Salvage Nodal Radiotherapy as Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer Detected by Positron Emission Tomography Shows Favorable Outcome in Long-Term Follow-Up
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Singh, R.; Wang, M.; Chinchilli, V.M.; Trifiletti, D.M.; Ost, P.; Siva, S.; Meng, M.B.; Tchelebi, L.; Zaorsky, N.G. Safety and Survival Rates Associated with Ablative Stereotactic Radiotherapy for Patients with Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021, 7, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; Macmillan, T.; Grzeda, M.T.; Peacock, J.; Summers, J.; Eddy, S.; Coker, B.; Patrick, H.; Powell, H.; Berry, L.; et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: A prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021, 22, 98–106. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Goetghebeur, E.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J. Clin. Oncol. 2020, 38 (Suppl. 6), 10. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs. Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecouvet, F.E.; Oprea-Lager, D.-E.; Liu, Y.; Ost, P.; Bidaut, L.; Collette, L.; Deroose, C.; Goffin, K.; Herrmann, K.; Hoekstra, O.S.; et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: A consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 2018, 19, e534–e545. [Google Scholar] [CrossRef]
- Alberts, I.L.; Seide, S.E.; Mingels, C.; Bohn, K.P.; Shi, K.; Zacho, H.D.; Rominger, A.; Afshar-Oromieh, A. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: A systematic review and network meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989. [Google Scholar] [CrossRef]
- Cytawa, W.; Hartrampf, P.; Lass, P.; Kircher, M.; Polat, B.; Buck, A.K.; Lapa, C. PSMA Theranostics: A “Must Have” in Every Prostate Cancer Center. Illustration of Two Clinical Cases and Review of the Literature. Clin. Genitourin Cancer 2021, 19, e235–e247. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölscher, T.; Baumann, M.; Kotzerke, J.; Zöphel, K.; Paulsen, F.; Müller, A.-C.; Zips, D.; Thomas, C.; Wirth, M.; Troost, E.G.C.; et al. Local Control after Locally Ablative, Image-Guided Radiotherapy of Oligometastases Identified by Gallium-68-PSMA-Positron Emission Tomography in Castration-Sensitive Prostate Cancer Patients (OLI-P). Cancers 2022, 14, 2073. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Decaestecker, K.; De Meerleer, G.; Lambert, B.; Delrue, L.; Fonteyne, V.; Claeys, T.; De Vos, F.; Huysse, W.; Hautekiet, A.; Maes, G.; et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat. Oncol. 2014, 9, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bleser, E.; Jereczek-Fossa, B.A.; Pasquier, D.; Zilli, T.; Van As, N.; Siva, S.; Fodor, A.; Dirix, P.; Gomez-Iturriaga, A.; Trippa, F.; et al. Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur. Urol. 2019, 76, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M.; Aebersold, D.M.; Böhmer, D.; Flentje, M.; Ghadjar, P.; Schmidt-Hegemann, N.-S.; Höcht, S.; Hölscher, T.; Müller, A.-C.; Niehoff, P.; et al. Radiotherapy in nodal oligorecurrent prostate cancer. Strahlenther Onkol. 2021, 197, 575–580. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.-L.; Supiot, S.; Belkacemi, Y.; Peiffert, D.; Allouache, N.; et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 4 May 2022).
- Therneau, T.; Crowson, C.; Atkinson, E. Multi-State Models and Competing Risks. CRAN-R. 2020. Available online: https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf (accessed on 24 July 2022).
- Fodor, A.; Berardi, G.; Fiorino, C.; Picchio, M.; Busnardo, E.; Kirienko, M.; Incerti, E.; Dell’Oca, I.; Cozzarini, C.; Mangili, P.; et al. Toxicity and efficacy of salvage carbon 11-choline positron emission tomography/computed tomography-guided radiation therapy in patients with lymph node recurrence of prostate cancer. BJU Int. 2017, 119, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, G.; Mariucci, C.; Tenti, M.V.; Bini, V.; Alì, E.; Saldi, S.; Palumbo, I.; Bellavita, R.; Aristei, C. Salvage radiotherapy in patients affected by oligorecurrent pelvic nodal prostate cancer. Clin. Transl. Oncol. 2020, 22, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, P.; Trapp, C.; von Bestenbostel, R.; Eze, C.; Ganswindt, U.; Li, M.; Unterrainer, M.; Zacherl, M.J.; Ilhan, H.; Beyer, L.; et al. Outcome after PSMA-PET/CT-based salvage radiotherapy for nodal recurrence after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The Impact of (68)Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Mao, J.; Yang, J.; Wang, T.; Zhao, Z.H. Comparing the diagnostic performance of radiotracers in prostate cancer biochemical recurrence: A systematic review and meta-analysis. Eur. Radiol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Krebs, M.; Polat, B.; Linke, F.; Eiber, M.; Samnick, S.; Lapa, C.; Lassmann, M.; Riedmiller, H.; Czernin, J.; et al. 68Ga-PSMA-PET/CT in Patients with Biochemical Prostate Cancer Recurrence and Negative 18F-Choline-PET/CT. Clin. Nucl. Med. 2016, 41, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supiot, S.; Vaugier, L.; Pasquier, D.; Buthaud, X.; Magné, N.; Peiffert, D.; Sargos, P.; Crehange, G.; Pommier, P.; Loos, G.; et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur. Urol. 2021, 80, 405–414. [Google Scholar] [CrossRef]
- Kroeze, S.G.; Henkenberens, C.; Schmidt-Hegemann, N.S.; Vogel, M.M.E.; Kirste, S.; Becker, J.; Burger, I.A.; Derlin, T.; Bartenstein, P.; Eiber, M.; et al. Prostate-specific Membrane Antigen Positron Emission Tomography-detected Oligorecurrent Prostate Cancer Treated with Metastases-directed Radiotherapy: Role of Addition and Duration of Androgen Deprivation. Eur. Urol. Focus 2021, 7, 309–316. [Google Scholar] [CrossRef] [Green Version]
- De Bruycker, A.; Spiessens, A.; Dirix, P.; Koutsouvelis, N.; Semac, I.; Liefhooghe, N.; Gomez-Iturriaga, A.; Everaerts, W.; Otte, F.; Papachristofilou, A.; et al. PEACE V—Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): A study protocol for a randomized controlled phase II trial. BMC Cancer 2020, 20, 406. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.; Staal, F.H.E.; Brouwer, C.L.; Langendijk, J.A.; de Jong, I.J.; van Moorselaar, R.J.A.; Schuit, E.; Verzijlbergen, J.F.; Smeenk, R.J.; Aluwini, S. Androgen Deprivation therapy for Oligo-recurrent Prostate cancer in addition to radioTherapy (ADOPT): Study protocol for a randomised phase III trial. BMC Cancer 2022, 22, 482. [Google Scholar] [CrossRef]
- Farolfi, A.; Ilhan, H.; Gafita, A.; Calais, J.; Barbato, F.; Weber, M.; Afshar-Oromieh, A.; Spohn, F.; Wetter, A.; Rischpler, C.; et al. Mapping Prostate Cancer Lesions Before and After Unsuccessful Salvage Lymph Node Dissection Using Repeat PSMA PET. J. Nucl. Med. 2020, 61, 1037–1042. [Google Scholar] [CrossRef]
- Plata Bello, A.; Touijer, K.A. The future of salvage lymph node dissection in the prostate-specific membrane antigen era. BJU Int. 2021, 128, 652–653. [Google Scholar] [CrossRef]
- Kretschmer, A.; Milow, J.; Eze, C.; Buchner, A.; Li, M.; Westhofen, T.; Fuchs, F.; Rogowski, P.; Trapp, C.; Ganswindt, U.; et al. Patient-Reported and Oncological Outcomes of Salvage Therapies for PSMA-Positive Nodal Recurrent Prostate Cancer: Real-Life Experiences and Implications for Future Trial Design. Front. Oncol. 2021, 11, 708595. [Google Scholar] [CrossRef]
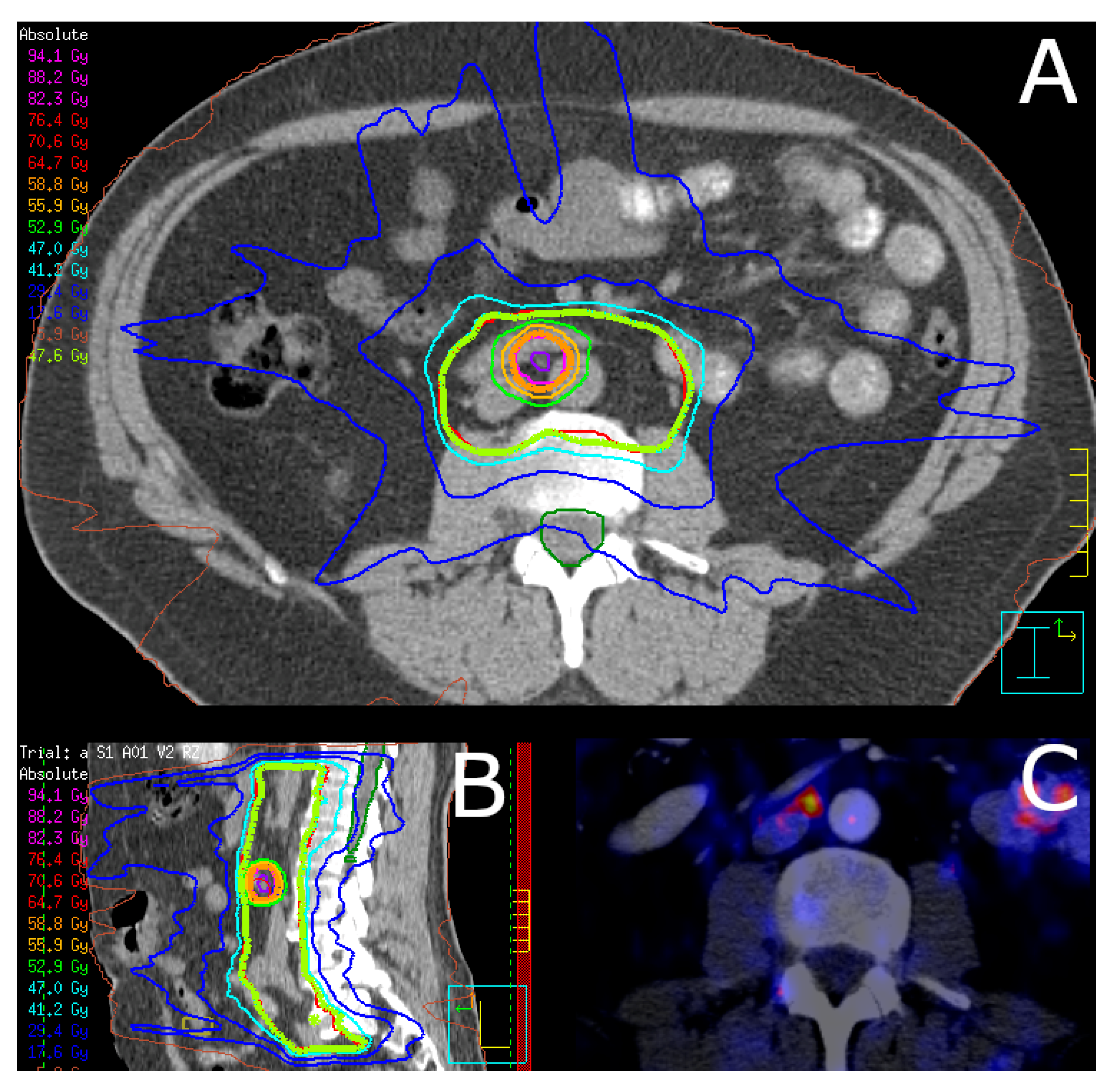
| Characteristics | Overall | No ADT | ADT | p Value |
|---|---|---|---|---|
| Patients, n (%) | 95 (100) | 38 (40) | 57 (60) | |
| Follow-up (months) | 47.1 (24.3, 75.0) | 45.7 (24.6, 70.3) | 50.4 (23.6, 79.9) | 0.605 |
| KPS (%) | 90 (90, 100) | 100 (90, 100) | 90 (90, 100) | 0.248 |
| Age at SNRT start (years) | 70.6 (66.3, 75.0) | 70.4 (63.8, 74.5) | 72.3 (67.9, 75.2) | 0.364 |
| PSA at primary diagnosis (ng/mL) | 10.9 (6.7, 17.7) | 8.9 (6.5, 14.0) | 11.4 (7.4, 24.6) | 0.102 |
| PSA at primary diagnosis, n (%) | 0.120 | |||
| <10 ng/mL | 45 (47.4) | 21 (55.3) | 24 (42.1) | |
| 10–20 ng/mL | 26 (27.4) | 11 (28.9) | 15 (26.3) | |
| >20 ng/mL | 20 (21.1) | 4 (10.5) | 16 (28.1) | |
| N/A | 4 (4.2) | 2 (5.3) | 2 (3.5) | |
| PSA at SNRT start (ng/mL) | 2.3 (0.6, 4.9) | 1.44 (0.5, 3.8) | 3.0 (0.7, 5.3) | 0.272 |
| Gleason-Score | 0.977 | |||
| ≤6 | 13 (13.7) | 5 (13.2) | 8 (14.0) | |
| 7 | 45 (47.4) | 18 (47.4) | 27 (47.4) | |
| ≥8 | 36 (37.9) | 15 (39.5) | 21 (36.8) | |
| N/A | 1 (1.1) | 0 (0) | 1 (1.8) | |
| Initial stage, n (%) | 0.576 | |||
| ≤T2a | 11 (11.6) | 6 (15.8) | 5 (8.8) | |
| T2b | 5 (5.3) | 2 (5.3) | 3 (5.3) | |
| ≥T2c | 79 (83.2) | 30 (78.9) | 49 (86.0) | |
| Initial D’Amico risk class, n (%) | 0.229 | |||
| Low | 3 (3.2) | 2 (5.3) | 1 (1.8) | |
| Intermediate | 6 (6.3) | 4 (10.5) | 2 (3.5) | |
| High | 86 (90.5) | 32 (84.2) | 54 (94.7) | |
| Initial treatment before SNRT | 0.629 | |||
| Surgery only | 33 (34.7) | 11 (28.9) | 22 (38.6) | |
| Surgery + adjuvant RT | 15 (15.8) | 8 (21.1) | 7 (12.3) | |
| Surgery + salvage RT | 35 (36.8) | 14 (36.8) | 21 (36.8) | |
| Primary RT | 12 (12.6) | 5 (13.2) | 7 (12.3) | |
| PET imaging, n (%) | 0.480 | |||
| PSMA PET | 67 (70.5) | 29 (76.3) | 38 (66.7) | |
| Choline PET | 27 (28.4) | 9 (23.7) | 18 (31.6) | |
| Conventional | 1 (1.1) | 0 (0) | 1 (1.8) | |
| PET-positive lymph node metastases, n (%) | 0.476 | |||
| 1 | 49 (51.6) | 21 (55.3) | 28 (49.1) | |
| 2 | 21 (22.1) | 12 (31.6) | 9 (15.8) | |
| 3 | 5 (5.3) | 2 (5.3) | 3 (5.3) | |
| ≥4 | 20 (21.1) | 3 (7.9) | 17 (29.8) | |
| SNRT location | 0.253 | |||
| Pelvic (N1) | 65 (68.4) | 30 (78.9) | 35 (61.4) | |
| Extrapelvic (M1a) | 18 (18.9) | 6 (15.8) | 12 (21.1) | |
| Pelvic + extrapelvic (N1 + M1a) | 12 (12.6) | 2 (5.3) | 10 (17.5) | |
| Biochemical Progression | Univariate | Multivariate | Multivariate AIC | |||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | p Value * | HR (95% CI) | p Value * | HR (95% CI) | p Value * |
| PSA at RT start | 1.05 (1.02–1.08) | 0.03 | 1.02 (0.96–1.09) | 0.44 | 1.04 (1.00–1.08) | 0.05 |
| Number of LN ≥ 4 | ||||||
| No | Ref | Ref | ||||
| Yes | 1.39 (0.66–2.96) | 0.40 | 1.49 (0.56–3.96) | 0.42 | ||
| Gleason score ≥ 8 | ||||||
| No | Ref | Ref | ||||
| Yes | 1.00 (0.49–2.04) | 1.00 | 1.17 (0.53–2.58) | 0.70 | ||
| Age at start of RT | 1.05 (1.00–1.10) | 0.06 | 1.04 (0.99–1.10) | 0.09 | 1.05 (1.00–1.10) | 0.06 |
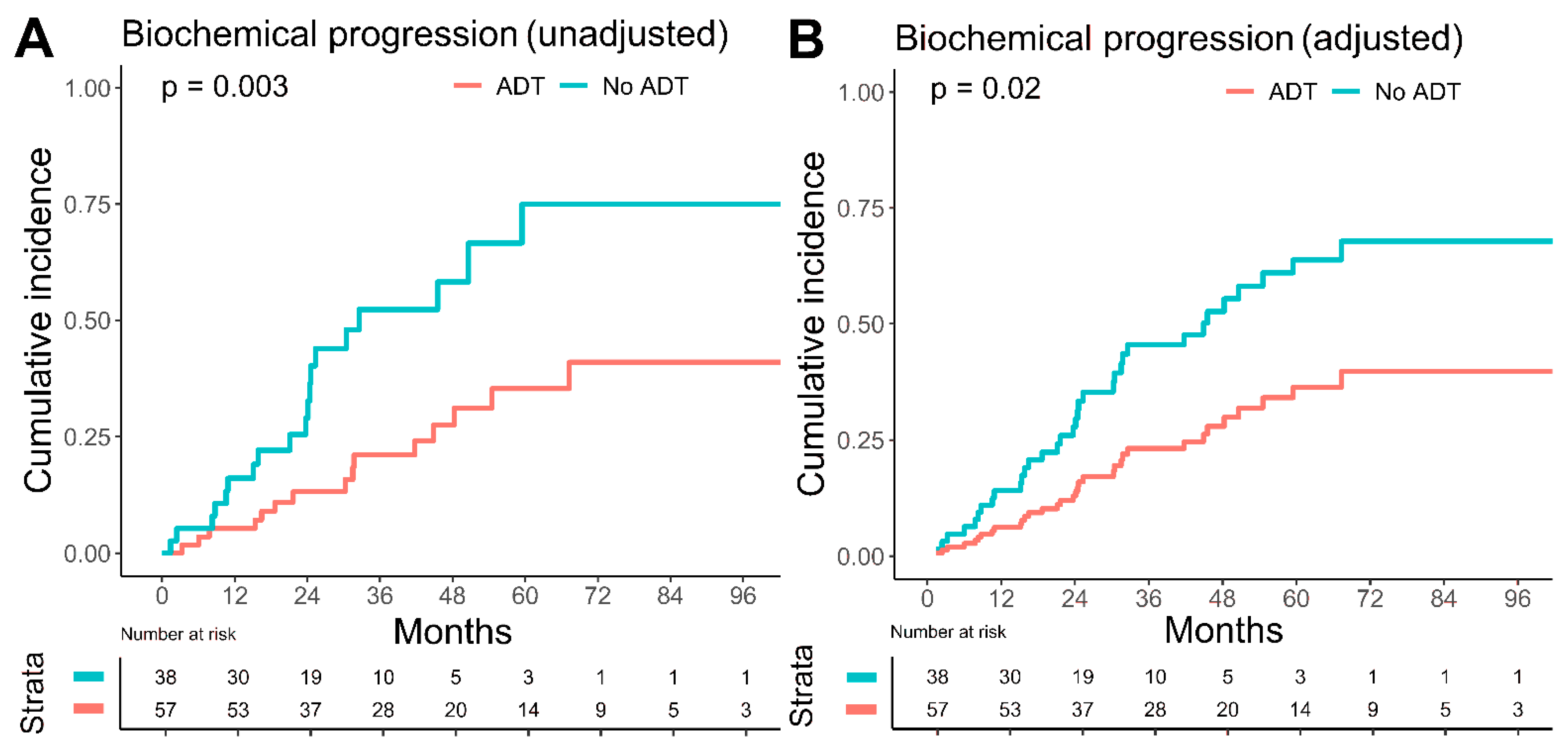
| Concomitant ADT | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.39 (0.19–0.76) | 0.07 | 0.41 (0.19–0.86) | 0.02 | 0.42 (0.21–0.84) | 0.01 |
| Extrapelvic disease | ||||||
| No | Ref | Ref | ||||
| Yes | 1.08 (0.54–2.14) | 0.80 | 0.89 (0.33–2.39) | 0.82 | ||
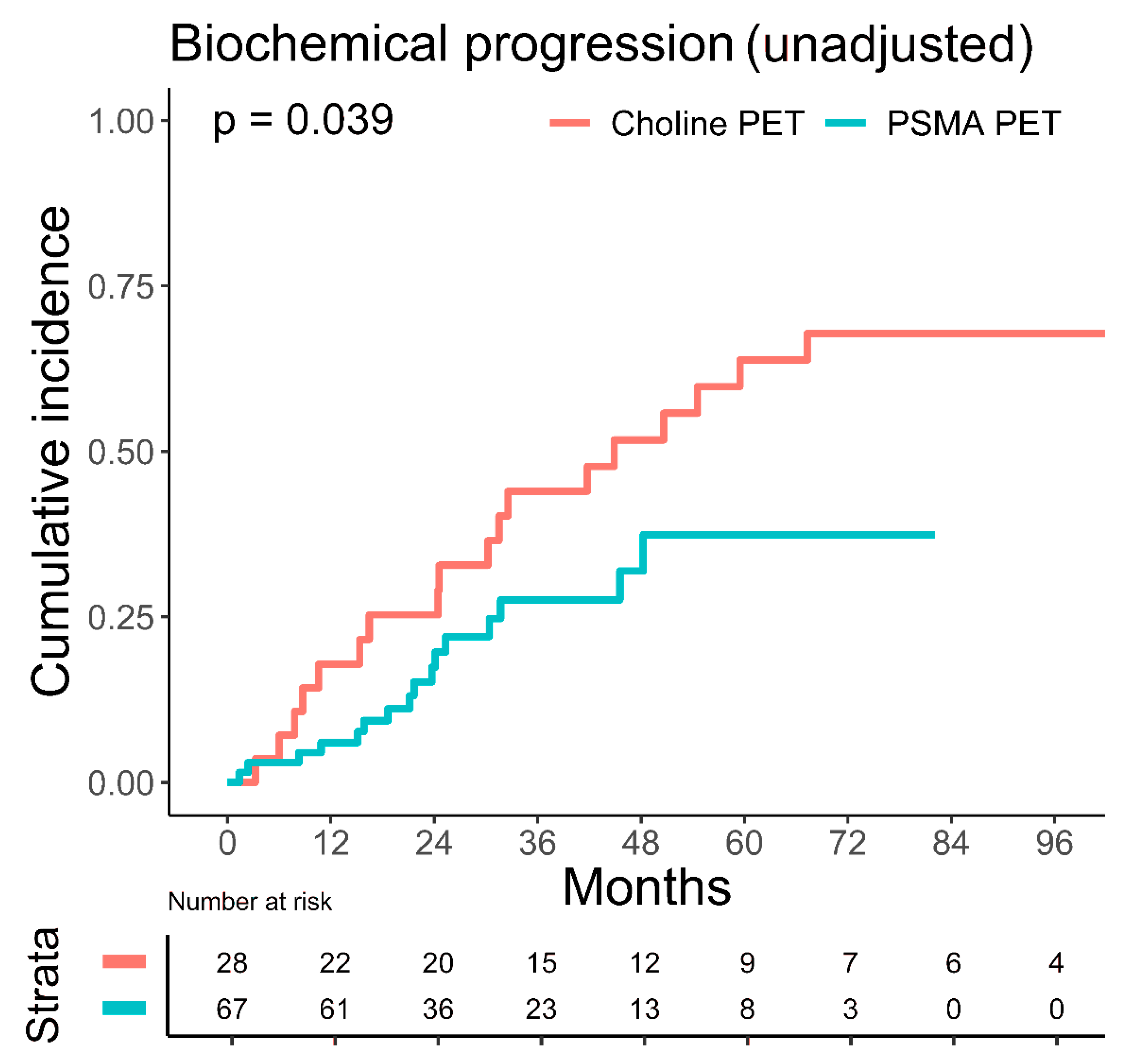
| PET imaging | ||||||
| Choline PET | Ref | Ref | ||||
| PSMA PET | 0.52 (0.26–1.03) | 0.06 | 0.62 (0.29–1.33) | 0.22 | ||
| Metastatic disease progression | Univariate | Multivariate | Multivariate AIC | |||
| Variables | HR (95% CI) | p Value * | HR (95% CI) | p Value * | HR (95% CI) | p Value * |
| PSA at RT start | 1.05 (1.01–1.09) | 0.02 | 1.03 (1.00–1.07) | 0.07 | 1.04 (1.01–1.08) | 0.02 |
| Number of LN ≥ 4 | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 2.12 (0.94–4.77) | 0.10 | 2.02 (0.68–5.98) | 0.20 | 2.35 (0.92–6.03) | 0.07 |
| Gleason score ≥ 8 | ||||||
| No | Ref | Ref | ||||
| Yes | 0.73 (0.32–1.69) | 0.50 | 0.90 (0.36–2.24) | 0.82 | ||
| Age at start of RT | 1.02 (0.98–1.06) | 0.50 | 1.00 (0.95–1.04) | 0.91 | ||
| Concomitant ADT | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.54 (0.25–1.17) | 0.10 | 0.36 (0.15–0.85) | 0.02 | 0.39 (0.17–0.90) | 0.03 |
| Extrapelvic disease | ||||||
| No | Ref | Ref | ||||
| Yes | 1.83 (0.86–3.90) | 0.10 | 1.61 (0.53–4.91) | 0.40 | ||
| PET imaging | ||||||
| Choline PET | Ref | Ref | Ref | |||
| PSMA PET | 0.62 (0.26–1.45) | 0.20 | 0.53 (0.24–1.16) | 0.11 | 0.55 (0.24–1.24) | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamihardja, J.; Zehner, L.; Hartrampf, P.; Lisowski, D.; Kneitz, S.; Cirsi, S.; Razinskas, G.; Flentje, M.; Polat, B. Salvage Nodal Radiotherapy as Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer Detected by Positron Emission Tomography Shows Favorable Outcome in Long-Term Follow-Up. Cancers 2022, 14, 3766. https://doi.org/10.3390/cancers14153766
Tamihardja J, Zehner L, Hartrampf P, Lisowski D, Kneitz S, Cirsi S, Razinskas G, Flentje M, Polat B. Salvage Nodal Radiotherapy as Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer Detected by Positron Emission Tomography Shows Favorable Outcome in Long-Term Follow-Up. Cancers. 2022; 14(15):3766. https://doi.org/10.3390/cancers14153766
Chicago/Turabian StyleTamihardja, Jörg, Leonie Zehner, Philipp Hartrampf, Dominik Lisowski, Susanne Kneitz, Sinan Cirsi, Gary Razinskas, Michael Flentje, and Bülent Polat. 2022. "Salvage Nodal Radiotherapy as Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer Detected by Positron Emission Tomography Shows Favorable Outcome in Long-Term Follow-Up" Cancers 14, no. 15: 3766. https://doi.org/10.3390/cancers14153766
APA StyleTamihardja, J., Zehner, L., Hartrampf, P., Lisowski, D., Kneitz, S., Cirsi, S., Razinskas, G., Flentje, M., & Polat, B. (2022). Salvage Nodal Radiotherapy as Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer Detected by Positron Emission Tomography Shows Favorable Outcome in Long-Term Follow-Up. Cancers, 14(15), 3766. https://doi.org/10.3390/cancers14153766






