Conti, L.; Dallanoce, C.; Tata, A.M. The Combined Treatment with Chemotherapeutic Agents and the Dualsteric Muscarinic Agonist Iper-8-Naphthalimide Affects Drug Resistance in Glioblastoma Stem Cells. Cells 2021, 10, 1877. [Google Scholar] [CrossRef]
- Bavo, F.; Pucci, S.; Fasoli, F.; Lammi, C.; Moretti, M.; Mucchietto, V.; Lattuada, D.; Viani, P.; De Palma, C.; Budriesi, R.; et al. Potent Antiglioblastoma Agents by Hybridizing the Onium-Alkyloxy-Stilbene Based Structures of an alpha 7-nAChR, alpha 9-nAChR Antagonist and of a Pro-Oxidant Mitocan. J. Med. Chem. 2018, 61, 10531–10544. [Google Scholar] [CrossRef]
- Pucci, S.; Bolchi, C.; Bavo, F.; Pallavicini, M.; De Palma, C.; Renzi, M.; Fucile, S.; Benfante, R.; Di Lascio, S.; Lattuada, D.; et al. Evidence of a dual mechanism of action underlying the anti-proliferative and cytotoxic effects of ammonium-alkyloxy-stilbene-based alpha7- and alpha9-nicotinic ligands on glioblastoma cells. Pharmacol. Res. 2022, 175, 105959. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Deneka-Hannemann, S.; Jarosz, B.; Zgrajka, W.; Stoma, F.; Trojanowski, T.; Turski, W.A.; Rzeski, W. Kynurenic acid inhibits proliferation and migration of human glioblastoma T98G cells. Pharmacol. Rep. 2014, 66, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, D.N.; Ramaswamy, P.; Prasad, C.; Srinivas, D.; Goswami, K. Glioblastoma invasion and NMDA receptors: A novel prospect. Physiol. Int. 2019, 106, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, G.; Konac, E.; Dere, U.A.; Emmez, H. Targeting Cancer Cell Metabolism with Metformin, Dichloroacetate and Memantine in Glioblastoma (GBM). Turk. Neurosurg. 2021, 31, 233–237. [Google Scholar] [CrossRef]
- Cacciatore, I.; Fornasari, E.; Marinelli, L.; Eusepi, P.; Ciulla, M.; Ozdemir, O.; Tatar, A.; Turkez, H.; Di Stefano, A. Memantine-derived drugs as potential antitumor agents for the treatment of glioblastoma. Eur. J. Pharm. Sci. 2017, 109, 402–411. [Google Scholar] [CrossRef]
- Salmaggi, A.; Corno, C.; Maschio, M.; Donzelli, S.; D’Urso, A.; Perego, P.; Ciusani, E. Synergistic Effect of Perampanel and Temozolomide in Human Glioma Cell Lines. J. Pers. Med. 2021, 11, 390. [Google Scholar] [CrossRef]
- Lange, F.; Weßlau, K.; Porath, K.; Hörnschemeyer, J.; Bergner, C.; Krause, B.J.; Mullins, C.S.; Linnebacher, M.; Köhling, R.; Kirschstein, T. AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PLoS ONE 2019, 14, e0211644. [Google Scholar] [CrossRef]
- Eyüpoglu, I.Y.; Hahnen, E.; Heckel, A.; Siebzehnrübl, F.A.; Buslei, R.; Fahlbusch, R.; Blümcke, I. Malignant glioma-induced neuronal cell death in an organotypic glioma invasion model. Technical note. J. Neurosurg. 2005, 102, 738–744. [Google Scholar] [CrossRef]
- Sperling, S.; Aung, T.; Martin, S.; Rohde, V.; Ninkovic, M. Riluzole: A potential therapeutic intervention in human brain tumor stem-like cells. Oncotarget 2017, 8, 96697–96709. [Google Scholar] [CrossRef]
- Dailey, C.B.; Wroblewska, B.; Wolfe, B.B.; Wroblewski, J.T. The Role of Metabotropic Glutamate Receptor 1 Dependent Signaling in Glioma Viability. J. Pharmacol. Exp. Ther. 2018, 367, 59–70. [Google Scholar] [CrossRef]
- Sehm, T.; Fan, Z.; Ghoochani, A.; Rauh, M.; Engelhorn, T.; Minakaki, G.; Dorfler, A.; Klucken, J.; Buchfelder, M.; Eyupoglu, I.Y.; et al. Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget 2016, 7, 36021–36033. [Google Scholar] [CrossRef]
- Bhat, K.; Saki, M.; Cheng, F.; He, L.; Zhang, L.; Ioannidis, A.; Nathanson, D.; Tsang, J.; Bensinger, S.J.; Nghiemphu, P.L.; et al. Dopamine Receptor Antagonists, Radiation, and Cholesterol Biosynthesis in Mouse Models of Glioblastoma. J. Natl. Cancer Inst. 2021, 113, 1094–1104. [Google Scholar] [CrossRef]
- Kast, R.E. Adding perphenazine to increase effectiveness of standard glioblastoma chemoirradiation. J. Buon 2020, 25, 1676–1686. [Google Scholar]
- Suzuki, S.; Yamamoto, M.; Sanomachi, T.; Togashi, K.; Sugai, A.; Seino, S.; Yoshioka, T.; Kitanaka, C.; Okada, M. Brexpiprazole, a Serotonin-Dopamine Activity Modulator, Can Sensitize Glioma Stem Cells to Osimertinib, a Third-Generation EGFR-TKI, via Survivin Reduction. Cancers 2019, 11, 947. [Google Scholar] [CrossRef]
- Frampton, J.E. Brexpiprazole: A Review in Schizophrenia. Drugs 2019, 79, 189–200. [Google Scholar] [CrossRef]
- Hosseindoost, S.; Hashemizadeh, S.; Gharaylou, Z.; Dehpour, A.R.; Javadi, S.A.H.; Arjmand, B.; Hadjighassem, M. β2-Adrenergic Receptor Stimulation Upregulates Cx43 Expression on Glioblastoma Multiforme and Olfactory Ensheathing Cells. J. Mol. Neurosci. MN 2020, 70, 1451–1460. [Google Scholar] [CrossRef]
- Drill, M.; Powell, K.L.; Kan, L.K.; Jones, N.C.; O’Brien, T.J.; Hamilton, J.A.; Monif, M. Inhibition of purinergic P2X receptor 7 (P2X7R) decreases granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in U251 glioblastoma cells. Sci. Rep. 2020, 10, 14844. [Google Scholar] [CrossRef]
- Kan, L.K.; Seneviratne, S.; Drummond, K.J.; Williams, D.A.; O’Brien, T.J.; Monif, M. P2X7 receptor antagonism inhibits tumour growth in human high-grade gliomas. Purinergic Signal. 2020, 16, 327–336. [Google Scholar] [CrossRef]
- Bi, L.; Liu, Y.; Yang, Q.; Zhou, X.; Li, H.; Liu, Y.; Li, J.; Lu, Y.; Tang, H. Paris saponin H inhibits the proliferation of glioma cells through the A1 and A3 adenosine receptor-mediated pathway. Int. J. Mol. Med. 2021, 47, 1. [Google Scholar] [CrossRef]
- Southan, G.J.; Szabó, C.; Thiemermann, C. Isothioureas: Potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995, 114, 510–516. [Google Scholar] [CrossRef]
- Jafarian-Tehrani, M.; Louin, G.; Royo, N.C.; Besson, V.C.; Bohme, G.A.; Plotkine, M.; Marchand-Verrecchia, C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide Biol. Chem. 2005, 12, 61–69. [Google Scholar] [CrossRef]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2019, 125, 424–433. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Kreisl, T.N.; Kim, L.; Duic, J.P.; Butman, J.A.; Albert, P.S.; Fine, H.A. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer 2010, 116, 1776–1782. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ye, X.; Chamberlain, M.; Mikkelsen, T.; Batchelor, T.; Desideri, S.; Piantadosi, S.; Fisher, J.; Fine, H.A. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: A multicenter phase II trial. J. Clin. Oncol. 2009, 27, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Ye, X.; Piantadosi, S.; Desideri, S.; Nabors, L.B.; Rosenfeld, M.; Fisher, J. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010, 16, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Bhat, K.; Ioannidis, A.; Zhang, L.; Nguyen, N.T.; Allen, J.E.; Nghiemphu, P.L.; Cloughesy, T.F.; Liau, L.M.; Kornblum, H.I.; et al. Effects of the DRD2/3 antagonist ONC201 and radiation in glioblastoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 161, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Arrillaga-Romany, I.; Odia, Y.; Prabhu, V.V.; Tarapore, R.S.; Merdinger, K.; Stogniew, M.; Oster, W.; Allen, J.E.; Mehta, M.; Batchelor, T.T.; et al. Biological activity of weekly ONC201 in adult recurrent glioblastoma patients. Neuro Oncol. 2020, 22, 94–102. [Google Scholar] [CrossRef]
- Chi, A.S.; Tarapore, R.S.; Hall, M.D.; Shonka, N.; Gardner, S.; Umemura, Y.; Sumrall, A.; Khatib, Z.; Mueller, S.; Kline, C.; et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J. Neuro-Oncol. 2019, 145, 97–105. [Google Scholar] [CrossRef]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Buccarelli, M.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Anticancer Properties of the Antipsychotic Drug Chlorpromazine and Its Synergism With Temozolomide in Restraining Human Glioblastoma Proliferation In Vitro. Front. Oncol. 2021, 11, 635472. [Google Scholar] [CrossRef]
- Chen, V.C.; Hsieh, Y.H.; Chen, L.J.; Hsu, T.C.; Tzang, B.S. Escitalopram oxalate induces apoptosis in U-87MG cells and autophagy in GBM8401 cells. J. Cell. Mol. Med. 2018, 22, 1167–1178. [Google Scholar] [CrossRef]
- Elgoyhen, A.B.; Johnson, D.S.; Boulter, J.; Vetter, D.E.; Heinemann, S. Alpha 9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994, 79, 705–715. [Google Scholar] [CrossRef]
- Drescher, D.G.; Ramakrishnan, N.A.; Drescher, M.J.; Chun, W.; Wang, X.; Myers, S.F.; Green, G.E.; Sadrazodi, K.; Karadaghy, A.A.; Poopat, N.; et al. Cloning and characterization of alpha9 subunits of the nicotinic acetylcholine receptor expressed by saccular hair cells of the rainbow trout (Oncorhynchus mykiss). Neuroscience 2004, 127, 737–752. [Google Scholar] [CrossRef]
- Morley, B.J.; Li, H.S.; Hiel, H.; Drescher, D.G.; Elgoyhen, A.B. Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Brain Res. Mol. Brain Res. 1998, 53, 78–87. [Google Scholar] [CrossRef]
- Taguchi, C.; Inazu, M.; Saiki, I.; Yara, M.; Hara, N.; Yamanaka, T.; Uchino, H. Functional analysis of [methyl-(3)H]choline uptake in glioblastoma cells: Influence of anti-cancer and central nervous system drugs. Biochem. Pharmacol. 2014, 88, 303–312. [Google Scholar] [CrossRef]
- Wei, X.L.; Zhan, C.Y.; Shen, Q.; Fu, W.; Xie, C.; Gao, J.; Peng, C.M.; Zheng, P.; Lu, W.Y. A D-Peptide Ligand of Nicotine Acetylcholine Receptors for Brain-Targeted Drug Delivery. Angew. Chem. Int. Ed. 2015, 54, 3023–3027. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Gras, G.; Samah, B.; Hubert, A.; Léone, C.; Porcheray, F.; Rimaniol, A.C. EAAT expression by macrophages and microglia: Still more questions than answers. Amino Acids 2012, 42, 221–229. [Google Scholar] [CrossRef]
- Osipova, E.D.; Semyachkina-Glushkovskaya, O.V.; Morgun, A.V.; Pisareva, N.V.; Malinovskaya, N.A.; Boitsova, E.B.; Pozhilenkova, E.A.; Belova, O.A.; Salmin, V.V.; Taranushenko, T.E.; et al. Gliotransmitters and cytokines in the control of blood-brain barrier permeability. Rev. Neurosci. 2018, 29, 567–591. [Google Scholar] [CrossRef]
- Serpe, C.; Monaco, L.; Relucenti, M.; Iovino, L.; Familiari, P.; Scavizzi, F.; Raspa, M.; Familiari, G.; Civiero, L.; D’Agnano, I.; et al. Microglia-Derived Small Extracellular Vesicles Reduce Glioma Growth by Modifying Tumor Cell Metabolism and Enhancing Glutamate Clearance through miR-124. Cells 2021, 10, 2066. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Elmaci, I. Targeting nitric oxide and NMDA receptor-associated pathways in treatment of high grade glial tumors. Hypotheses for nitro-memantine and nitrones. Nitric Oxide 2018, 79, 68–83. [Google Scholar] [CrossRef]
- Sontheimer, H. A role for glutamate in growth and invasion of primary brain tumors. J. Neurochem. 2008, 105, 287–295. [Google Scholar] [CrossRef]
- Pereira, M.S.L.; Klamt, F.; Thome, C.C.; Worm, P.V.; de Oliveira, D.L. Metabotropic glutamate receptors as a new therapeutic target for malignant gliomas. Oncotarget 2017, 8, 22279–22298. [Google Scholar] [CrossRef]
- Yamada, T.; Tsuji, S.; Nakamura, S.; Egashira, Y.; Shimazawa, M.; Nakayama, N.; Yano, H.; Iwama, T.; Hara, H. Riluzole enhances the antitumor effects of temozolomide via suppression of MGMT expression in glioblastoma. J. Neurosurg. 2020, 134, 701–710. [Google Scholar] [CrossRef]
- He, M.; Luo, M.; Chen, S.; Li, K.; Zheng, M.; Weng, Y.; Pi, R.; Liu, A. Combined treatment of fasudil and glutamate decreased the viability of human glioblastoma cells by excitotoxicity through NMDAR in vitro. Int. J. Clin. Exp. Med. 2015, 8, 18434–18440. [Google Scholar]
- Verbruggen, L.; Sprimont, L.; Bentea, E.; Janssen, P.; Gharib, A.; Deneyer, L.; De Pauw, L.; Lara, O.; Sato, H.; Nicaise, C.; et al. Chronic Sulfasalazine Treatment in Mice Induces System xc (-)-Independent Adverse Effects. Front. Pharmacol. 2021, 12, 625699. [Google Scholar] [CrossRef]
- Dahlmanns, M.; Yakubov, E.; Chen, D.S.; Sehm, T.; Rauh, M.; Savaskan, N.; Wrosch, J.K. Chemotherapeutic xCT inhibitors sorafenib and erastin unraveled with the synaptic optogenetic function analysis tool. Cell Death Discov. 2017, 3, 11. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Y.L.; Wang, H.Y.; Zhu, M.; Guo, D.; Wang, G.L.; Gao, Y.T.; Yang, Z.; Li, T.; Yang, C.Y.; et al. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca(2+) permeable AMPA receptor-system x(c)(-) pathway. Toxicol. In Vitro 2017, 44, 57–65. [Google Scholar] [CrossRef]
- Blanchart, A.; Fernando, R.; Häring, M.; Assaife-Lopes, N.; Romanov, R.A.; Andäng, M.; Harkany, T.; Ernfors, P. Endogenous GAB(AA) receptor activity suppresses glioma growth. Oncogene 2017, 36, 777–786. [Google Scholar] [CrossRef]
- El-Habr, E.A.; Dubois, L.G.; Burel-Vandenbos, F.; Bogeas, A.; Lipecka, J.; Turchi, L.; Lejeune, F.X.; Coehlo, P.L.; Yamaki, T.; Wittmann, B.M.; et al. A driver role for GABA metabolism in controlling stem and proliferative cell state through GHB production in glioma. Acta Neuropathol. 2017, 133, 645–660. [Google Scholar] [CrossRef]
- Panosyan, E.H.; Lin, H.J.; Koster, J.; Lasky, J.L., 3rd. In search of druggable targets for GBM amino acid metabolism. BMC Cancer 2017, 17, 162. [Google Scholar] [CrossRef]
- Patil, V.; Pal, J.; Mahalingam, K.; Somasundaram, K. Global RNA editome landscape discovers reduced RNA editing in glioma: Loss of editing of gamma-amino butyric acid receptor alpha subunit 3 (GABRA3) favors glioma migration and invasion. PeerJ 2020, 8, e9755. [Google Scholar] [CrossRef]
- Hujber, Z.; Horváth, G.; Petővári, G.; Krencz, I.; Dankó, T.; Mészáros, K.; Rajnai, H.; Szoboszlai, N.; Leenders, W.P.J.; Jeney, A.; et al. GABA, glutamine, glutamate oxidation and succinic semialdehyde dehydrogenase expression in human gliomas. J. Exp. Clin. Cancer Res. 2018, 37, 271. [Google Scholar] [CrossRef]
- Caragher, S.P.; Hall, R.R.; Ahsan, R.; Ahmed, A.U. Monoamines in glioblastoma: Complex biology with therapeutic potential. Neuro Oncol. 2018, 20, 1014–1025. [Google Scholar] [CrossRef]
- Eshleman, J.S.; Carlson, B.L.; Mladek, A.C.; Kastner, B.D.; Shide, K.L.; Sarkaria, J.N. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002, 62, 7291–7297. [Google Scholar] [PubMed]
- Wang, J.D.; Cao, Y.L.; Li, Q.; Yang, Y.P.; Jin, M.; Chen, D.; Wang, F.; Wang, G.H.; Qin, Z.H.; Hu, L.F.; et al. A pivotal role of FOS-mediated BECN1/Beclin 1 upregulation in dopamine D2 and D3 receptor agonist-induced autophagy activation. Autophagy 2015, 11, 2057–2073. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Mak, T.W. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer 2004, 4, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Brotchi, J.; Kiss, R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J. Clin. Oncol. 2005, 23, 2411–2422. [Google Scholar] [CrossRef]
- Lefranc, F.; Facchini, V.; Kiss, R. Proautophagic drugs: A novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist 2007, 12, 1395–1403. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Qin, T.; Wang, C.; Chen, X.; Duan, C.; Zhang, X.; Zhang, J.; Chai, H.; Tang, T.; Chen, H.; Yue, J.; et al. Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicol. Appl. Pharmacol. 2015, 286, 112–123. [Google Scholar] [CrossRef]
- Rahman, A.; Fazal, F. Blocking NF-κB: An inflammatory issue. Proc. Am. Thorac. Soc. 2011, 8, 497–503. [Google Scholar] [CrossRef]
- Trudler, D.; Weinreb, O.; Mandel, S.A.; Youdim, M.B.; Frenkel, D. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J. Neurochem. 2014, 129, 434–447. [Google Scholar] [CrossRef]
- Yeung, Y.T.; McDonald, K.L.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef]
- Rubí, B.; Maechler, P. Minireview: New roles for peripheral dopamine on metabolic control and tumor growth: Let’s seek the balance. Endocrinology 2010, 151, 5570–5581. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Madhukar, N.S.; Gilvary, C.; Kline, C.L.B.; Oster, S.; El-Deiry, W.S.; Elemento, O.; Doherty, F.; VanEngelenburg, A.; Durrant, J.; et al. Dopamine Receptor D5 is a Modulator of Tumor Response to Dopamine Receptor D2 Antagonism. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2305–2313. [Google Scholar] [CrossRef]
- Kast, R.E.; Ellingson, B.M.; Marosi, C.; Halatsch, M.E. Glioblastoma treatment using perphenazine to block the subventricular zone’s tumor trophic functions. J. Neuro-Oncol. 2014, 116, 207–212. [Google Scholar] [CrossRef][Green Version]
- Lieb, K.; Biersack, L.; Waschbisch, A.; Orlikowski, S.; Akundi, R.S.; Candelario-Jalil, E.; Hull, M.; Fiebich, B.L. Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J. Neurochem. 2005, 93, 549–559. [Google Scholar] [CrossRef]
- Goswami, S.; Gupta, A.; Sharma, S.K. Interleukin-6-mediated autocrine growth promotion in human glioblastoma multiforme cell line U87MG. J. Neurochem. 1998, 71, 1837–1845. [Google Scholar] [CrossRef]
- Kast, R.E. Glioblastoma chemotherapy adjunct via potent serotonin receptor-7 inhibition using currently marketed high-affinity antipsychotic medicines. Br. J. Pharmacol. 2010, 161, 481–487. [Google Scholar] [CrossRef]
- Abadi, B.; Shahsavani, Y.; Faramarzpour, M.; Rezaei, N.; Rahimi, H.R. Antidepressants with anti-tumor potential in treating glioblastoma: A narrative review. Fundam. Clin. Pharmacol. 2021, 36, 35–48. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Petrosyan, E.; Fares, J.; Cordero, A.; Rashidi, A.; Arrieta, V.A.; Kanojia, D.; Lesniak, M.S. Repurposing Autophagy Regulators in Brain Tumors. Int. J. Cancer 2022, 151, 167–180. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; Lo Furno, D.; Zappalà, A.; Valable, S.; Li Volti, G.; Tibullo, D.; Vicario, N.; Parenti, R. Connexin 43 and Sonic Hedgehog Pathway Interplay in Glioblastoma Cell Proliferation and Migration. Biology 2021, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, S.; Spray, D.C. Glioblastoma-Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion. Mol. Cancer Res. MCR 2022, 20, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Introduction to Purinergic Signalling in the Brain. Adv. Exp. Med. Biol. 2020, 1202, 1–12. [Google Scholar] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Braganhol, E.; Wink, M.R.; Lenz, G.; Battastini, A.M.O. Purinergic Signaling in Glioma Progression. Adv. Exp. Med. Biol. 2020, 1202, 87–108. [Google Scholar]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Morrone, F.B.; Horn, A.P.; Stella, J.; Spiller, F.; Sarkis, J.J.; Salbego, C.G.; Lenz, G.; Battastini, A.M. Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J. Neuro-Oncol. 2005, 71, 135–140. [Google Scholar] [CrossRef]
- Gehring, M.P.; Kipper, F.; Nicoletti, N.F.; Sperotto, N.D.; Zanin, R.; Tamajusuku, A.S.; Flores, D.G.; Meurer, L.; Roesler, R.; Filho, A.B.; et al. P2X7 receptor as predictor gene for glioma radiosensitivity and median survival. Int. J. Biochem. Cell Biol. 2015, 68, 92–100. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, M.; Xiao, J.; Zou, J.; Huang, Q.; Yang, K.; Zhang, B.; Yang, F.; Liu, S.; Wang, H.; et al. Paris Saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother. Pharmacol. 2014, 73, 807–818. [Google Scholar] [CrossRef]
- Yu, Q.; Li, Q.; Lu, P.; Chen, Q. Polyphyllin D induces apoptosis in U87 human glioma cells through the c-Jun NH2-terminal kinase pathway. J. Med. Food 2014, 17, 1036–1042. [Google Scholar] [CrossRef]
- Pang, D.; Li, C.; Yang, C.; Zou, Y.; Feng, B.; Li, L.; Liu, W.; Geng, Y.; Luo, Q.; Chen, Z.; et al. Polyphyllin VII Promotes Apoptosis and Autophagic Cell Death via ROS-Inhibited AKT Activity, and Sensitizes Glioma Cells to Temozolomide. Oxidative Med. Cell. Longev. 2019, 2019, 1805635. [Google Scholar] [CrossRef]
- Li, J.; Tian, Y.; Wu, A. Neuropeptide Y receptors: A promising target for cancer imaging and therapy. Regen Biomater. 2015, 2, 215–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.Y.; Chen, W.C.; Shi, Y.C.; Wang, C.M.; Lin, S.; He, H.F. Regulation of neuropeptide Y in body microenvironments and its potential application in therapies: A review. Cell Biosci. 2021, 11, 151. [Google Scholar] [CrossRef]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef]
- Hofmann, S.; Maschauer, S.; Kuwert, T.; Beck-Sickinger, A.G.; Prante, O. Synthesis and in vitro and in vivo evaluation of an (18)F-labeled neuropeptide Y analogue for imaging of breast cancer by PET. Mol. Pharm. 2015, 12, 1121–1130. [Google Scholar] [CrossRef]
- Reubi, J.C.; Gugger, M.; Waser, B.; Schaer, J.C. Y(1)-mediated effect of neuropeptide Y in cancer: Breast carcinomas as targets. Cancer Res. 2001, 61, 4636–4641. [Google Scholar]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Duarte, L.F.; Reyes, A.; Farias, M.A.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M.; Gonzalez, P.A. Crosstalk Between Epithelial Cells, Neurons and Immune Mediators in HSV-1 Skin Infection. Front. Immunol. 2021, 12, 662234. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Glioma and Neurokinin-1 Receptor Antagonists: A New Therapeutic Approach. Anticancer Agents Med. Chem. 2019, 19, 92–100. [Google Scholar] [CrossRef]
- Melani, R.; Von Itter, R.; Jing, D.; Koppensteiner, P.; Ninan, I. Opposing effects of an atypical glycinergic and substance P transmission on interpeduncular nucleus plasticity. Neuropsychopharmacology 2019, 44, 1828–1836. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, Y.J.; Jung, H.J. Discovery of a New CaMKII-Targeted Synthetic Lethal Therapy against Glioblastoma Stem-like Cells. Cancers 2022, 14, 1315. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. (225)Ac- and (213)Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Pilip, A.; Rius, M.; Bruchertseifer, F.; Apostolidis, C.; Weis, M.; Bonelli, M.; Laurenza, M.; Królicki, L.; Morgenstern, A. In vitro evaluation of (225) Ac-DOTA-substance P for targeted alpha therapy of glioblastoma multiforme. Chem. Biol. Drug Des. 2018, 92, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, S.; Bernhardt, P.; Uusijärvi, H.; Good, S.; Plasswilm, L.; Buitrago-Téllez, C.; Müller-Brand, J.; Mäcke, H.; Merlo, A. Individual voxelwise dosimetry of targeted 90Y-labelled substance P radiotherapy for malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Forrer, F.; Kneifel, S.; Sailer, M.; Mariani, L.; Mäcke, H.; Müller-Brand, J.; Merlo, A. Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA-substance P--results from a phase I study. J. Neurooncol. 2010, 100, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with (213)Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with (213)Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Lam-Himlin, D.; Espey, M.G.; Perry, G.; Smith, M.A.; Castellani, R.J. Malignant glioma progression and nitric oxide. Neurochem. Int. 2006, 49, 764–768. [Google Scholar] [CrossRef]
- Nomura, Y. NF-kappaB activation and IkappaB alpha dynamism involved in iNOS and chemokine induction in astroglial cells. Life Sci. 2001, 68, 1695–1701. [Google Scholar] [CrossRef]
- Pucci, S.; Zoli, M.; Clementi, F.; Gotti, C. alpha9-Containing Nicotinic Receptors in Cancer. Front. Cell. Neurosci. 2021, 15, 805123. [Google Scholar] [CrossRef]
- Belotti, Y.; Tolomeo, S.; Yu, R.; Lim, W.T.; Lim, C.T. Prognostic Neurotransmitter Receptors Genes Are Associated with Immune Response, Inflammation and Cancer Hallmarks in Brain Tumors. Cancers 2022, 14, 2544. [Google Scholar] [CrossRef]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2021, 68, 258–278. [Google Scholar] [CrossRef]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.-J.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef]
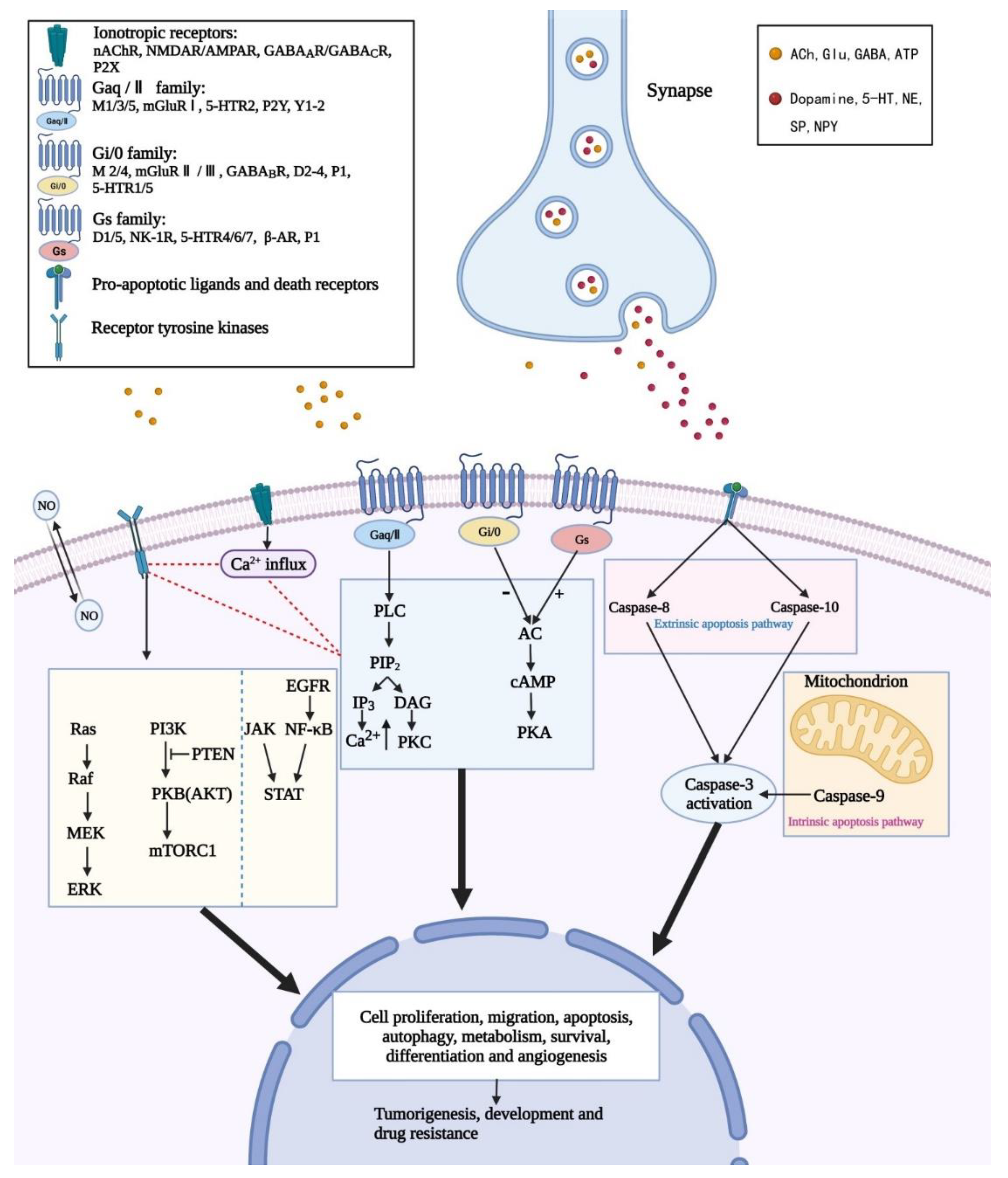
| Neurotransmitters | Receptors | Physiological Functions | References | Pathological Functions | References |
|---|---|---|---|---|---|
| Acetylcholine | mAChR M1–M5 nAChR |
| [20,21,22] |
| [27,28,29,30,31,32,33,34,35,36] |
| Glutamate | NMDAR AMPAR mGluR I–III |
| [23,37,38,39] |
| [40,41,42,43,44,45,46,47,48,49,50] |
| GABA | GABAAR GABABR GABACR |
| [25,51] |
| [12,52,53,54] |
| Dopamine | DRD1-DRD5 |
| [55,56,57] |
| [58] |
| Serotonin | 5-HTR 1–7 |
| [59] |
| [60] |
| Norepinephrine/ Noradrenaline | β-AR |
| [61] |
| [62,63,64] |
| Purinergic | P1,P2 |
| [65] |
| [66] |
| Neuropeptide Y | Y1, Y2, Y4, Y5 |
| [24,67] |
| [68,69] |
| Substance P | NK-1R |
| [70] |
| [71,72,73,74,75,76] |
| NO | - |
| [77] |
| [78,79,80,81,82,83,84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Chen, L.; Liang, J.; Huang, Q.; Sun, H. Neurotransmitters: Potential Targets in Glioblastoma. Cancers 2022, 14, 3970. https://doi.org/10.3390/cancers14163970
Huang Q, Chen L, Liang J, Huang Q, Sun H. Neurotransmitters: Potential Targets in Glioblastoma. Cancers. 2022; 14(16):3970. https://doi.org/10.3390/cancers14163970
Chicago/Turabian StyleHuang, Qiqi, Lishi Chen, Jianhao Liang, Qiongzhen Huang, and Haitao Sun. 2022. "Neurotransmitters: Potential Targets in Glioblastoma" Cancers 14, no. 16: 3970. https://doi.org/10.3390/cancers14163970
APA StyleHuang, Q., Chen, L., Liang, J., Huang, Q., & Sun, H. (2022). Neurotransmitters: Potential Targets in Glioblastoma. Cancers, 14(16), 3970. https://doi.org/10.3390/cancers14163970





