Linear Tumor Regression of Rectal Cancer in Daily MRI during Preoperative Chemoradiotherapy: An Insight of Tumor Regression Velocity for Personalized Cancer Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Diagnosis
2.3. Treatment
2.4. Follow-Up
2.5. Daily Tumor Volumetry
2.6. Primary Endpoint
2.7. Secondary Endpoint
2.8. Statistical Analyses
3. Results
3.1. Tumor, Patient, and Treatment Characteristics
3.2. Treatment Outcome
3.3. Primary Endpoint
3.4. Secondary Endpoint
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, D.H.; Bekaii-Saab, T. Watch and Wait in Rectal Cancer: Who’s In and Who’s Out? J. Oncol. Pract. 2019, 15, 133–134. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Gurski, L.A. NCCN guidelines insights: Rectal cancer, version 6.2020: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Briffaux, A.; Collette, L. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results—EORTC 22921. J. Clin. Oncol. 2005, 23, 5620–5627. [Google Scholar] [CrossRef]
- Bostel, T.; Dreher, C.; Wollschläger, D.; Mayer, A.; König, F.; Bickelhaupt, S.; Schlemmer, H.P.; Huber, P.E.; Sterzing, F.; Bäumer, P.; et al. Exploring MR regression patterns in rectal cancer during neoadjuvant radiochemotherapy with daily T2-and diffusion-weighted MRI. Radiat. Oncol. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Capirci, C.; Valentini, V.; Cionini, L.; De Paoli, A.; Rodel, C.; Glynne-Jones, R.; Coco, C.; Romano, M.; Mantello, G.; Palazzi, S.; et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int. J. Radiat. Oncol. * Biol. * Phys. 2008, 72, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cedermark, B.; Johansson, H.; Rutqvist, L.E. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. Cancer 1995, 75, 2269–2275. [Google Scholar] [CrossRef]
- Cercek, A.; Roxburgh, C.S.; Strombom, P.; Smith, J.J.; Temple, L.K.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef] [PubMed]
- Chirivella, I.; Bermejo, B.; Insa, A.; Perez-Fidalgo, A.; Magro, A.; Rosello, S.; Garcia-Garre, E.; Martin, P.; Bosch, A.; Lluch, A. Impact of chemotherapy dose-related factors on survival in breast cancer patients treated with adjuvant anthracycline-based chemotherapy. J. Clin. Oncol. 2006, 24, 668. [Google Scholar] [CrossRef]
- Dulskas, A.; Gaizauskas, V.; Kildusiene, I.; Samalavicius, N.E.; Smailyte, G. Improvement of survival over time for colorectal cancer patients: A population-based study. J. Clin. Med. 2020, 9, 4038. [Google Scholar] [CrossRef]
- Fertil, B.; Malaise, E. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: Analysis of 101 published survival curves. Int. J. Radiat. Oncol. * Biol. * Phys. 1985, 11, 1699–1707. [Google Scholar] [CrossRef]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.D.; Nijkamp, J.; Duppen, J.C.; Rasch, C.R.; Thomas, C.R.; Wang, S.J.; Okunieff, P.; Jones, W.E.; Baseman, D.; Patel, S.; et al. Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. Int. J. Radiat. Oncol. * Biol. * Phys. 2011, 79, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G. Next-generation in vivo modeling of human cancers. Front. Oncol. 2018, 8, 429. [Google Scholar] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; e Sousa, A.H.S., Jr. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hasan, S.; Renz, P.; Wegner, R.E.; Finley, G.G.; Raj, M.S.; Monga, D.K.; McCormick, J.; Kirichenko, A.V. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A National Cancer Database (NCDB) analysis. Ann. Surg. 2020, 271, 716. [Google Scholar]
- Heald, R.; Ryall, R. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 327, 1479–1482. [Google Scholar]
- Kang, J.H.; Kim, Y.C.; Kim, H.; Hur, H.; Kim, J.S.; Min, B.S.; Kim, H.; Lim, J.S.; Seong, J.; Keum, K.C.; et al. Tumor volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: The impact of the volume reduction ratio on the prediction of pathologic complete response. Int. J. Radiat. Oncol. * Biol. * Phys. 2010, 76, 1018–1025. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; Van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [PubMed]
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar]
- Looney, W. Lymphatic distribution of the colon and rectum. Am. J. Surg. 1939, 46, 143–148. [Google Scholar]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.; A Calvo, F.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Moher, D.; Dulberg, C.S.; Wells, G.A. Statistical power, sample size, and their reporting in randomized controlled trials. Jama 1994, 272, 122–124. [Google Scholar] [CrossRef]
- Nishino, M.; Jagannathan, J.P.; Ramaiya, N.H.; Van den Abbeele, A.D. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. Am. J. Roentgenol. 2010, 195, 281–289. [Google Scholar]
- O’Connell, E.; Reynolds, I.; McNamara, D.; Prehn, J.; Burke, J. Microsatellite instability and response to neoadjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2020, 34, 57–62. [Google Scholar]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic resonance imaging–detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Caravatta, L.; Di Tommaso, M.; Fasciolo, D.; Gasparini, L.; Di Guglielmo, F.C.; Augurio, A.; Vinciguerra, A.; Vecchi, C.; Genovesi, D. Cone-beam computed tomography for organ motion evaluation in locally advanced rectal cancer patients. La Radiol. Med. 2021, 126, 147–154. [Google Scholar]
- Sakorafas, G.H.; Zouros, E.; Peros, G. Applied vascular anatomy of the colon and rectum: Clinical implications for the surgical oncologist. Surg. Oncol. 2006, 15, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Sathyakumar, K.; Chandramohan, A.; Masih, D.; Jesudasan, M.R.; Pulimood, A.; Eapen, A. Best MRI predictors of complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. Br. J. Radiol. 2016, 89, 20150328. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Gambacorta, M.A.; Barbaro, B.; Chiloiro, G.; Coco, C.; Das, P.; Fanfani, F.; Joye, I.; Kachnic, L.; Maingon, P.; et al. International consensus guidelines on clinical target volume delineation in rectal cancer. Radiother. Oncol. 2016, 120, 195–201. [Google Scholar] [CrossRef]
- Van Den Begin, R.; Kleijnen, J.-P.; Engels, B.; Philippens, M.; Van Asselen, B.; Raaymakers, B.; Reerink, O.; De Ridder, M.; Intven, M. Tumor volume regression during preoperative chemoradiotherapy for rectal cancer: A prospective observational study with weekly MRI. Acta Oncol. 2018, 57, 723–727. [Google Scholar] [CrossRef]
- Weiner, L.M.; Lotze, M.T. Tumor-cell death, autophagy, and immunity. N. Engl. J. Med. 2012, 366, 1156–1158. [Google Scholar] [CrossRef]
- White, I.; Hunt, A.; Bird, T.; Settatree, S.; Soliman, H.; Mcquaid, D.; Dearnaley, D.; Lalondrelle, S.; Bhide, S. Interobserver variability in target volume delineation for CT/MRI simulation and MRI-guided adaptive radiotherapy in rectal cancer. Br. J. Radiol. 2021, 94, 20210350. [Google Scholar] [CrossRef]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
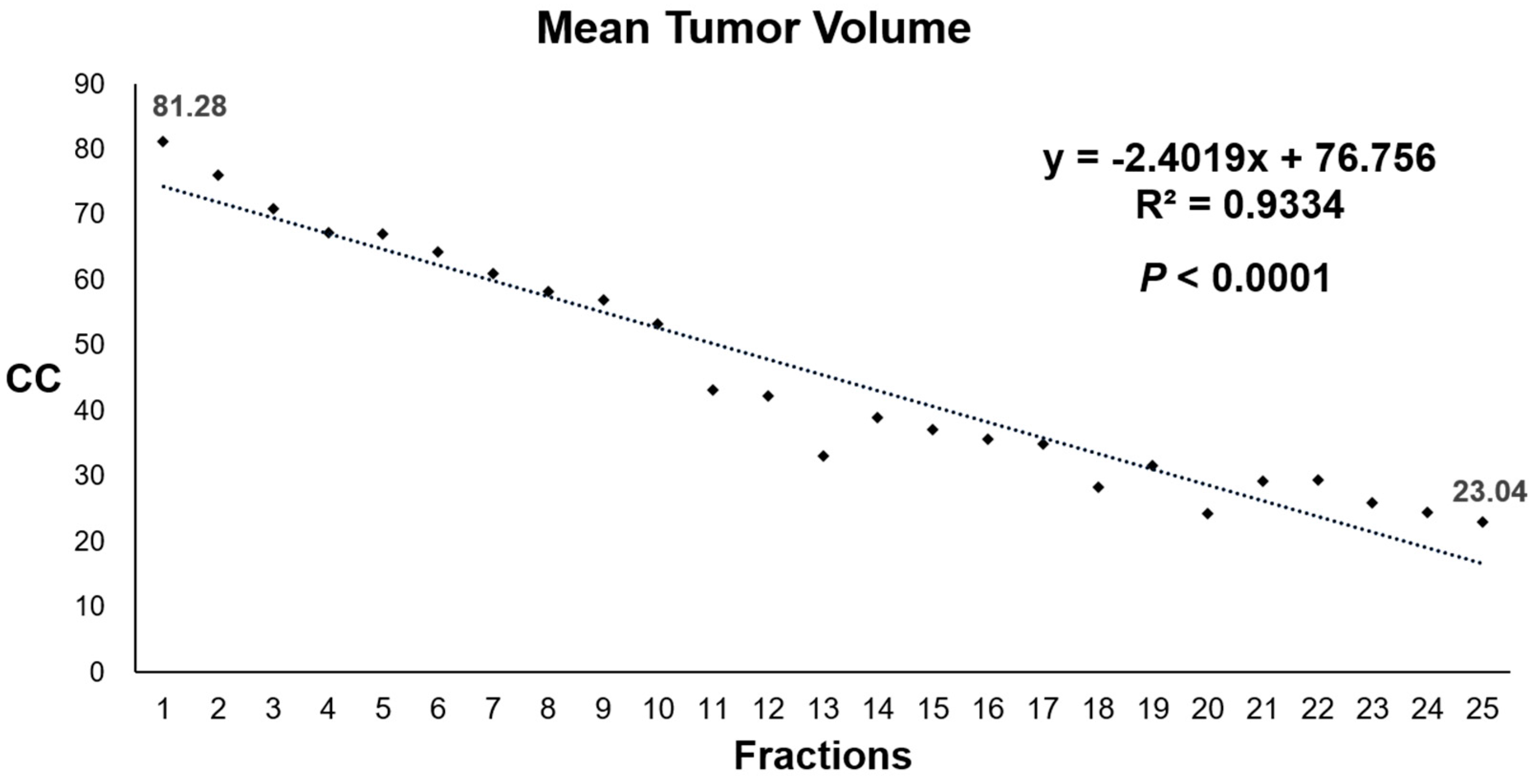

| Total N = 20 (%) | Groups According to Tumor Regression Velocity | p | ||
|---|---|---|---|---|
| Rapid Regressors N = 9 (%) | Slow Regressors N = 11 (%) | |||
| Tumor regression velocity (cc) per fraction | <0.001 | |||
| R2 (p) | 2.40 0.93 (<0.001) | 4.58 0.90 (<0.001) | 0.78 0.97 (<0.001) | |
| Mean | 2.49 | 4.58 | 0.78 | |
| SD | 3.48 | 4.43 | 0.40 | |
| Median | 1.52 | 3.18 | 0.85 | |
| Range | 0.23–14.13 | 1.52–14.13 | 0.23–1.41 | |
| Age (median 64, range: 47–87) | 1.000 | |||
| <64 | 11 (55) | 4 (44.4) | 5 (45.5) | |
| ≥64 | 9 (45) | 5 (55.6) | 6 (54.5) | |
| Sex | 0.479 | |||
| Male | 18 (90) | 9 (100) | 9 (81.8) | |
| Female | 2 (10) | 0 (0) | 2 (18.2) | |
| Clinical T stage | 0.642 | |||
| T3 | 13 (65) | 5 (55.6) | 8 (72.7) | |
| T4 | 7 (35) | 4 (44.4) | 2 (27.3) | |
| Clinical N stage | 0.370 | |||
| N0 | 1 (5) | 0 (0) | 1 (9.1) | |
| N1 | 9 (45) | 3 (33.3) | 6 (54.5) | |
| N2 | 10 (50) | 6 (66.7) | 4 (36.4) | |
| Tumor size (median 5, range: 3–9) | 0.092 | |||
| ≤5 cm | 10 (50) | 2 (22.2) | 7 (63.6) | |
| >5 cm | 10 (50) | 7 (77.8) | 4 (36.4) | |
| Anal verge (median 6.8, range: 1–12) | 0.591 | |||
| ≤5 cm | 5 (25) | 1 (11.1) | 3 (27.3) | |
| >5 cm | 15 (75) | 8 (88.9) | 8 (72.7) | |
| CEA (median 8, range: 1–92.3 ng/mL) | 0.642 | |||
| <3.5 ng/mL | 6 (30) | 2 (22.2) | 4 (36.4) | |
| ≥3.5 ng/mL | 14 (70) | 7 (77.8) | 7 (63.6) | |
| Differentiation | 1.000 | |||
| Well | 3 (15) | 1 (11.1) | 2 (18.2) | |
| Moderate | 13 (65) | 5 (55.6) | 8 (72.7) | |
| Unavailable | 4 (20) | 3 (33.3) | 1 (9.1) | |
| Response rate (RECIST v1.1) | 0.770 | |||
| Complete response | 1 (5) | 0 (0) | 1 (9.1) | |
| Partial response | 16 (80) | 7 (77.8) | 9 (81.8) | |
| Stable disease | 3 (15) | 2 (22.2) | 1 (9.1) | |
| Resection rate | 1.000 | |||
| Surgery | 14 (70) | 6 (66.7) | 8 (72.7) | |
| Observation | 6 (30) | 3 (33.3) | 3 (27.3) | |
| Adjuvant chemotherapy | 0.854 | |||
| FOLFOX | 6 (30) | 2 (22.2) | 4 (36.4) | |
| Capecitabine | 7 (35) | 3 (33.3) | 4 (36.4) | |
| None | 7 (35) | 4 (44.4) | 3 (27.3) | |
| Resected Patients N = 14 (%) | Rapid Regressors N = 6 (%) | Slow Regressors N = 8 (%) | p | |
| yp T stage (N = 14) | 0.385 | |||
| T1 | 2 (14.3) | 1 (16.7) | 1 (12.5) | |
| T2 | 1 (7.1) | 1 (16.7) | 0 (0) | |
| T3 | 10 (71.4) | 3 (50) | 7 (87.5) | |
| T4 | 1 (7.1) | 1 (16.7) | 0 (0) | |
| yp N stage (N = 14) | 0.083 | |||
| N0 | 7 (50) | 6 (100) | 3 (37.5) | |
| N1 | 6 (42.9) | 0 (0) | 2 (25) | |
| N2 | 1 (7.1) | 0 (0) | 3 (37.5) | |
| Lymphatic invasion (N = 14) | 1.000 | |||
| Yes | 1 (7.1) | 0 (0) | 1 (12.5) | |
| No | 13 (92.9) | 6 (100) | 7 (87.5) | |
| Vascular invasion (N = 14) | 0.209 | |||
| Yes | 3 (21.4) | 0 (0) | 3 (37.5) | |
| No | 11 (78.6) | 6 (100) | 5 (67.5) | |
| Perineural invasion (N = 14) | 0.627 | |||
| Yes | 6 (42.9) | 2 (33.3) | 4 (50) | |
| No | 8 (57.1) | 4 (66.7) | 4 (50) | |
| Margin (N = 14) | 1.000 | |||
| Positive | 2 (14.3) | 1 (16.7) | 1 (12.5) | |
| Negative | 12 (85.7) | 5 (83.3) | 7 (87.5) | |
| Group According to Tumor Regression Velocity | Patient (N = 20) | Tumor Regression Velocity (cc) per Fraction | Tumor Regression Rate (%) | DFS (Months) |
|---|---|---|---|---|
| Rapid regressors (N = 9) | 1 | 14.13 | 36.98 | 40.5 |
| 2 | 9.87 | 74.67 | 7.5 | |
| 3 | 3.96 | 73.60 | 36.1 | |
| 4 | 3.35 | 31.70 | 31.6 | |
| 5 | 3.18 | 74.39 | 32.6 | |
| 6 | 1.98 | 80.88 | 33.7 | |
| 7 | 1.65 | 71.38 | 36.0 | |
| 8 | 1.59 | 94.06 | 38.2 | |
| 9 | 1.52 | 79.99 | 35.6 | |
| Mean SD (SE) | 4.58 4.43 | 72.58 17.99 | 36.8 3.5 | |
| Slow regressors (N = 11) | 10 | 1.41 | 59.79 | 9.5 |
| 11 | 1.10 | 49.11 | 21.2 | |
| 12 | 1.07 | 79.21 | 34.0 | |
| 13 | 1.05 | 47.76 | 33.4 | |
| 14 | 1.02 | 70.06 | 38.0 | |
| 15 | 0.85 | 64.13 | 39.3 | |
| 16 | 0.82 | 80.94 | 34.5 | |
| 17 | 0.46 | 67.12 | 37.7 | |
| 18 | 0.36 | 74.52 | 6.3 | |
| 19 | 0.26 | 82.90 | 32.4 | |
| 20 | 0.23 | 39.29 | 36.5 | |
| Mean SD (SE) | 0.78 0.40 | 64.98 14.61 | 31.9 3.8 | |
| p | <0.001 | 0.272 | 0.400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.-Y.; Lee, S.-W.; Hong, J.H.; Kang, H.J.; Lee, S.J.; Kim, M.; Kim, J.-H.; Kwak, Y.-K. Linear Tumor Regression of Rectal Cancer in Daily MRI during Preoperative Chemoradiotherapy: An Insight of Tumor Regression Velocity for Personalized Cancer Therapy. Cancers 2022, 14, 3749. https://doi.org/10.3390/cancers14153749
Sung S-Y, Lee S-W, Hong JH, Kang HJ, Lee SJ, Kim M, Kim J-H, Kwak Y-K. Linear Tumor Regression of Rectal Cancer in Daily MRI during Preoperative Chemoradiotherapy: An Insight of Tumor Regression Velocity for Personalized Cancer Therapy. Cancers. 2022; 14(15):3749. https://doi.org/10.3390/cancers14153749
Chicago/Turabian StyleSung, Soo-Yoon, Sea-Won Lee, Ji Hyung Hong, Hye Jin Kang, So Jung Lee, Myungsoo Kim, Ji-Hoon Kim, and Yoo-Kang Kwak. 2022. "Linear Tumor Regression of Rectal Cancer in Daily MRI during Preoperative Chemoradiotherapy: An Insight of Tumor Regression Velocity for Personalized Cancer Therapy" Cancers 14, no. 15: 3749. https://doi.org/10.3390/cancers14153749
APA StyleSung, S.-Y., Lee, S.-W., Hong, J. H., Kang, H. J., Lee, S. J., Kim, M., Kim, J.-H., & Kwak, Y.-K. (2022). Linear Tumor Regression of Rectal Cancer in Daily MRI during Preoperative Chemoradiotherapy: An Insight of Tumor Regression Velocity for Personalized Cancer Therapy. Cancers, 14(15), 3749. https://doi.org/10.3390/cancers14153749






