Can National Registries Contribute to Predict the Risk of Cancer? The Cancer Risk Assessment Model (CRAM)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design
2.2. Data Sources
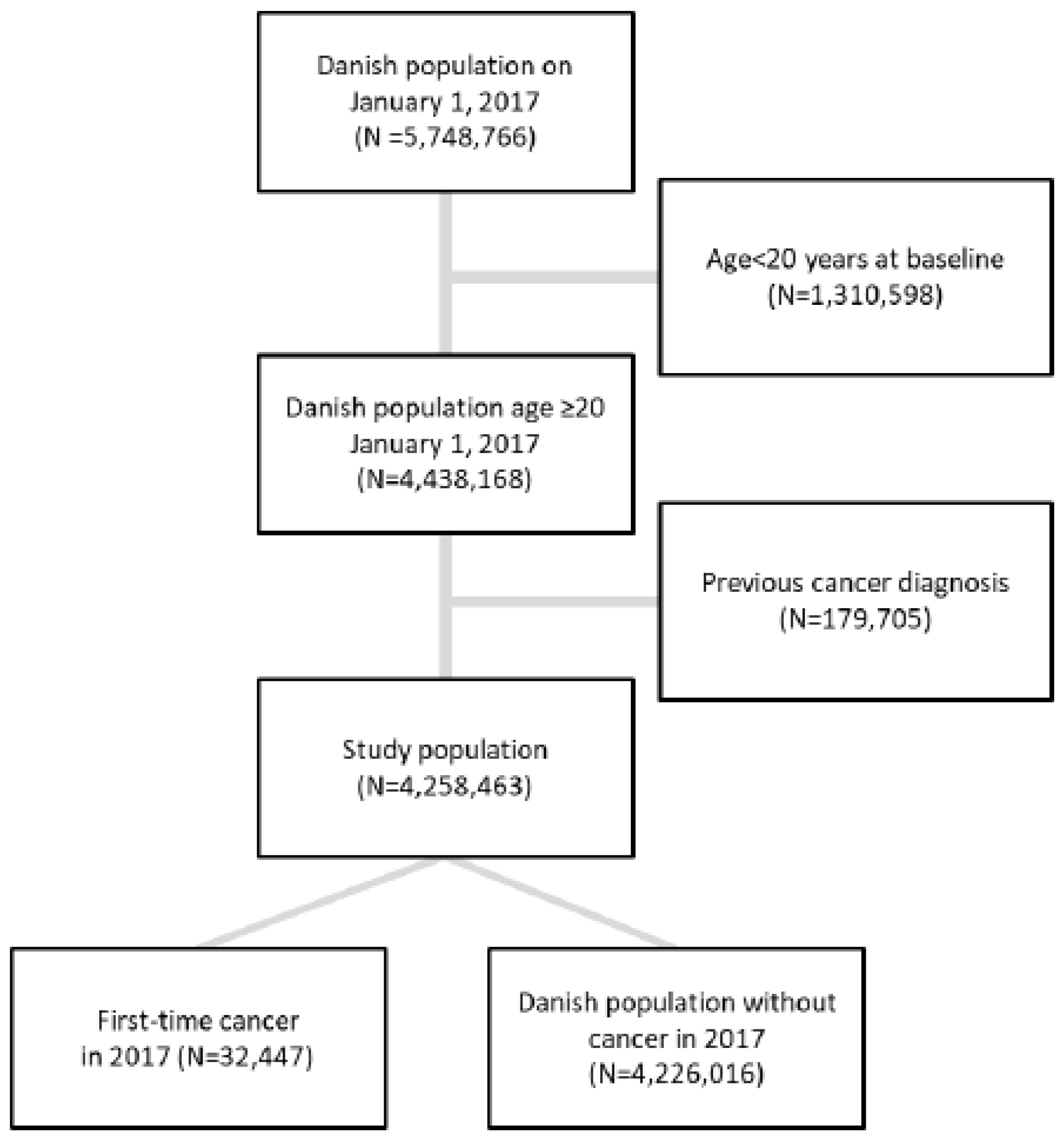
2.3. Study Population
2.4. Outcome (Cancer)
2.5. Conditions of Interest (Exposure)
2.6. Statistical Analysis
3. Results
3.1. Cancer Outcome
3.2. Conditions Related to Cancer and Development of the Predictive Model (CRAM)
3.3. Validation of CRAM
4. Discussion
4.1. Strengths and Limitations
4.2. Comparison with theExisting Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vuong, K.; McGeechan, K.; Armstrong, B.K.; Cust, A.E. Risk prediction models for incident primary cutaneous melanoma: A systematic review. JAMA Dermatol. 2014, 150, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Kendall, B.J.; Pandeya, N.; Whiteman, D.C. A model to determine absolute risk for esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 138–144.e2. [Google Scholar] [CrossRef] [PubMed]
- Frank, L. Epidemiology. When an entire country is a cohort. Science 2000, 287, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Frank, L. Epidemiology. The epidemiologist’s dream: Denmark. Science 2003, 301, 163. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.S.; Hamilton, P.; Hulme, C.T.; Meads, D.M.; Jones, H.; Newsham, A.; Marti, J.; Smith, A.; Mason, H.; Velikova, G.; et al. Costs of cancer care for use in economic evaluation: A UK analysis of patient-level routine health system data. Br. J. Cancer 2015, 112, 948–956. [Google Scholar] [CrossRef]
- Torring, M.L.; Falborg, A.Z.; Jensen, H.; Neal, R.D.; Weller, D.; Reguilon, I.; Menon, U.; Vedsted, P.; Almberg, S.S.; Anandan, C.; et al. Advanced-stage cancer and time to diagnosis: An International Cancer Benchmarking Partnership (ICBP) cross-sectional study. Eur. J. Cancer Care 2019, 28, e13100. [Google Scholar] [CrossRef]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, W. The CAPER studies: Five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br. J. Cancer 2009, 101 (Suppl. S2), S80–S86. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.; Haastrup, P.F.; Balasubramaniam, K.; Elnegaard, S.; Christensen, R.D.; Storsveen, M.M.; Søndergaard, J.; Jarbøl, D.E. Predictive values of colorectal cancer alarm symptoms in the general population: A nationwide cohort study. Br. J. Cancer 2019, 120, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.; Haastrup, P.F.; Balasubramaniam, K.; Christensen, R.D.; Sondergaard, J.; Jarbol, D.E. Predictive values of upper gastrointestinal cancer alarm symptoms in the general population: A nationwide cohort study. BMC Cancer 2018, 18, 440. [Google Scholar] [CrossRef] [Green Version]
- Haastrup, P.F.; Jarbol, D.E.; Balasubramaniam, K.; Saetre, L.M.S.; Sondergaard, J.; Rasmussen, S. Predictive values of lung cancer alarm symptoms in the general population: A nationwide cohort study. NPJ Prim. Care Respir. Med. 2020, 30, 15. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, I.K.; Andersen, J.S. Predictive value of the official cancer alarm symptoms in general practice—A systematic review. Dan. Med. J. 2015, 62, A5034. [Google Scholar]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundboll, J.; Laugesen, K.; Ehrenstein, V.; Sørensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baadsgaard, M.; Quitzau, J. Danish registers on personal income and transfer payments. Scand. J. Public Health 2011, 39 (Suppl. S7), 103–105. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.M.; Rasmussen, A.W. Danish Education Registers. Scand. J. Public Health 2011, 39 (Suppl. S7), 91–94. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Pedersen, L.; Sorensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Gjerstorff, M.L. The Danish Cancer Registry. Scand. J. Public Health 2011, 39 (Suppl. S7), 42–45. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [Green Version]
- Pottegard, A.; Schmidt, S.A.J.; Wallach-Kildemoes, H.; Sorensen, H.T.; Hallas, J.; Schmidt, M. Data Resource Profile: The Danish National Prescription Registry. Int. J. Epidemiol. 2017, 46, 798–798f. [Google Scholar] [CrossRef]
- Andersen, J.S.; Olivarius Nde, F.; Krasnik, A. The Danish National Health Service Register. Scand. J. Public Health 2011, 39 (Suppl. S7), 34–37. [Google Scholar] [CrossRef]
- Rubin, K.H.; Moller, S.; Holmberg, T.; Bliddal, M.; Sondergaard, J.; Abrahamsen, B. A New Fracture Risk Assessment Tool (FREM) Based on Public Health Registries. J. Bone Miner. Res. 2018, 33, 1967–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, L.C.; Ersboll, A.K. When the entire population is the sample: Strengths and limitations in register-based epidemiology. Eur. J. Epidemiol. 2014, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Development and validation of risk prediction algorithms to estimate future risk of common cancers in men and women: Prospective cohort study. BMJ Open 2015, 5, e007825. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.P.; Teare, M.D.; Stevens, J.; Archer, R. Risk Prediction Models for Lung Cancer: A Systematic Review. Clin. Lung Cancer 2016, 17, 95–106. [Google Scholar] [CrossRef]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; et al. Developing a Diagnostic Multivariable Prediction Model for Urinary Tract Cancer in Patients Referred with Haematuria: Results from the IDENTIFY Collaborative Study. IDENTIFY Study Group. Eur. Urol. Focus 2022, 24, S2405-4569(22)00129-8. [Google Scholar] [CrossRef]
- Ferrario, M.M.; Veronesi, G.; Chambless, L.E.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Salomaa, V.; Borglykke, A.; Hart, N.; Söderberg, S.; Cesana, G.; et al. The contribution of educational class in improving accuracy of cardiovascular risk prediction across European regions: The MORGAM Project Cohort Component. Heart 2014, 100, 1179–1187. [Google Scholar] [CrossRef]
- Ohm, J.; Skoglund, P.H.; Discacciati, A.; Sundstrom, J.; Hambraeus, K.; Jernberg, T.; Svensson, P. Socioeconomic status predicts second cardiovascular event in 29,226 survivors of a first myocardial infarction. Eur. J. Prev. Cardiol. 2018, 25, 985–993. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: Cohort study. BMJ 2017, 359, j5019. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; He, T.; Li, H.; Guo, X.; Zhang, Z. Improve individual treatment by comparing treatment benefits: Cancer artificial intelligence survival analysis system for cervical carcinoma. J. Transl. Med. 2022, 20, 293. [Google Scholar] [CrossRef]
- Boehm, K.M.; Aherne, E.A.; Ellenson, L.; Nikolovski, I.; Alghamdi, M.; Vázquez-García, I.; Zamarin, D.; Roche, K.L.; Liu, Y.; Patel, D.; et al. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat. Cancer 2022, 3, 723–733. [Google Scholar] [CrossRef]
- Huang, R.J.; Kwon, N.S.; Tomizawa, Y.; Choi, A.Y.; Hernandez-Boussard, T.; Hwang, J.H. A Comparison of Logistic Regression Against Machine Learning Algorithms for Gastric Cancer Risk Prediction Within Real-World Clinical Data Streams. JCO Clin. Cancer Inform. 2022, 6, e2200039. [Google Scholar] [CrossRef] [PubMed]

| Cases (N (%)) | Controls (N (%)) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women (n = 12,018) | Men (n = 12,318) | Women (n = 1,604,172) | Men (n = 1,565,340) | ||||||
| Development (n = 7973) | Validation (n = 4045) | Development (n = 8251) | Validation (n = 4067) | Development (n = 1,069,726) | Validation (n = 534,446) | Development (n = 1,043,282) | Validation (n = 522,058) | ||
| Age (Median (Q1–Q3) | 67.8 (57.4–75.8) | 67.9 (56.8–76.2) | 69.7 (61.7–75.8) | 69.5 (61.8–75.7) | 49.7 (35.1–64.8) | 49.7 (35.0–64.8) | 48.2 (33.9–62.0) | 48.2 (33.9–62.0) | |
| Age categories | |||||||||
| Age 20.0–39.9 | 381 (4.8) | 213 (5.3) | 220 (2.7) | 120 (3.0) | 350,901 (32.8) | 175,890 (32.9) | 364,505 (34.9) | 182,329 (34.9) | |
| Age 40.0–59.9 | 2040 (25.6) | 1051 (26.0) | 1524 (18.5) | 743 (18.3) | 375,185 (35.1) | 186,510 (34.9) | 384,620 (36.9) | 192,888 (36.9) | |
| Age 60.0–79.9 | 4330 (54.3) | 2173 (53.7) | 5315 (64.4) | 2629 (64.6) | 275,353 (25.7) | 138,047 (25.8) | 254,286 (24.4) | 126,906 (24.3) | |
| Age ≥80.0 | 1222 (15.3) | 608 (15.0) | 1192 (14.4) | 575 (14.1) | 68,287 (6.4) | 33,999 (6.4) | 39,871 (3.8) | 19,935 (3.8) | |
| Marital status | |||||||||
| Married or living with someone | 4746 (59.3) | 2418 (59.8) | 5956 (72.2) | 2930 (72.0) | 705,074(65.9) | 352,347 (65.9) | 707,332 (67.8) | 353,782 (67.8) | |
| Living alone | 3227 (40.5) | 1627 (40.2) | 2295 (27.8) | 1137 (28.0) | 364,652 (34.1) | 182,099 (34.1) | 335,950 (32.2) | 168,276 (32.2) | |
| Ethnicity | |||||||||
| Danish | 7481 (93.8) | 3800 (93.9) | 7837 (95.0) | 3856 (94.8) | 929,056 (86.8) | 464,024 (86.8) | 902,004 (86.5) | 451,317 (86.4) | |
| Immigrant | 469 (5.9) | 233 (5.8) | 405 (4.9) | 205 (5.0) | 126,935 (11.9) | 63,477 (11.9) | 126,756 (12.1) | 63,635 (12.2) | |
| Descendant | 23 (0.3) | 12 (0.3) | 9 (0.1) | 6 (0.1) | 13,735 (1.3) | 6945 (1.3) | 14,522 (1.4) | 7106 (1.4) | |
| Country of origin | |||||||||
| Denmark | 7481 (93.8) | 3800 (93.9) | 7837 (98.3) | 3856 (95.3) | 929,056 (86.8) | 464,024 (86.8) | 902,004 (86.5) | 451,317 (86.4) | |
| Western countries | 264 (3.3) | 127 (3.1) | 206 (2.6) | 111 (2.7) | 54,419 (5.1) | 27,090 (5.1) | 57,819 (5.5) | 28,813 (5.5) | |
| Non-Western countries | 228 (2.9) | 118 (2.9) | 208 (2.6) | 100 (2.5) | 86,238 (8.1) | 43,320 (8.1) | 83,446 (8.0) | 41,923 (8.0) | |
| Unknown or missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (0.0) | 12 (0.0) | 13 (0.0) | 5 (0.0) | |
| Income # | |||||||||
| High tertile | 2106 (26.4) | 1038 (25.7) | 3018 (36.6) | 1521 (37.4) | 285,158 (26.7) | 143,399 (26.8) | 418,974 (40.2) | 209,573 (40.1) | |
| Middle tertile | 2895 (36.3) | 1493 (36.9) | 2492 (30.2) | 1288 (31.7) | 393,342 (36.8) | 196,379 (36.7) | 311,220 (29.8) | 155,220 (29.7) | |
| Low tertile | 2972 (37.3) | 1514 (37.4) | 2741 (33.2) | 1258 (30.9) | 391,180 (36.6) | 194,648 (36.4) | 313,038 (30.0) | 157,244 (30.1) | |
| Unknown or missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 46 (0.0) | 20 (0.0) | 50 (0.0) | 21 (0.0) | |
| Occupational status | |||||||||
| Employed | 2413 (30.3) | 1258 (31.1) | 2428 (29.4) | 1239 (30.5) | 550,737 (51.5) | 275,445 (51.5) | 635,210 (60.9) | 318,155 (60.9) | |
| Unemployed or on welfare payment | 64 (0.8) | 149 (3.7) | 30 (0.4) | 119 (2.9) | 76,566 (7.2) | 40,173 (7.5) | 63,911 (6.1) | 31,336 (6.0) | |
| Education | 292 (3.7) | 24 (0.6) | 255 (3.1) | 15 (0.4) | 80,826 (7.6) | 38,207 (7.1) | 62,085 (6.0) | 31,830 (6.1) | |
| Early retirement | 744 (9.3) | 336 (8.3) | 611 (7.4) | 316 (7.8) | 73,383 (6.9) | 36,833 (6.9) | 59,022 (5.7) | 29,557 (5.7) | |
| Retirement pension | 4360 (54.7) | 2211 (54.7) | 4832 (58.6) | 2349 (57.8) | 244,640 (22.9) | 122,007 (22.8) | 183,363 (17.6) | 91,385 (17.5) | |
| Unknown or missing | 100 (1.3) | 67 (1.7) | 95 (1.2) | 29 (0.7) | 43,574 (4.1) | 21,781 (4.1) | 39,691 (3.8) | 19,795 (3.8) | |
| Education | |||||||||
| High education | 2784 (34.9) | 1397 (34.5) | 2446 (29.6) | 1220 (30.0) | 251,820 (23.5) | 124,765 (23.3) | 238,119 (22.8) | 119,297 (22.9) | |
| Medium education | 2927 (36.7) | 1549 (38.3) | 3765 (45.6) | 1854 (45.6) | 414,171 (38.7) | 207,506 (38.8) | 472,263 (45.3) | 236,730 (45.3) | |
| Low education | 2091 (26.2) | 1010 (25.0) | 1840 (22.3) | 899 (22.1) | 358,649 (33.5) | 179,490 (33.6) | 283,304 (27.2) | 141,404 (27.1) | |
| Unknown or missing | 171 (2.1) | 89 (2.2) | 200 (2.4) | 94 (2.3) | 45,086 (4.2) | 22,685 (4.2) | 49,596 (4.8) | 24,627 (4.7) | |
| Dead in year 2017 | 1071 (13.4) | 560 (13.8) | 1268 (15.4) | 620 (15.2) | 9135 (0.9) | 4501 (0.8) | 8427 (0.8) | 4325 (0.8) | |
| Comorbidity | |||||||||
| Charlson = 0 | 6500 (81.5) | 3307 (81.8) | 6588 (79.8) | 3292 (80.9) | 968,598 (90.5) | 484,189 (90.6) | 958,628 (91.9) | 479,665 (91.9) | |
| Charlson = 1–2 | 1281 (16.1) | 620 (15.3) | 1324 (16.0) | 609 (15.0) | 90,170 (8.4) | 44,776 (8.4) | 72,649 (7.0) | 36,293 (7.0) | |
| Charlson ≥ 3 | 192 (2.4) | 118 (2.9) | 339 (4.1) | 166 (4.1) | 10,958 (1.0) | 5481 (1.0) | 12,005 (1.2) | 6100 (1.2) | |
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Age Categories | ||
| Age 20–29 | Ref | |
| Age 30–34 | 2.15 (1.65–2.80) | <0.001 |
| Age 35–39 | 3.00 (2.35–3.82) | <0.001 |
| Age 40–44 | 4.67 (3.75–5.81) | <0.001 |
| Age 45–49 | 6.88 (5.59–8.48) | <0.001 |
| Age 50–54 | 9.38 (7.66–11.48) | <0.001 |
| Age 55–59 | 13.00 (10.65–15.87) | <0.001 |
| Age 60–64 | 17.47 (14.35–21.26) | <0.001 |
| Age 65–69 | 23.07 (18.99–28.03) | <0.001 |
| Age 70–74 | 25.81 (21.25–31.35) | <0.001 |
| Age 75–79 | 28.17 (23.10–34.36) | <0.001 |
| Age 80–84 | 32.23 (26.32–39.47) | <0.001 |
| Age 85–89 | 25.44 (20.47–31.61) | <0.001 |
| Age 90–94 | 18.02 (13.88–23.38) | <0.001 |
| Age 95–99 | 11.90 (7.56–18.74) | <0.001 |
| Age +100 | 11.74 (3.71–37.15) | <0.001 |
| ICD-10 codes | ||
| T26 (Burns and corrosion confined to the eye and adnexa) | 2.43 (1.43–4.13) | 0.001 |
| O28 (Abnormal findings on antenatal screening of the mother) | 2.06 (1.29–3.29) | 0.003 |
| D05 (Carcinoma in situ of the breast) | 2.04 (1.52–2.73) | <0.001 |
| E64 (Sequelae of malnutrition and other nutritional deficiencies) | 1.77 (1.17–2.66) | 0.006 |
| K70 (Alcoholic liver disease) | 1.71 (1.22–2.41) | 0.002 |
| K13 (Other diseases of the lip and oral mucosa) | 1.69 (1.21–2.36) | 0.002 |
| J90 (Pleural effusion, not elsewhere classified) | 1.64 (1.16–2.33) | 0.005 |
| K83 (Other diseases of the biliary tract) | 1.62 (1.18–2.23) | 0.003 |
| D22 (Melanocytic naevi) | 1.52 (1.17–1.97) | 0.002 |
| C44 (skin cancer, other type) | 1.48 (1.30–1.70) | <0.001 |
| N60 (Benign mammary dysplasia) | 1.40 (1.16–1.69) | <0.001 |
| J44 (Other chronic obstructive pulmonary disease) | 1.39 (1.26–1.54) | <0.001 |
| I73 (Other peripheral vascular diseases) | 1.36 (1.17–1.58) | <0.001 |
| D24 (Benign neoplasm of the breast) | 1.36 (1.13–1.65) | 0.001 |
| D12 (Benign neoplasm of the colon, rectum, anus and anal canal) | 1.31 (1.17–1.46) | <0.001 |
| G40 (Epilepsy) | 1.34 (1.09–1.65) | 0.006 |
| E04 (Other nontoxic goiter) | 1.28 (1.11–1.47) | <0.001 |
| R00 (Abnormalities of the heartbeat) | 1.24 (1.06–1.46) | 0.009 |
| D25 (Leiomyoma of uterus) | 1.24 (1.06–1.47) | 0.009 |
| VRK (Perioperative bleeding (ml)) | 0.76 (0.62–0.92) | 0.006 |
| M15 (Polyarthrosis) | 0.63 (0.47–0.85) | 0.002 |
| F03 (Unspecified dementia) | 0.45 (0.29–0.68) | <0.001 |
| G30 (Alzheimer’s disease) | 0.37 (0.21–0.65) | 0.001 |
| F22 (Persistent delusional disorders) | 0.32 (0.14–0.72) | 0.006 |
| ATC codes | ||
| N07 (Other nervous system drugs) | 1.39 (1.26–1.54) | <0.001 |
| L04 (Immunosuppressants) | 1.21 (1.07–1.38) | 0.004 |
| C08 (Calcium channel blockers) | 1.08 (1.02–1.14) | 0.008 |
| N02 (Analgesics) | 1.07 (1.02–1.13) | 0.004 |
| M05 (Drugs for treatment of bone diseases) | 0.89 (0.82–0.96) | 0.002 |
| A06 (Drugs for constipation) | 0.79 (0.71–0.88) | <0.001 |
| Practicing specialists (Year(s) before cancer diagnosis) | ||
| Child psychiatry (3 years) | 46.64 (6.32–344.33) | <0.001 |
| Plastic surgery (1 year) | 1.24 (1.06–1.45) | 0.008 |
| Internal medicine (4 years) | 0.87 (0.80–0.95) | 0.002 |
| Psychiatry (5 years) | 0.82 (0.72–0.94) | 0.003 |
| GP consultations and procedures (Year(s) before cancer diagnosis) | ||
| GP spirometry (4 years) | 1.11 (1.04–1.17) | 0.001 |
| GP spirometry (1 year) | 1.09 (1.02–1.15) | 0.008 |
| GP point-of-care hemoglobin (1 year) | 1.04 (1.01–1.06) | 0.001 |
| GP consultation (1 year) | 1.02 (1.02–1.03) | <0.001 |
| GP consultation (2 years) | 0.99 (0.98–0.99) | <0.001 |
| GP point-of-care hemoglobin (6 years) | 0.96 (0.93–0.99) | 0.007 |
| Constant (baseline odds) | 0.0005648 (0.0004691–0.0006799) | <0.001 |
| Variables | OR (95% CI) | p-Value |
|---|---|---|
| Age Categories | ||
| Age 20–29 | Ref | |
| Age 30–34 | 1.68 (1.19–2.38) | 0.003 |
| Age 35–39 | 2.63 (1.94–3.58) | <0.001 |
| Age 40–44 | 3.47 (2.61–4.60) | <0.001 |
| Age 45–49 | 5.84 (4.50–7.57) | <0.001 |
| Age 50–54 | 10.41 (8.16–13.29) | <0.001 |
| Age 55–59 | 18.79 (14.81–23.84) | <0.001 |
| Age 60–64 | 29.98 (23.72–37.90) | <0.001 |
| Age 65–69 | 42.80 (33.92–54.02) | <0.001 |
| Age 70–74 | 57.24 (45.37–72.20) | <0.001 |
| Age 75–79 | 59.19 (46.76–74.92) | <0.001 |
| Age 80–84 | 64.60 (50.78–82.17) | <0.001 |
| Age 85–89 | 66.24 (51.47–85.26) | <0.001 |
| Age 90–94 | 55.49 (41.02–75.07) | <0.001 |
| Age 95–99 | 51.39 (30.50–86.57) | <0.001 |
| Age +100 | 78.90 (18.95–328.52) | <0.001 |
| ICD-10 codes | ||
| B18 (Chronic viral hepatitis) | 2.21 (1.63–3.00) | <0.001 |
| T23 (Burns and corrosion of the wrist and hand) | 1.77 (1.20–2.62) | 0.004 |
| K83 (Other diseases of the biliary tract) | 1.78 (1.29–2.46) | <0.001 |
| R79 (Other abnormal findings of blood chemistry) | 1.66 (1.46–1.90) | <0.001 |
| K70 (Alcoholic liver disease) | 1.63 (1.27–2.08) | <0.001 |
| F10 (Mental and behavioral disorders due to use of alcohol) | 1.51 (1.34–1.70) | <0.001 |
| E04 (Other nontoxic goiter) | 1.46 (1.15–1.87) | 0.002 |
| T18 (Foreign body in the alimentary tract) | 1.45 (1.11–1.89) | 0.007 |
| F17 (Mental and behavioral disorders due to use of tobacco) | 1.36 (1.16–1.59) | <0.001 |
| R91 (Abnormal findings on diagnostic imaging of the lung) | 1.34 (1.17–1.53) | <0.001 |
| D12 (Benign neoplasm of the colon, rectum, anus and anal canal) | 1.32 (1.21–1.45) | <0.001 |
| I70 (Atherosclerosis) | 1.28 (1.10–1.49) | 0.001 |
| T81 (Complications of procedures, not elsewhere classified) | 1.20 (1.05–1.37) | 0.006 |
| K57 (Diverticular disease of the intestine) | 0.85 (0.76–0.95) | 0.005 |
| R29 (Other symptoms and signs involving the nervous and musculoskeletal systems) | 0.80 (0.70–0.92) | 0.002 |
| F00 (Dementia in Alzheimer’s disease) | 0.39 (0.23–0.68) | 0.001 |
| ATC codes | ||
| N07 (Other nervous system drugs) | 1.22 (1.10–1.34) | <0.001 |
| R03 (Adrenergics, inhalants) | 1.12 (1.05–1.20) | 0.001 |
| C08 (Calcium channel blockers) | 1.11 (1.05–1.17) | <0.001 |
| N02 (Analgesics) | 1.07 (1.02–1.13) | 0.004 |
| A12 (Mineral supplements) | 0.89 (0.83–0.97) | 0.006 |
| R01 (Nasal preparations) | 0.86 (0.80–0.93) | <0.001 |
| A06 (Drugs for constipation) | 0.84 (0.75–0.94) | 0.002 |
| Practicing specialists (Year(s) before cancer diagnosis) | ||
| Paediatrics (8 years) | 1.26 (1.08–1.47) | 0.003 |
| Surgery (1 year) | 1.17 (1.06–1.28) | 0.001 |
| Dermatologist (3 years) | 1.05 (1.02–1.07) | <0.001 |
| Ear specialist (1 year) | 1.06 (1.02–1.10) | 0.001 |
| Ear specialist (4 years) | 0.95 (0.91–0.99) | 0.009 |
| Radiology Copenhagen (3 years) | 0.90 (0.84–0.96) | 0.002 |
| GP contacts or procedures | ||
| GP spirometry (5 years) | 1.21 (1.05–1.38) | 0.007 |
| GP spirometry (6 years) | 1.10 (1.03–1.17) | 0.002 |
| GP Spirometry (2 years) | 1.10 (1.03–1.16) | 0.002 |
| GP urine examination (1 year) | 1.07 (1.04–1.09) | <0.001 |
| GP laboratory test (10 years) | 1.06 (1.02–1.10) | 0.003 |
| GP blood sample (1 year) | 1.04 (1.03–1.05) | <0.001 |
| GP C-reactive protein testing (1 yr) | 1.04 (1.02–1.06) | <0.001 |
| GP telephone consultation (1 year) | 1.01 (1.00–1.02) | 0.002 |
| GP e-mail consultation (1 year) | 0.98 (0.97–0.99) | <0.001 |
| GP urine examination (4 years) | 0.95 (0.92–0.98) | <0.001 |
| Out of hours services, telephone consultation (2 years) | 0.95 (0.91–0.98) | 0.006 |
| Out of hours service, consultation (6 years) | 0.90 (0.84–0.97) | 0.006 |
| GP peak flow (9 years) | 0.79 (0.66–0.94) | 0.008 |
| Constant (baseline odds) | 0.0003711 (0.0002961–0.0004651) | <0.001 |
| Men | Women | |||
|---|---|---|---|---|
| Development Cohort | Validation Cohort | Development Cohort | Validation Cohort | |
| Model | AUC (95% confidence interval) | |||
| Age | 0.81 (0.81–0.82) | 0.81 (0.80–0.81) | 0.75 (0.74–0.75) | 0.74 (0.74–0.75) |
| SES | 0.75 (0.75–0.76) | 0.75 (0.74–0.76) | 0.70 (0.70–0.71) | 0.70 (0.69–0.701) |
| Model A | 0.82 (0.82–0.83) | 0.82 (0.81–0.82) | 0.76 (0.75–0.76) | 0.75 (0.74–0.75) |
| Model B | 0.825 (0.82–0.83) | 0.82 (0.81–0.82) | 0.76 (0.76–0.77) | 0.75 (0.74–0.76) |
| Absolute Predictive Performance of Model A | ||||
|---|---|---|---|---|
| Validation cohort | Model A | |||
| Gender | Men | Women | ||
| Individuals | 526,125 | 526,125 | ||
| Total cancer cases | 4067 | 4045 | ||
| Risk cut-off | 1% | 5% | 1% | 5% |
| Number of subjects predicted above cutoff | 151,668 | 2550 | 163,438 | 439 |
| Cancer cases detected | 3247 | 96 | 2,77 | 11 |
| Positive Predictive value | 2.1% | 3.8% | 1.6% | 2.4% |
| Sensitivity | 78.8% | 2.4% | 66.2% | 0.3% |
| Odds ratio | 9.78 | 4.96 | 4.49 | 3.34 |
| (95%CI) | (9.05;10.57) | (4.00; 6.10) | (4.20; 4.80) | (1.65; 6.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarbøl, D.E.; Hyldig, N.; Möller, S.; Wehberg, S.; Rasmussen, S.; Balasubramaniam, K.; Haastrup, P.F.; Søndergaard, J.; Rubin, K.H. Can National Registries Contribute to Predict the Risk of Cancer? The Cancer Risk Assessment Model (CRAM). Cancers 2022, 14, 3823. https://doi.org/10.3390/cancers14153823
Jarbøl DE, Hyldig N, Möller S, Wehberg S, Rasmussen S, Balasubramaniam K, Haastrup PF, Søndergaard J, Rubin KH. Can National Registries Contribute to Predict the Risk of Cancer? The Cancer Risk Assessment Model (CRAM). Cancers. 2022; 14(15):3823. https://doi.org/10.3390/cancers14153823
Chicago/Turabian StyleJarbøl, Dorte E., Nana Hyldig, Sören Möller, Sonja Wehberg, Sanne Rasmussen, Kirubakaran Balasubramaniam, Peter F. Haastrup, Jens Søndergaard, and Katrine H. Rubin. 2022. "Can National Registries Contribute to Predict the Risk of Cancer? The Cancer Risk Assessment Model (CRAM)" Cancers 14, no. 15: 3823. https://doi.org/10.3390/cancers14153823
APA StyleJarbøl, D. E., Hyldig, N., Möller, S., Wehberg, S., Rasmussen, S., Balasubramaniam, K., Haastrup, P. F., Søndergaard, J., & Rubin, K. H. (2022). Can National Registries Contribute to Predict the Risk of Cancer? The Cancer Risk Assessment Model (CRAM). Cancers, 14(15), 3823. https://doi.org/10.3390/cancers14153823








