1. Introduction
The use of radiation therapy (RT) to treat cancer has increased [
1,
2]. Ionizing radiation exposure to the heart and vasculature is associated with an increased risk of atherosclerotic cardiovascular (ASCVD) events among long-term survivors of multiple cancer types [
3,
4,
5,
6,
7,
8,
9]. The benefits of statin therapy for patients at risk for vascular events is well established [
10,
11]. This is particularly true in populations in which traditional cardiovascular (CV) risk may be elevated, including those with cancer [
12,
13]. The ACC/AHA pooled cohort equations (PCE), the Framingham Risk Score (FRS) models, and the United States Preventative Services Taskforce (USPSTF) recommendations are used to predict ASCVD risk and to provide guidance on the initiation of statin therapy for primary prevention of ASCVD [
11,
14,
15]. These models are standard risk prediction models for ASCVD events among the general population [
11,
14,
15]. The accuracy of these models for risk prediction of ASCVD among patients receiving RT involving the vasculature is unclear. Therefore, our aim was to evaluate the efficacy of the PCE and FRS models and of the USPSTF recommendations in predicting ASCVD events among patients receiving RT. We studied patients with head and neck cancers (HNCA), as this represents the sixth most common type of cancer worldwide, with nearly 600,000 persons diagnosed each year, with a particular increase among younger patients. The increased incidence in young patients is due to the shift in risk factors from smoking-related cancer to human papilloma virus–related cancer [
16,
17]. Additionally, RT is a standard primary treatment for HNCAs, where overall survival rates have improved.
2. Materials and Methods
This was a retrospective study. The total cohort included 1011 consecutive patients receiving radiation therapy for HNCA from 2001–2012 at Massachusetts General Hospital, Boston, Massachusetts. From these patients, 723 non-statin treated patients were identified and formed the final cohort for the main analysis. The study was approved by the hospital’s institutional review board. The requirement for written informed consent was waived due to the study design.
2.1. Covariates of Interest
Data on covariates including age, sex, race, and body mass index (BMI) were extracted retrospectively from the electronic medical records. Pre-existing CV disease and risk factors including hypertension, dyslipidemia, diabetes mellitus, tobacco use, coronary artery disease (CAD), stroke, cardiomyopathy, heart failure (HF), and atrial fibrillation or flutter were recorded. Information on cardiovascular and chemotherapy medications was also obtained via chart review. Statin eligibility was determined based on ACC/AHA PCE, USPSTF, and the Framingham risk scores [
11,
14,
15]. The USPSTF recommendations for a statin for primary prevention are as follows: For adults aged 40–75 yrs. with no history of CVD, 1 or higher CVD risk factors, and a calculated 10-year CVD event risk of 10% or higher. The PCE approach recommends a statin for primary prevention for the following: individuals with primary elevations of LDL > 190 mg/dL, individuals 40 to 75 yrs. of age with diabetes and 70 to 189 mg/dL without clinical ASCVD, and individuals without clinical ASCVD or diabetes who are 40 to 75 yrs. of age with LDL 70 to 189 mg/dL and a 10-yr ASCVD risk of 7.5% or higher. The FRS model recommends a statin for primary prevention for the following: individuals with a FRS of 20% or higher, individuals with a FRS of 10% to 19% with LDL-C 3.5 mmol/L or higher, individuals with a LDL-C less than 3.5 mmol/L with non-HDL 4.3 mmol/L or higher or men 50 yrs. of age or older and women 60 yrs. of age or older with a 1-risk factor.
2.2. Outcome of Interest
Our outcome of interest was the occurrence an ASCVD event, defined as the composite of incident non-fatal myocardial infarction (MI), coronary heart disease (CHD), stroke, and cardiovascular death. When a patient had multiple ASCVD events, the date of the earliest event was defined as the incident ASCVD event. All subject charts were individually reviewed for incident ASCVD events. All outcome events were adjudicated using standard definitions and were confirmed by review of the EHR by the study team blinded to cancer, cardiac, medication, and RT variables. The source of the standard ASCVD risk calculator was the ACC/AHA ASCVD risk estimator tool (
https://tools.acc.org/ldl/ascvd_risk_estimator/index.html#!/calulate/estimator/ (accessed on 30 April 2017)).
2.3. Statistical Analysis
Descriptive statistics were used to summarize patient characteristics, wherein continuous variables were presented as mean and SD or median (interquartile range, IQR), as appropriate, based on normality, and categorical variables were presented as percentages. Continuous data were compared with the use of unpaired Student t-tests or Wilcoxon rank sum tests, as appropriate. Categorical data were compared using the chi-square or the Fisher exact test. The association between statin eligibility and events was determined using unadjusted and adjusted Cox proportional hazard models (for presence or absence of an ASCVD event as binary variable) for the calculation of hazard ratios. We used Fine and Grey models for competing risk analysis. To calculate the incidence rate ratio (IRR), first, we tested our data for dispersion by calculating the mean and variance of the count outcome of the ratio of variance where mean = 1.1, which is close to “1”. We further confirmed by looking at the ratio of value of the deviance to the degree of freedom in the goodness of the fit model of our analysis which was also close to 1, indicating that our count outcome was not over- or under-dispersed. Therefore, we used Poisson regression analysis (incorporating all ACSVD events outcomes as count variable) for the calculation of IRRs. Time-to-event analysis survival analysis (Kaplan–Meier) curves and the log-rank test were used to compare the event rates between statin eligible and statin non-eligible groups across risk prediction models. Statistical significance was defined using a p-value ≤ 0.05. The rate of events per 1000 person-years were calculated by standard formula. Statistical analyses were performed using SPSS software version 24 (IBM Corp., Armonk, NY, USA).
4. Discussion
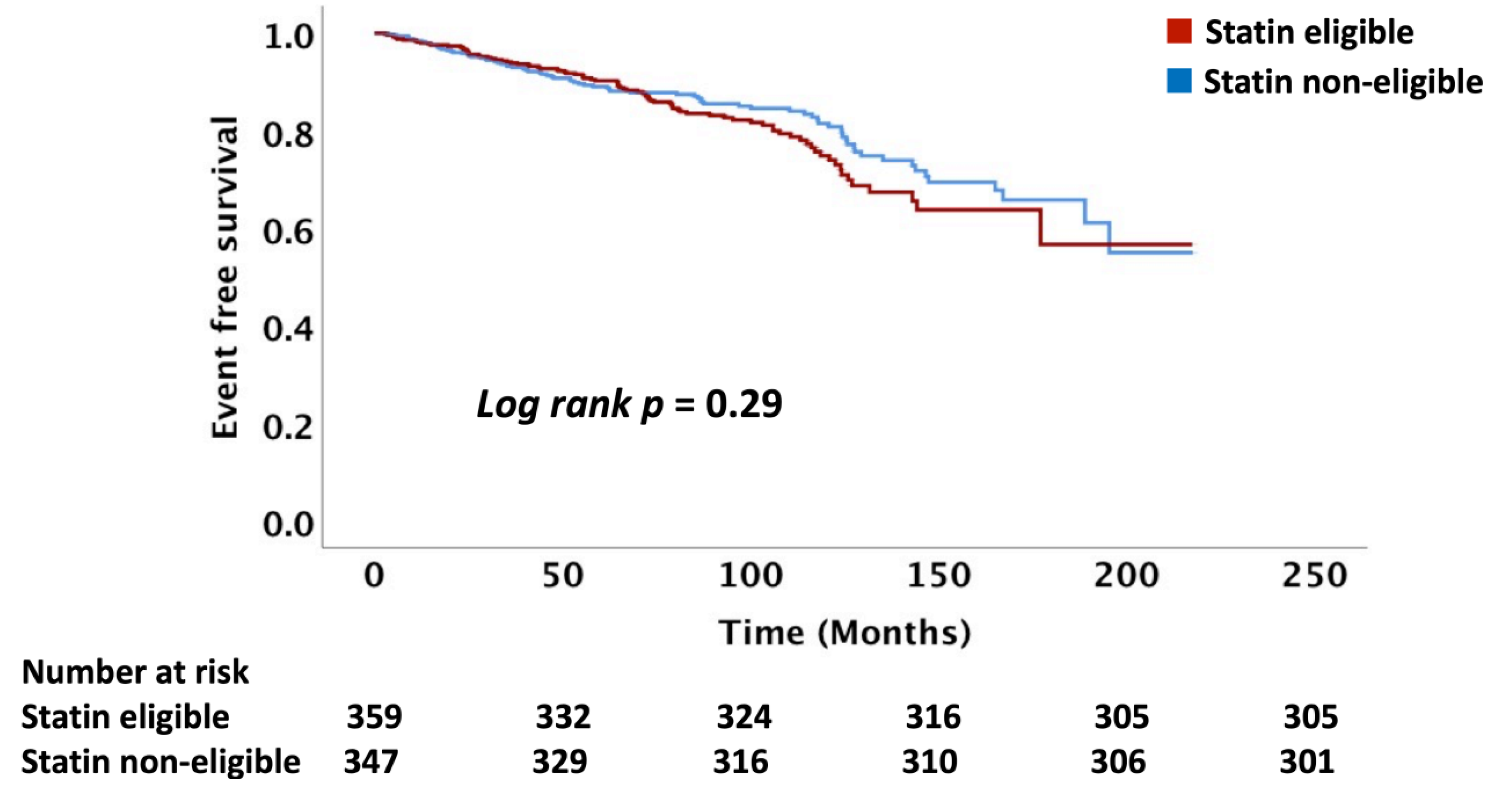
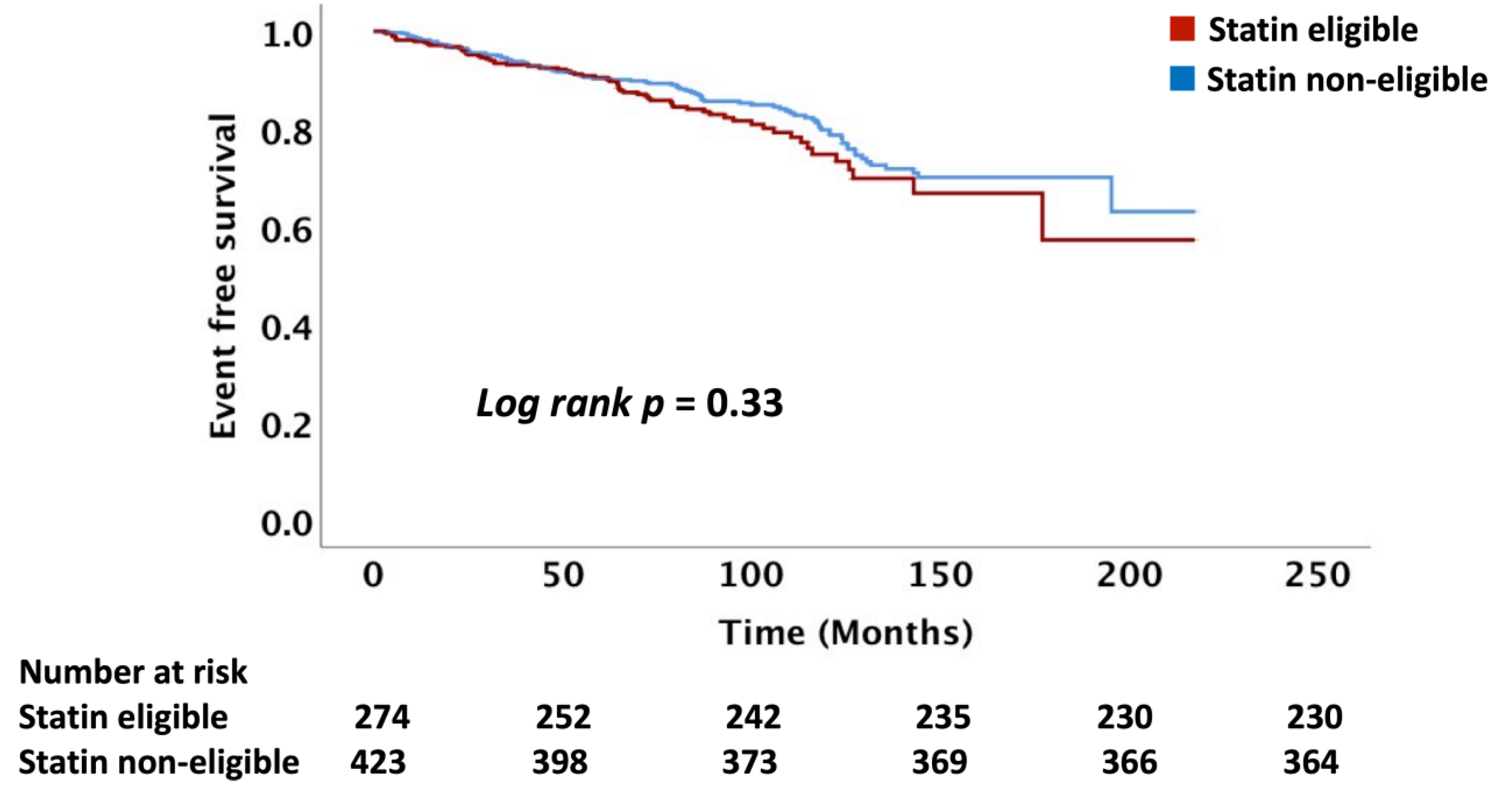
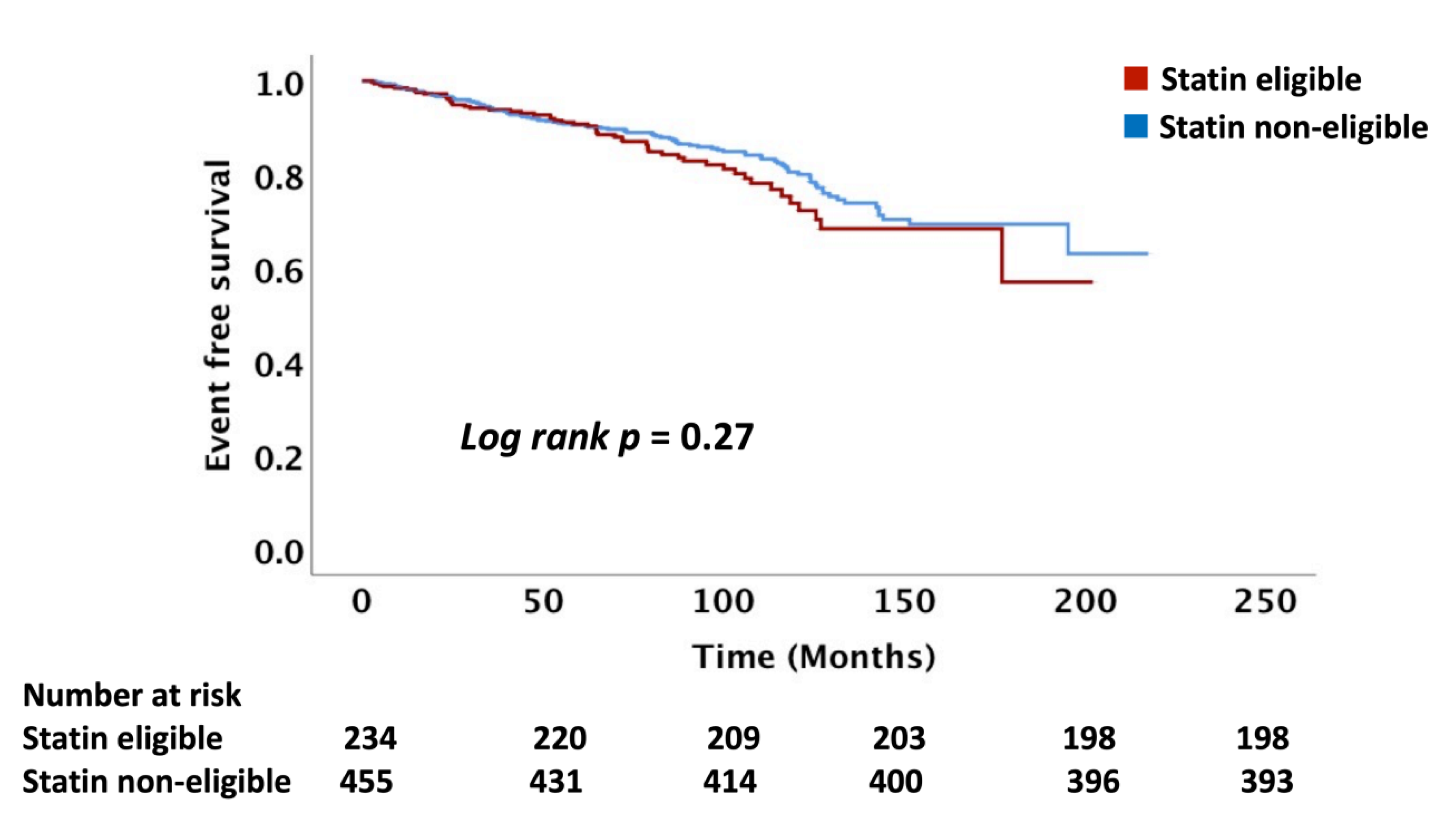
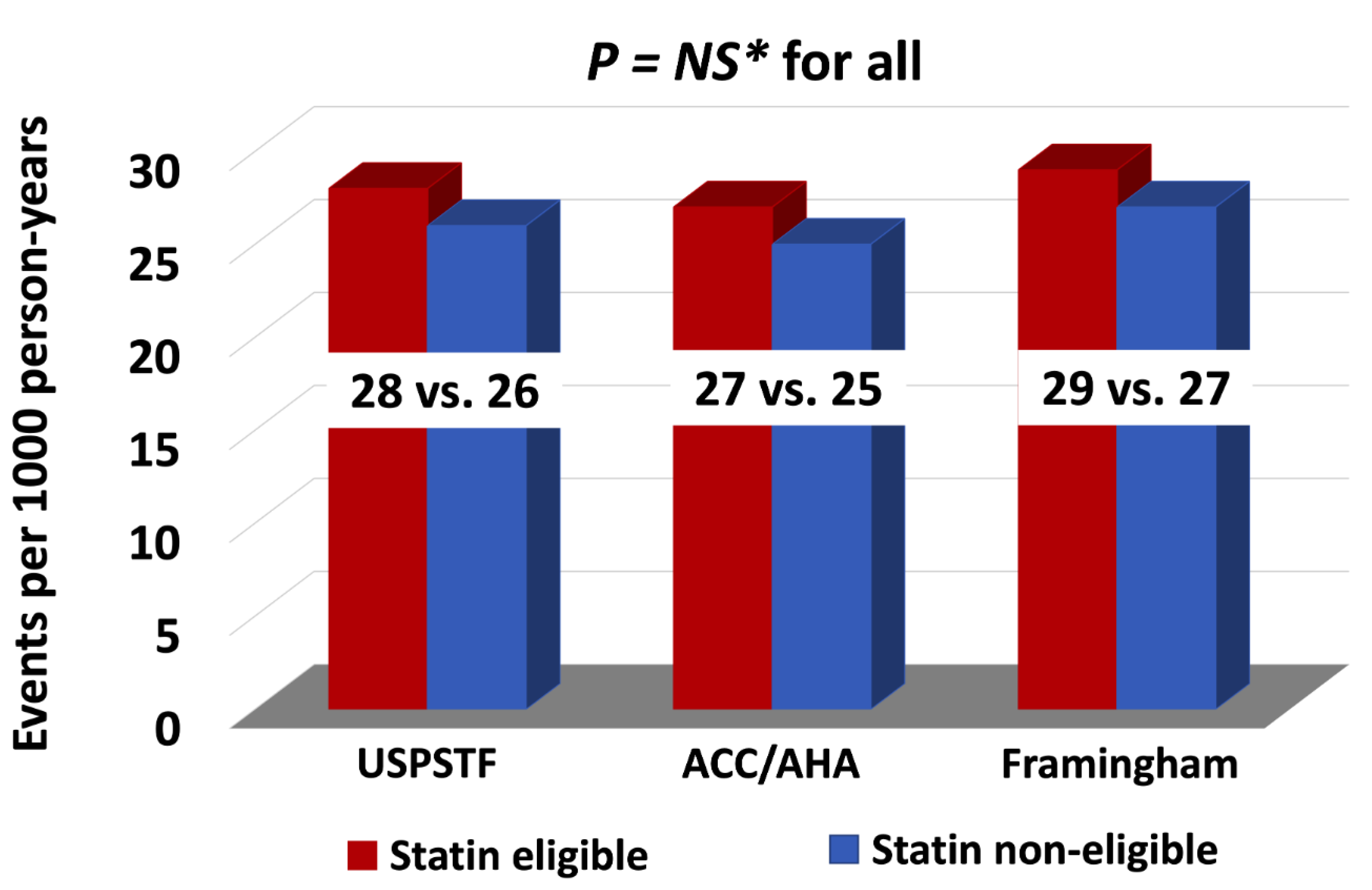
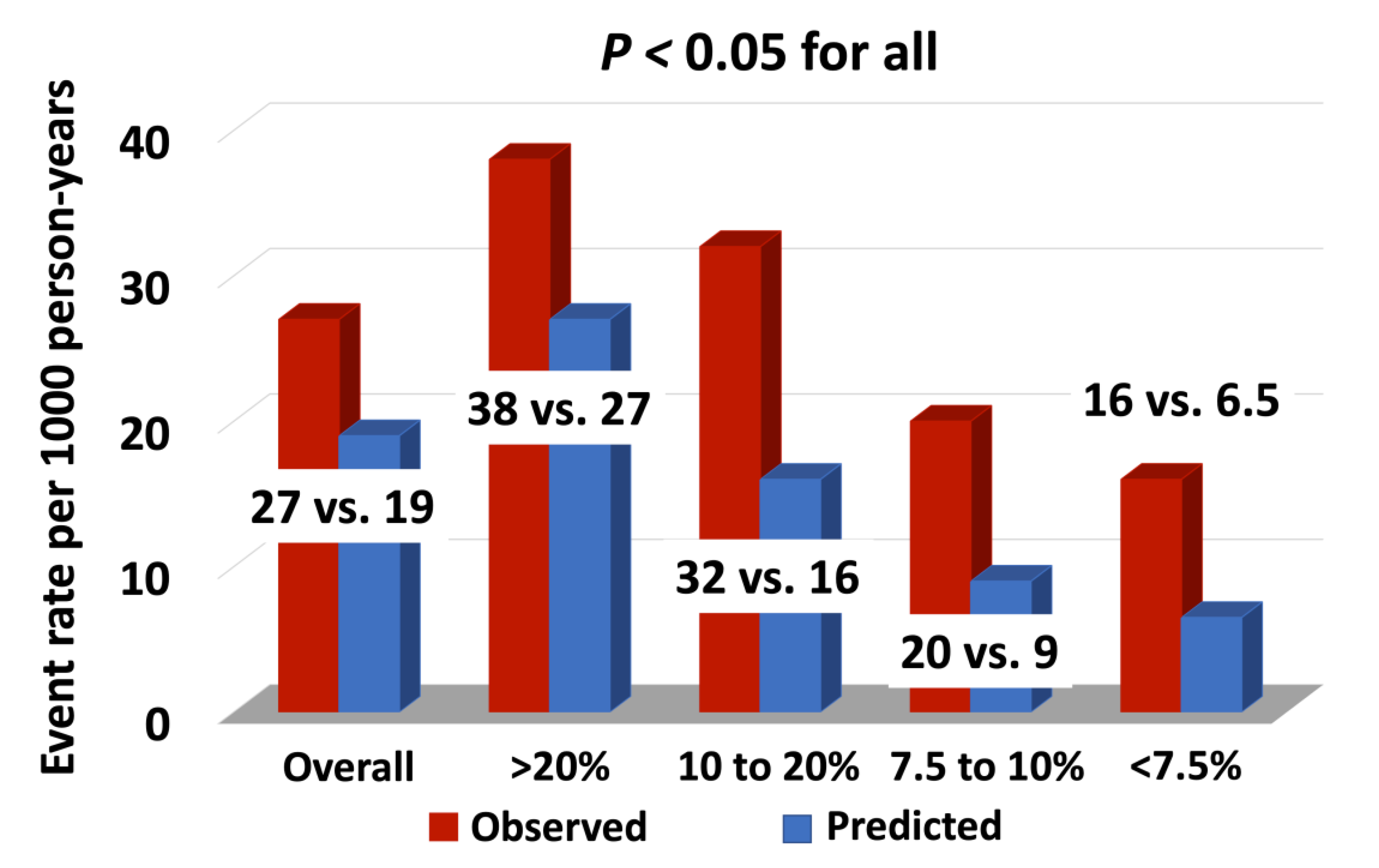
In this study, we present data on the role of the major standard CV risk prediction models, including the ACC/AHA PCE, USPSTF, and FRS, in predicting long-term ASCVD risk among patients with head and neck cancers treated with RT. The observed rate of ASCVD was nearly 70% higher than that predicted following RT. Our data demonstrated that these risk scores do not reliably differentiate between those who will and those who will not have an ASCVD event after RT. Even in those patients predicted to have the lowest risk of ASCVD, an event rate of 10% or more was observed in follow-up, an event rate that would traditionally be considered high-risk.
The association between RT and ASCVD is well-established [
3,
18,
19]. For example, studies among patients with Hodgkin’s lymphoma treated with RT have demonstrates a relative risk of 3 for cardiac death and of 5.5 for acute MI [
20,
21]. Similarly, an early Breast Cancer Trialists’ Collaborative Group analysis found that the risk of death from coronary heart disease increased by three percent per Gray (Gy) increase in the mean radiation dose [
18]; moreover, a population-based study of more than 2000 women in Denmark and Sweden by Darby et al., noted that coronary events increased by 7.4% per Gy of the mean RT dose regardless of the presence of cardiac risk factors [
18]. Among patients with HNCA, RT to the neck has been linked to accelerated carotid atherosclerosis. For example, in a meta-analysis that included 8 case-control studies and randomized clinical trials, Bashar et al. demonstrated that the incidence of extra-cranial carotid artery stenosis was 4–7-fold higher among the patients who received RT for HNCA compared to those with HNCA who did not receive RT [
22]. This accelerated atherosclerosis with RT for HNCA is associated with ASCVD events. For example, Van Aken et al., in a prospective study involving 750 HNCA patients, showed an independent dose–effect relationship between RT dose to the neck and ischemic cerebrovascular events (HR = 1.11, AUC = 0.68) [
23]. In our study cohort the overall ASCVD event rate per 1000 person-years was also high at 27 events per 1000-person years, with 18% of patients having an ASCVD event, complementary to the previously published data [
20,
21,
24].
There may be value to expanding the indications for statin therapy among patients with HNCA treated with RT as data have suggested beneficial effects of statin therapy on reducing the risk of vascular events among patients receiving RT to the head and neck. For example, in a study involving patients with HNCA who were receiving RT, we previously showed a nearly 60% reduction in ischemic stroke and TIA and a >50% relative risk reduction in the development of ischemic stroke alone [
24]. In another study involving 5718 patients with RT to the head, neck, and thorax, there was a 15% risk reduction in the primary outcome including cerebrovascular and cardiovascular events and 32% relative risk reduction in ischemic stroke alone among those on statins [
25]. Similarly, in a retrospective study involving 15 vascular surgery or interventional radiology centers in France, Favre et al. demonstrated that statins were associated with a reduction in re-stenosis after carotid stenting in patients who developed symptomatic carotid stenosis after RT involving the neck [
26]. The mechanism behind the favorable effects of statin against ASCVD with RT is probably mediated, in part, via an attenuation in the inflammatory process leading to reversal or stabilization in the atherosclerotic plaque burden [
27,
28,
29,
30,
31]. This is supported by complementary pre-clinical data supporting a beneficial effect of statins for the risk of ASCVD post-RT to the head and neck. Specifically, in vitro data evaluating the effect of pravastatin on RT-treated endothelial cells showed statin use was associated with reduction in inflammation [
32].
In 2016, the USTSPF released recommendations on statin use for the primary prevention of ASCVD. In these guidelines, individuals aged 40–75 years with 1 or more major ASCVD risk factors (hypertension, smoking, diabetes, or dyslipidemia) and a 10-year ASCVD risk equal of greater than 10% assessed by PCE were advised to initiate statin therapy as a primary prevention [
11]. Similarly, the ACC/AHA guidelines use a PCE risk-based approach for statin therapy prescription for primary prevention, identifying a lower threshold for initiating statin therapy compared with USTSPF recommendations. The ACC/AHA recommended statin for individuals with a 10-year ASCVD risk greater than or equal to 7.5% using the PCE calculator without requirement for the presence of 1 major ASCVD risk factor [
14]. The FRS is similar to the PCE; however, it is designed to estimate CAD alone and does not predict other significant atherosclerotic outcomes, such as CVA [
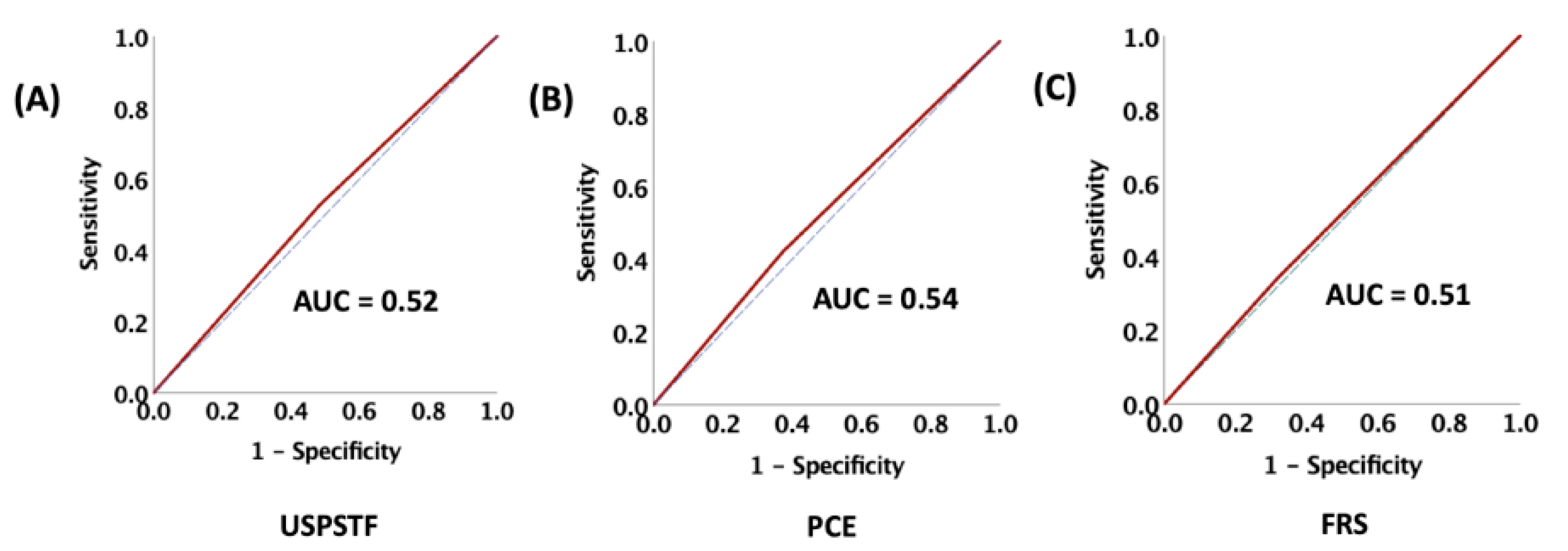
15]. There are no wide-spread risk prediction models for ASCVD events that incorporate cancer/cancer therapy as a major effect modifier. Our data show that there was no difference in the incidence of ASCVD events between the statin eligible and non-eligible patients based on the ACC/AHA, USPSTF, and FRS recommendations suggesting that the PCE and FRS models lack precision post-RT for differentiating those at risk. The mechanisms for the underestimation of ASCVD risk post-RT is not clearly understood, with data suggesting a key role for inflammation and acceleration of atherosclerosis driven by endothelial cell injury and microvascular injury [
33]. Furthermore, the pattern of plaque generation in the cardiovascular system appears to be different from that caused by conventional atherosclerosis [
34]. For this reason, these ASCVD risk prediction models may underestimate risk among patients who have received RT, especially for HNCA.
Study Limitations
This study needs to be interpreted within the context of the study design. The study was performed in a large cancer center, where study populations may differ from other community or suburban hospitals. The sample size is large and, while most of the patients were followed at our institution, we do not have access to the information on the ASCVD events that may have occurred in a different network, which would likely further increase ASCVD rates. We also could not adjust for cancer-related or cancer treatment–related factors such as RT dose and such unmeasured confounders may subsequently impact cardiovascular disease. However, our cohort had the same type of cancer with likely a similar treatment protocol, and it seems unlikely that eligibility for primary prevention with a statin would have impacted the RT dose. Survivor bias and loss of follow-up may have influenced the prevalence of statin eligibility and incident ASCVD risk in our cohort. Similarly, given that the risk of ASCVD was less known during the early experience with RT, adverse events may have gone uncaptured despite extensive search.