Grade 3–4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Objectives, and Participants
2.2. Categorization and Definition of irAEs
2.3. Data Collection and Baseline Measurements
2.4. Study Endpoints
2.5. Statistical Analysis
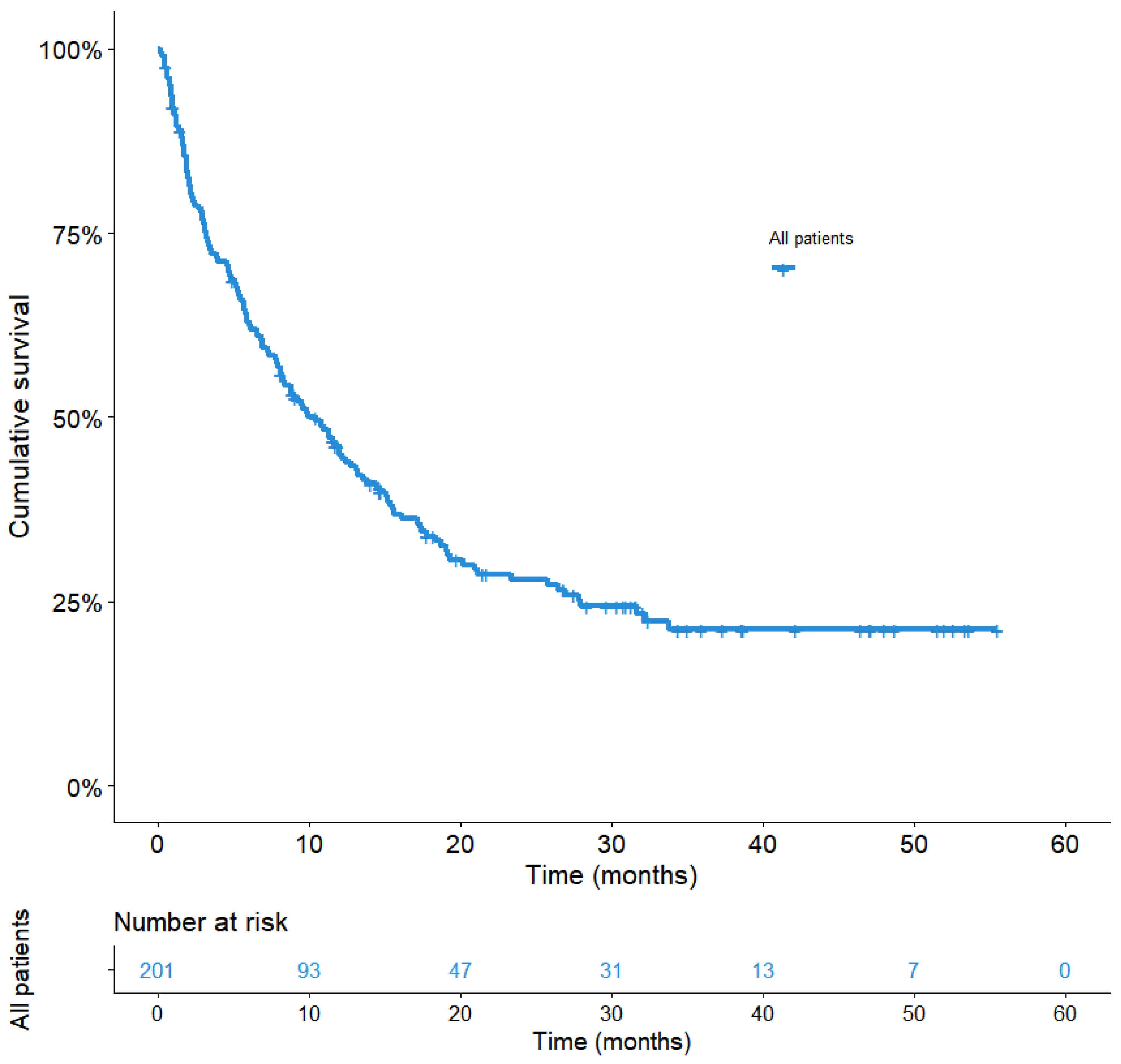
3. Results
3.1. Cohort Characteristics
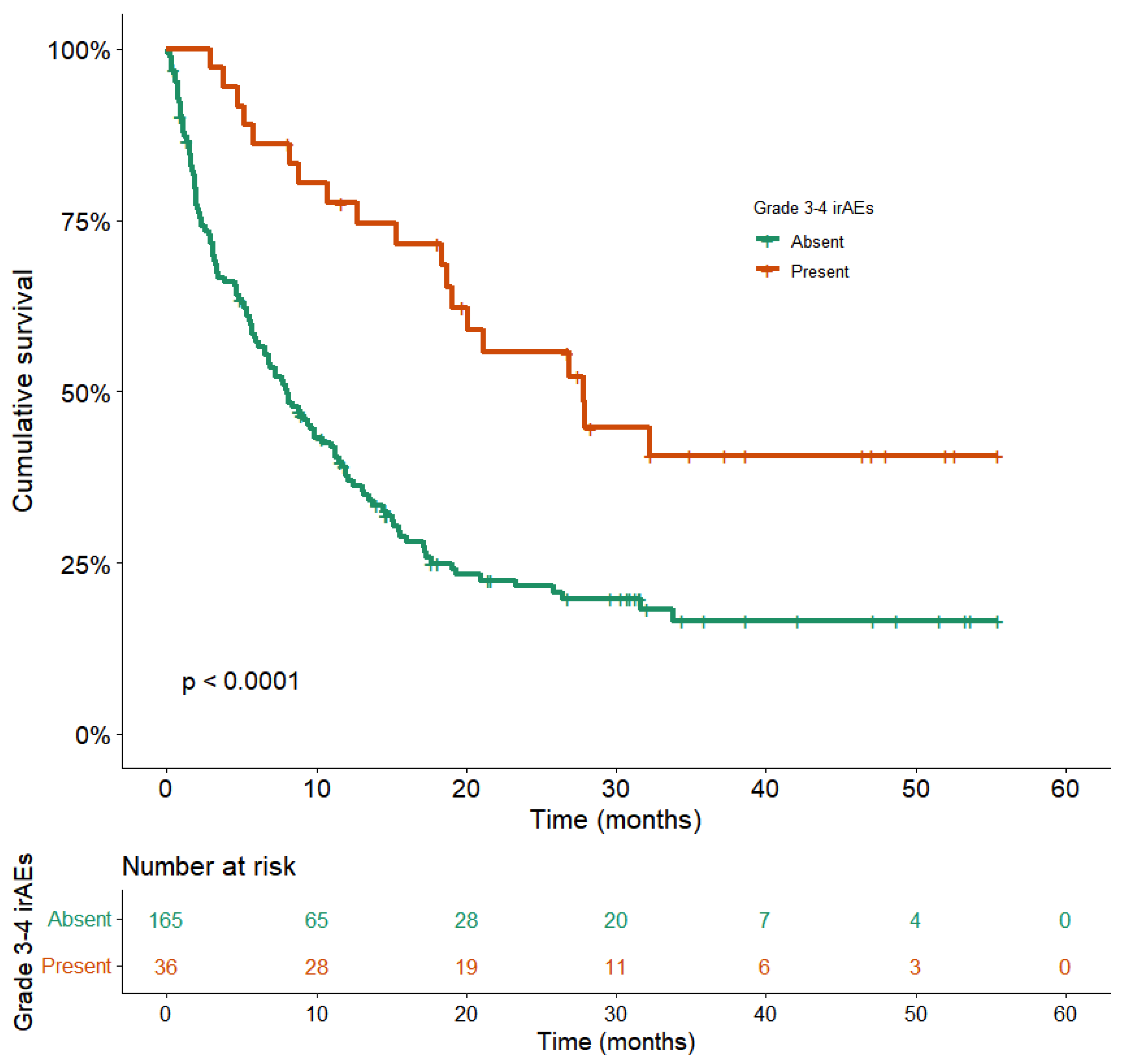
3.2. Grade 3–4 irAEs
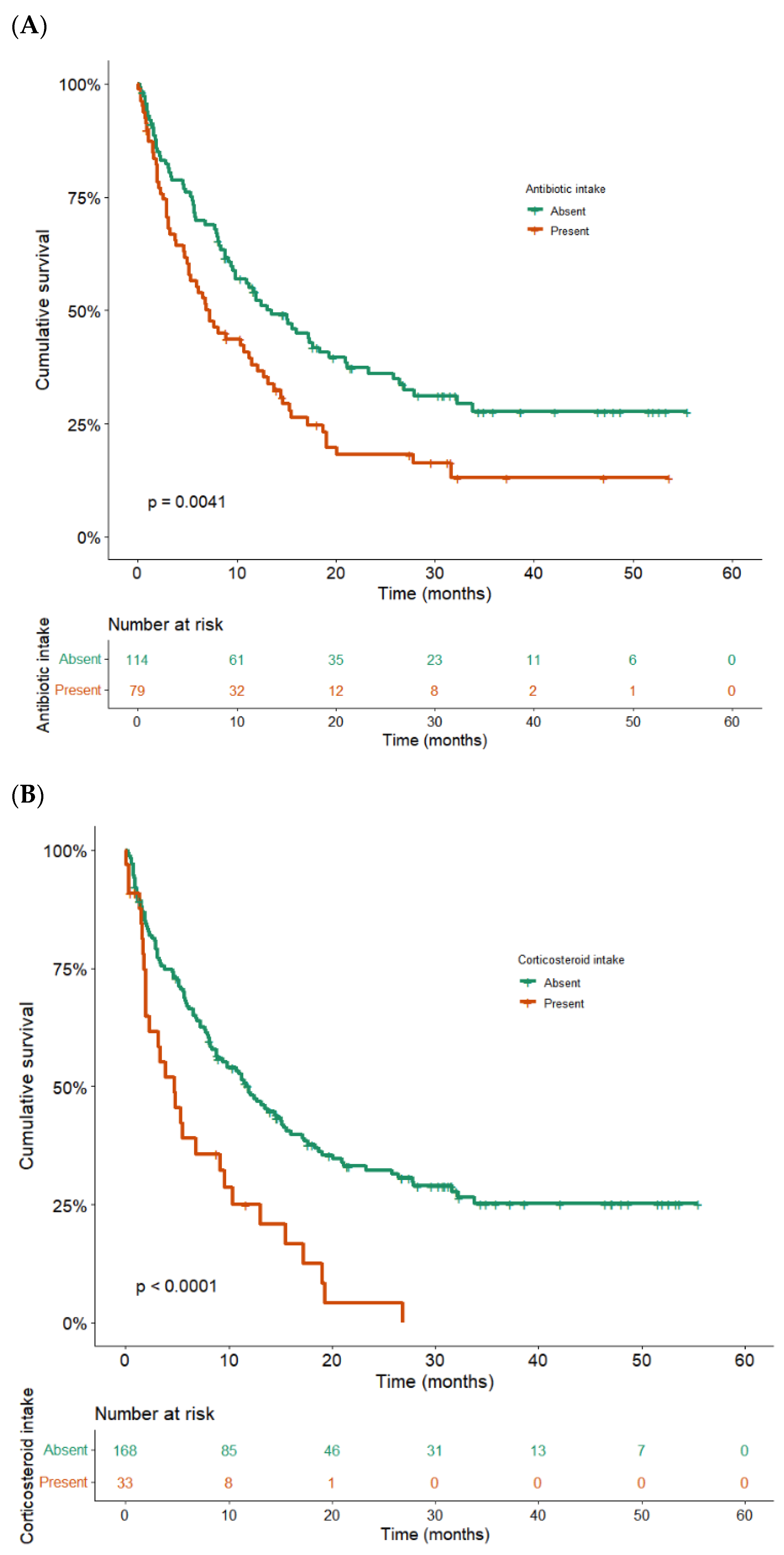
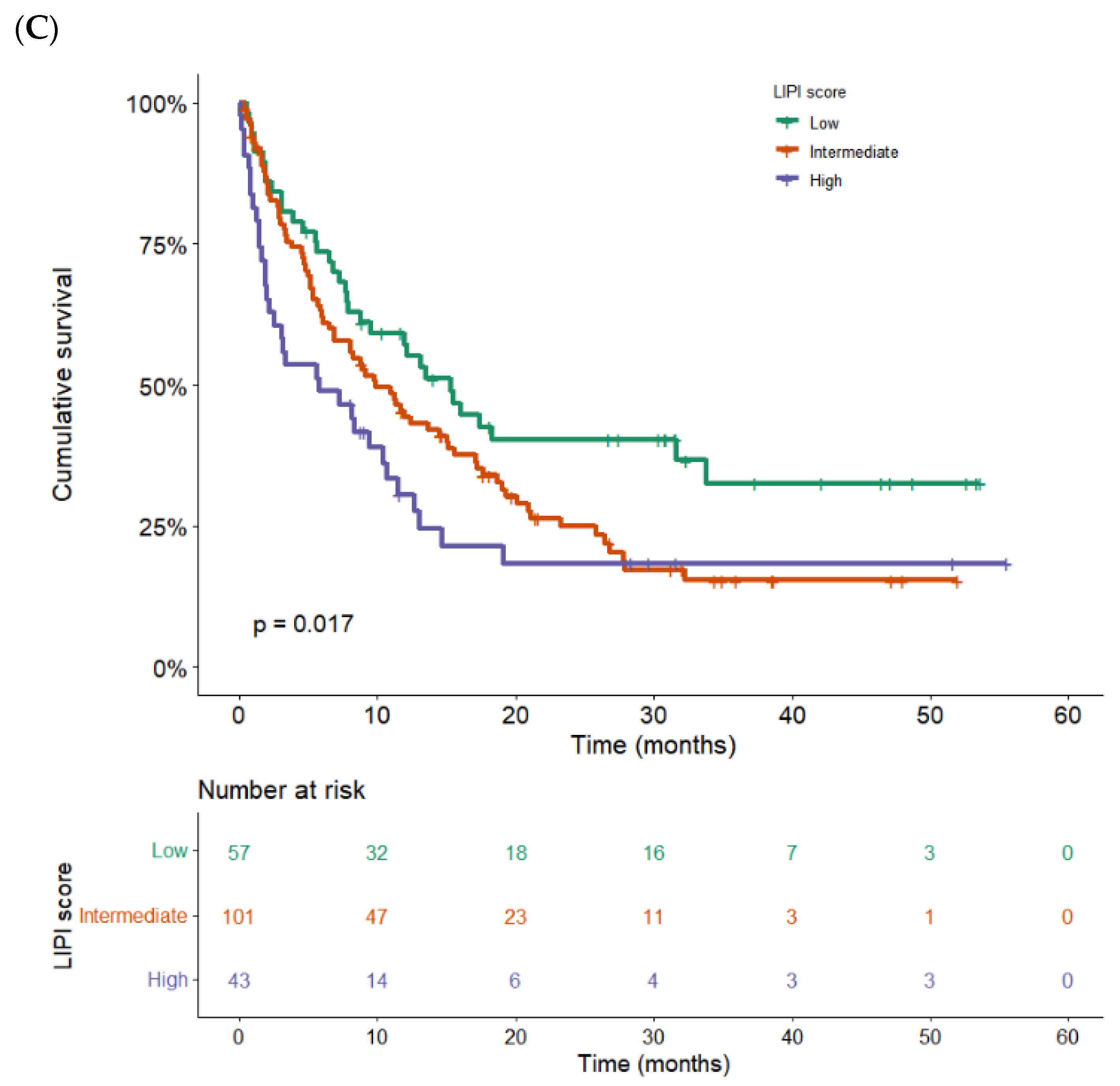
3.3. Correlation between Serious irAEs and Better Outcomes
3.4. Time to Next Treatment (TTNT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spini, A.; Gini, R.; Rosellini, P.; Singier, A.; Bellan, C.; Pascucci, A.; Leoncini, L.; Mathieu, C.; Martellucci, I.; Furiesi, F.; et al. First-Line Pharmacotherapies and Survival among Patients Diagnosed with Non-Resectable NSCLC: A Real-Life Setting Study with Gender Prospective. Cancers 2021, 13, 6129. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Lambotte, O.; Kostine, M.; Calabrese, L.; Suarez-Almazor, M.; Bingham, C.; Radstake, T.; Baldini, C.; Schaeverbeke, T.; Gottenberg, J.-E.; et al. Immune-related adverse events induced by cancer immunotherapies. big data analysis of 13,051 cases (immunocancer international registry). BMJ J. 2019, 78, 607–608. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492, Erratum in Lancet Oncol. 2018, 19, e668. Erratum in Lancet Oncol. 2018, 19, e581. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Q.; Qu, L.; Li, M.; Wang, L.; Mir, M.C.; Carbonara, U.; Pandolfo, S.D.; Black, P.C.; Paul, A.K.; et al. Adverse Events of Immune Checkpoint Inhibitors Therapy for Urologic Cancer Patients in Clinical Trials: A Collaborative Systematic Review and Meta-analysis. Eur. Urol. 2022, 81, 414–425. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients with Previously Treated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef]
- Eun, Y.; Kim, I.Y.; Sun, J.-M.; Lee, J.; Cha, H.-S.; Koh, E.-M.; Kim, H.; Lee, J. Risk Factors for Immune-Related Adverse Events Associated with Anti-PD-1 Pembrolizumab. Sci. Rep. 2019, 9, 14039–14046. [Google Scholar] [CrossRef]
- Sung, M.; Zer, A.; Walia, P.; Khoja, L.; Maganti, M.; Labbe, C.; Shepherd, F.A.; Bradbury, P.A.; Liu, G.; Leighl, N.B. Correlation of Immune-Related Adverse Events and Response from Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer. J. Thorac. Dis. 2020, 12, 2706–2712. [Google Scholar] [CrossRef]
- Griffith, S.D.; Miksad, R.A.; Calkins, G.; You, P.; Lipitz, N.G.; Bourla, A.B.; Williams, E.; George, D.J.; Schrag, D.; Khozin, S.; et al. Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association with Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set. JCO Clin. Cancer Inform. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Yu, W.; Patel, H.; Paranthaman, N.; et al. Pooled Analyses of Immune-Related Adverse Events (IrAEs) and Efficacy from the Phase 3 Trials IMpower130, IMpower132, and IMpower150. JCO 2021, 39, 9002. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 306–316. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.-A. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Bomze, D.; Hasan Ali, O.; Bate, A.; Flatz, L. Association Between Immune-Related Adverse Events During Anti–PD-1 Therapy and Tumor Mutational Burden. JAMA Oncol. 2019, 5, 1633–1635. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020: Featured Updates to the NCCN Guidelines. J. Nal. Compr. Cancer Network 2020, 18, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, C.; Farina, N.; Tomelleri, A.; Ferrara, R.; Lazzari, C.; De Luca, G.; Alessandra, B.; Signorelli, D.; Palmisano, A.; Vignale, D.; et al. Tocilizumab for the Treatment of Immune-Related Adverse Events: A Systematic Literature Review and a Multicentre Case Series. Eur. J. Intern. Med. 2021, 93, 87–94. [Google Scholar] [CrossRef]


| Characteristics (n = 201) | n (%) |
|---|---|
| Age at introduction of ICI (years) | |
| Mean | 63 |
| Median (IQR) | 64 (70–57) |
| Gender | |
| Male | 132 (66%) |
| Female | 69 (34%) |
| Hospital | |
| Bichat Hospital | 162 (81%) |
| Saint Joseph Foundation | 39 (19%) |
| Tobacco status | |
| Smoker/Former smoker | 190 (92%) |
| Non-smoker | 11 (6%) |
| Histology | |
| Adenocarcinoma | 120 (60%) |
| Squamous cell carcinoma | 55 (27%) |
| Others | 26 (13%) |
| Performance status | |
| 0 | 27 (13%) |
| 1 | 94 (47%) |
| 2 | 69 (34%) |
| 3 | 11 (6%) |
| Number of metastatic sites | |
| <3 | 102 (51%) |
| ≥3 | 99 (49%) |
| Brain metastases | |
| Present | 136 (68%) |
| Absent | 65 (32%) |
| Liver metastases | |
| Present | 33 (16%) |
| Absent | 168 (84%) |
| Stage at diagnosis/Stage at introduction of ICIs | |
| Stage III | 44/24 (22%/12%) |
| Stage IV | 144/176 (72%/87%) |
| Other | 13/1 (6%/1%) |
| History | |
| Chronic respiratory disease | 61 (30%) |
| Cardiovascular disease | 101 (50%) |
| Treatment with proton pump inhibitors, antibiotics, corticosteroids * | 73/79/33 (36%/39%/16%) |
| Treatment line | |
| ICI on the first line | 61 (30%) |
| Pretreatment with chemotherapy (≥ second line) | 140 (70%) |
| ICI type | |
| Nivolumab | 138 (69%) |
| Pembrolizumab | 51 (25%) |
| Nivolumab + Ipilimumab | 12 (6%) |
| PDL1 rate | |
| <1% | 76 (38%) |
| 1–50% | 37 (18%) |
| 50–75% | 47 (23%) ** |
| Multivariable Analysis | |||
|---|---|---|---|
| Variables | aHR | 95% CI | p-Value |
| Gender | 1.4 | 1.0–2.1 | 0.076 |
| Female | |||
| Male | |||
| PS at ICI initiation | 2.2 | 1.5–3.1 | <0.0001 |
| 0–1 | |||
| ≥2 | |||
| Antibiotic intake * | 1.6 | 1.1–2.3 | 0.008 |
| No | |||
| Yes | |||
| Corticosteroid intake * | 2.0 | 1.2–3.2 | 0.007 |
| No | |||
| Yes | |||
| Number of metastatic sites | 1.5 | 1.0–2.3 | 0.047 |
| <3 | |||
| ≥3 | |||
| Brain metastasis | 1.4 | 0.9–2.2 | 0.097 |
| No | |||
| Yes | |||
| Liver metastasis | 1.9 | 1.2–3.0 | 0.006 |
| No | |||
| Yes | |||
| LIPI score | - 2.1 1.8 | 1.3–3.2 1.1–3.1 | 0.005 - 0.001 0.017 |
| 0 | |||
| 1 | |||
| 2 | |||
| Grade 3–4 irAEs | 3.0 | 1.8–5.1 | <0.0001 |
| No | |||
| Yes | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guezour, N.; Soussi, G.; Brosseau, S.; Abbar, B.; Naltet, C.; Vauchier, C.; Poté, N.; Hachon, L.; Namour, C.; Khalil, A.; et al. Grade 3–4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study. Cancers 2022, 14, 3878. https://doi.org/10.3390/cancers14163878
Guezour N, Soussi G, Brosseau S, Abbar B, Naltet C, Vauchier C, Poté N, Hachon L, Namour C, Khalil A, et al. Grade 3–4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study. Cancers. 2022; 14(16):3878. https://doi.org/10.3390/cancers14163878
Chicago/Turabian StyleGuezour, Nadia, Ghassen Soussi, Solenn Brosseau, Baptiste Abbar, Charles Naltet, Charles Vauchier, Nicolas Poté, Lorry Hachon, Céline Namour, Antoine Khalil, and et al. 2022. "Grade 3–4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study" Cancers 14, no. 16: 3878. https://doi.org/10.3390/cancers14163878
APA StyleGuezour, N., Soussi, G., Brosseau, S., Abbar, B., Naltet, C., Vauchier, C., Poté, N., Hachon, L., Namour, C., Khalil, A., Trédaniel, J., Zalcman, G., & Gounant, V. (2022). Grade 3–4 Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer (NSCLC) Patients Are Correlated with Better Outcome: A Real-Life Observational Study. Cancers, 14(16), 3878. https://doi.org/10.3390/cancers14163878







