The Role of PSMA PET/CT in the Primary Diagnosis and Follow-Up of Prostate Cancer—A Practical Clinical Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Background
2.1. Prostate Specific Membrane Antigen
2.2. Physiological PSMA Expression
2.3. Pathological PSMA Expression or Tracer Uptake
2.4. Small-Molecule PSMA Inhibitors
2.5. Gallium-PSMA vs. Fluor-PSMA
2.6. Pharmacokinetics and Biodistribution
2.7. Clinical Factors and Predictors Influencing PSMA Expression or Predicting PET Positivity
2.8. Possible Options for Influencing PSMA Expression, Sensitivity or Specificity of PET/CT
2.8.1. Dual-Time-Point Acquisition
2.8.2. ADT Prior to PET/CT
2.8.3. Forced Diuresis
3. Clinical Application
3.1. PSMA PET/CT in Initial Staging
3.1.1. Primary T-Staging
| Authors (Year) | Comparison | Number of Patients | Sensitivity PSMA PET/CT | Sensitivity mpMRI | Specificity PSMA PET/CT | Specificity mpMRI | Localization Index Tumor PSMA PET/CT (Sensitivity/Specificity) | Localization Index Tumor mpMRI (Sensitivity/Specificity) |
|---|---|---|---|---|---|---|---|---|
| Kalapara et al. (2020) [68] | 68Ga-PSMA PET/CT vs. mpMRI | 205 | 0.94 | 0.95 | - | - | 0.91/- | 0.89/- |
| Sonni et al. (2021) [66] | 68Ga-PSMA PET/CT vs. mpMRI vs. PSMA PET/CT + mpMRI | 74 | 0.85 | 0.83 | - | - | 0.84/ 0.55 | 0.86/ 0.59 |
| Donato et al. (2019) [70] | 68Ga-PSMA PET/CT vs. mpMRI | 58 | Index lesions: 0.93 Bilateral disease: 0.42 Multifocal disease: 0.34 | Index lesions: 0.90 Bilateral disease: 0.21 Multifocal disease: 0.19 | - | - | - | - |
| Chen et al. (2019) [69] | 68Ga-PSMA PET/CT + mpMRI vs. 68Ga-PSMA PET/CT or mpMRI alone | 54 | 0.89 (95% CI: 0.79–0.96) | 0.76 (95% CI: 0.64–0.86) | 0.71 (95% CI: 0.49–0.87) | 0.88 (95% CI: 0.68–0.97) | - | - |
| Berger et al. (2018) [67] | 68Ga-PSMA PET/CT vs. mpMRI | 50 | Index lesions: 1.0 Secondary lesions: 0.935 | Index lesions: 0.94 Secondary lesions: 0.516 | - | - | 0.811 (95% CI: 0.76–0.86)/ 0.846 (95% CI: 0.79–0.90) | 0.648 (95% CI: 0.59–0.71)/ 0.827 (95% CI: 0.77–0.89) |
3.1.2. Primary N-Staging
68Ga-PSMA in Primary Nodal Staging
| Authors (Year) | Number of Studies Included (Patients) | Tracer | Lesion-Based Sensitivity | Lesion-Based Specificity | Patient-Based Sensitivity | Patient-Based Specificity |
|---|---|---|---|---|---|---|
| Stabile et al. (2022) [82] | 27 (2832) | 68Ga-PSMA, [64Cu]PSMA-617, [18F]rh-PSMA-7, [18F]DCFPyl, [18F]PSMA-1007 | - | - | 0.58 (95% CI: 0.50–0.66) | 0.95 (95% CI: 0.93–0.97) |
| Tu et al. (2020) [83] | 11 (904) | 68Ga-PSMA | 0.70 (95% CI: 0.49–0.85) | 0.99 (95% CI: 0.96–1.00) | 0.63 (95% CI: 0.46–0.78) | 0.93 (95% CI: 0.88–0.96) |
| Luiting et al. (2020), retr. [81] | 9 (696) | 68Ga-PSMA | 0.24–0.96 | 0.99–1.00 | 0.33–1.00 | 0.80–1.00 |
| Wang et al. (2021) [80] | 9 (640) | 68Ga-PSMA | - | - | 0.71 (95% CI: 0.48–0.86) | 0.92 (95% CI: 0.88–0.95) |
| Hope et al. (2019) [84] | 5 (266) | 68Ga-PSMA | 0.74 (95% CI: 0.51–0.89) | 0.96 (95% CI: 0.85–0.99) | - | - |
| Luiting et al. (2020), pros. [81] | 2 (63) | 68Ga-PSMA | 0.50–0.58 | 0.96–1.00 | 0.64–1.00 | 0.90–0.95 |
18F-PSMA in Primary Nodal Staging
| Author | Number of Patients | Tracer | Lesion-Based Sensitivity | Lesion-Based Specificity | Patient-Based Sensitivity | Patient-Based Specificity |
|---|---|---|---|---|---|---|
| Jansen et al. (2021) [90] | 117 | [18F]DCFPyL | - | - | 0.412 (95% CI: 0.194–0.665) | 0.94 (95% CI: 0.869–0.975) |
| Sprute et al. (2021) [86] | 96 (90.6% staged before primary treatment and 9.4% following biochemical recurrence) | [18F]PSMA-1007 | Overall: 0.712 LN > 3 mm: 0.817 | Overall: 0.995 LN > 3 mm: 0.996 | Overall: 0.735 LN > 3 mm: 0.859 | Overall: 0.994 LN > 3 mm: 0.995 |
| Malaspina et al. (2021) [87] | 79 | [18F]PSMA-1007 | - | - | 0.87 (95% CI: 0.71–0.95) | 0.98 (95% CI: 0.89–1.00) |
| Kroenke et al. (2020) [88] | 58 | [18F]rhPSMA-7 (PET/CT or PET/MRI) | 0.538 (95% CI: 0.413–0.660) (template based) | 0.969 (95% CI: 0.914–0.989) (template based) | 0.722 (95% CI: 0.465–0.903) | 0.925 (95% CI: 0.796–0.984) |
Summary—PSMA PET/CT in Primary Nodal Staging
3.1.3. Primary M-Staging
3.2. Biochemical Failure
3.3. PSMA-PET in the Situation of Biochemical Recurrence
3.3.1. Local Recurrence
3.3.2. Secondary N- and M-Staging
Metastasis-Directed Therapy Based on Results from PET/CT
3.3.3. How to Deal with Negative PSMA PET/CT despite Rising PSA?
3.4. PSMA PET/CT in Therapy
3.4.1. Optimal Time Point for PSMA PET/CT
- Short-term ADT in hormone-sensitive PCa: In a small study, Emmett et al. were able to show that in hormone-sensitive patients, a significant reduction of PSMA uptake in the PET occurs very early (within 9 days), so that an examination should ideally take place before the start of the therapy [147]; this was also recommended as part of a consensus statement based on the same study [148]. In individual cases, however, an initial increase in uptake by day 9 can occur even in hormone-sensitive patients, which is in line with the contradictory data mentioned above. It is possible that these cases represent initial hormone-resistant PCa tumor clones [147].
- Long-term ADT in hormone-sensitive PCa: If ADT has already been started in hormone-sensitive PCa, the same consensus statement does not recommend PSMA PET/CT within the first three months [148].
- Short-term ADT in castration-resistant PCa: In patients with castration-resistant PCa, Emmet et al. were also able to show that the tumors experience a significant change in PSMA uptake in PET/CT. Within 9 days—with a plateau of up to 28 days after the start of ADT—the uptake increased, so that the optimal time seems to lie within this period if the potentially increased sensitivity is used for initial diagnostics [147].
- Long-term ADT in castration-resistant PCa: In order to detect a therapeutic effect of ADT in castration-resistant PCa, a further examination should be performed in these patients after three months at the earliest [148].
3.4.2. PSMA PET/CT in Monitoring and Response Assessment of Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Kahn, D.; Williams, R.D.; Seldin, D.W.; Libertino, J.A.; Hirschhorn, M.; Dreicer, R.; Weiner, G.J.; Bushnell, D.; Gulfo, J. Radioimmunoscintigraphy with 111 Indium Labeled Cyt-356 for the Detection of Occult Prostate Cancer Recurrence. J. Urol. 1994, 152, 1490–1495. [Google Scholar] [CrossRef]
- Smith-Jones, P.M.; Vallabahajosula, S.; Goldsmith, S.J.; Navarro, V.; Hunter, C.J.; Bastidas, D.; Bander, N.H. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000, 60, 5237–5243. [Google Scholar]
- Maresca, K.P.; Hillier, S.M.; Femia, F.J.; Keith, D.; Barone, C.; Joyal, J.L.; Zimmerman, C.N.; Kozikowski, A.P.; Barrett, J.A.; Eckelman, W.C.; et al. A Series of Halogenated Heterodimeric Inhibitors of Prostate Specific Membrane Antigen (PSMA) as Radiolabeled Probes for Targeting Prostate Cancer. J. Med. Chem. 2009, 52, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Marquis, J.C.; Foss, C.A.; Nguyen, N.; Zimmerman, C.N.; Barrett, J.A.; Eckelman, W.C.; Pomper, M.G.; et al. Preclinical Evaluation of Novel Glutamate-Urea-Lysine Analogues That Target Prostate-Specific Membrane Antigen as Molecular Imaging Pharmaceuticals for Prostate Cancer. Cancer Res. 2009, 69, 6932–6940. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2012, 40, 486–495. [Google Scholar] [CrossRef]
- Verburg, F.A.; Heinzel, A.; Hänscheid, H.; Mottaghy, F.M.; Luster, M.; Giovanella, L. Nothing new under the nuclear sun: Towards 80 years of theranostics in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido–Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiess, A.P.; Banerjee, S.R.; Mease, R.C.; Rowe, S.P.; Rao, A.; Foss, C.A.; Chen, Y.; Yang, X.; Cho, S.Y.; Nimmagadda, S.; et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 241–268. [Google Scholar] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Rischpler, C.; Beck, T.I.; Okamoto, S.; Schlitter, A.M.; Knorr, K.; Schwaiger, M.; Gschwend, J.; Maurer, T.; Meyer, P.T.; Eiber, M. 68Ga-PSMA-HBED-CC Uptake in Cervical, Celiac, and Sacral Ganglia as an Important Pitfall in Prostate Cancer PET Imaging. J. Nucl. Med. 2018, 59, 1406–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccala, A.; Sercia, L.; Li, J.; Heston, W.; Zhou, M. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Neovasculature of Renal Neoplasms. Urology 2007, 70, 385–390. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of Prostate-Specific Membrane Antigen in Normal and Malignant Human Tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Töpfer, A.; Müller, J.; Muehlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First Clinicopathologic Evidence of a Non–PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019, 60, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, C.; Sathekge, M.; de Spiegeleer, B.; De Jonghe, P.J.; Debruyne, P.R.; Borms, M.; Beels, L.; Maes, A. PSMA Expression on Neovasculature of Solid Tumors. Histol. Histopathol. 2020, 35, 919–927. [Google Scholar] [CrossRef]
- Jiao, D.; Li, Y.; Yang, F.; Han, D.; Wu, J.; Shi, S.; Tian, F.; Guo, Z.; Xi, W.; Li, G.; et al. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Vasculature Predicts Poor Prognosis in Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00041. [Google Scholar] [CrossRef]
- de Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic diseases on PSMA PET imaging: A spectrum of benign and malignant findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef]
- van Boxtel, W.; Lütje, S.; van Engen-van Grunsven, I.C.; Verhaegh, G.W.; Schalken, J.A.; Jonker, M.A.; Nagarajah, J.; Gotthardt, M.; van Herpen, C.M.L. 68Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics 2020, 10, 2273–2283. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Shetty, D.; Patel, D.; Le, K.; Bui, C.; Mansberg, R. Pitfalls in Gallium-68 PSMA PET/CT Interpretation—A Pictorial Review. Tomography 2018, 4, 182–193. [Google Scholar] [CrossRef]
- Chandekar, K.R.; Tanigassalam, S.; Kavanal, A.J.; Singh, H.; Bhattacharya, A.; Mavuduru, R.S. [68Ga]Ga-PSMA-11 Small Bowel Uptake in Crohn’s Disease: Revisiting the “Non-specificity” of PSMA Ligands. Nucl. Med. Mol. Imaging 2022, 56, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; Kim, D.I.; Shepherd, B.; Gustafson, S.; Thomas, P. Intense Uptake in Amyloidosis of the Seminal Vesicles on 68Ga-PSMA PET Mimicking Locally Advanced Prostate Cancer. Clin. Nucl. Med. 2017, 42, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Hartrampf, P.E.; Petritsch, B.; Buck, A.K.; Serfling, S.E. Pitfalls in PSMA-PET/CT: Intensive bone marrow uptake in a case with polycythemia vera. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1669–1670. [Google Scholar] [CrossRef]
- de Souza, S.P.M.; Tobar, N.; Frasson, F.; Perini, E.A.; de Souza, C.A.; Delamain, M.T.; Ramos, C.D. Head-to-head comparison between 68Ga-PSMA and 18F-FDG-PET/CT in lymphomas: A Preliminary Analysis. Nucl. Med. Commun. 2021, 42, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Riondato, M.; Uccelli, L.; Giovacchini, G.; Giovannini, E.; Duce, V.; Ciarmiello, A. Toward the Discovery and Development of PSMA Targeted Inhibitors for Nuclear Medicine Applications. Curr. Radiopharm. 2020, 13, 63–79. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Strauss, D.S.; Sachpekidis, C.; Kopka, K.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Pharmacokinetic studies of [68Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: Detection, differences in temporal distribution and kinetic modelling by tissue type. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4472–4482. [Google Scholar] [CrossRef]
- Anttinen, M.; Ettala, O.; Malaspina, S.; Jambor, I.; Sandell, M.; Kajander, S.; Rinta-Kiikka, I.; Schildt, J.; Saukko, E.; Rautio, P.; et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur. Urol. Oncol. 2021, 4, 635–644. [Google Scholar] [CrossRef]
- Rauscher, I.; Kroenke, M.; König, M.; Gafita, A.; Maurer, T.; Horn, T.; Schiller, K.; Weber, W.; Eiber, M. Matched-Pair Comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: Frequency of Pitfalls and Detection Efficacy in Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2020, 61, 51–57. [Google Scholar] [CrossRef]
- Evangelista, L.; Maurer, T.; van der Poel, H.; Alongi, F.; Kunikowska, J.; Laudicella, R.; Fanti, S.; Hofman, M.S. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the Staging of Primary and Recurrent Prostate Cancer. A Systematic Review of the Literature. Eur. Urol. Oncol. 2022, 5, 273–282. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. RadioGraphics 2018, 38, 200–217. [Google Scholar] [CrossRef] [Green Version]
- Ceci, F.; Uprimny, C.; Nilica, B.; Geraldo, L.; Kendler, D.; Kroiss, A.; Bektic, J.; Horninger, W.; Lukas, P.; Decristoforo, C.; et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1284–1294. [Google Scholar] [CrossRef] [Green Version]
- Bravaccini, S.; Puccetti, M.; Bocchini, M.; Ravaioli, S.; Celli, M.; Scarpi, E.; De Giorgi, U.; Tumedei, M.M.; Raulli, G.; Cardinale, L.; et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018, 8, 4254. [Google Scholar] [CrossRef] [Green Version]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.-C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical Validation of PSMA Expression Measured by 68Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef]
- Gorges, T.M.; Riethdorf, S.; von Ahsen, O.; Nastał Y, P.; Röck, K.; Boede, M.; Peine, S.; Kuske, A.; Schmid, E.; Kneip, C.; et al. Heterogeneous PSMA expression on circulating tumor cells—A potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget 2016, 7, 34930–34941. [Google Scholar] [CrossRef] [Green Version]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of Prostate-Specific Membrane Antigen (PSMA) Expression in Prostate Carcinoma with Distant Metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Laudicella, R.; Rüschoff, J.H.; Ferraro, D.A.; Brada, M.D.; Hausmann, D.; Mebert, I.; Maurer, A.; Hermanns, T.; Eberli, D.; Rupp, N.J.; et al. Infiltrative growth pattern of prostate cancer is associated with lower uptake on PSMA PET and reduced diffusion restriction on mpMRI. Eur. J. Nucl. Med. Mol. Imaging, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Rüschoff, J.H.; Ferraro, D.A.; Muehlematter, U.J.; Laudicella, R.; Hermanns, T.; Rodewald, A.-K.; Moch, H.; Eberli, D.; Burger, I.A.; Rupp, N.J. What’s behind 68Ga-PSMA-11 uptake in primary prostate cancer PET? Investigation of histopathological parameters and immunohistochemical PSMA expression patterns. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4042–4053. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Pfister, D.; Drude, N.I.; Mottaghy, F.M.; Behrendt, F.F. PSA levels, PSA doubling time, Gleason score and prior therapy cannot predict measured uptake of [68Ga]PSMA-HBED-CC lesion uptake in recurrent/metastatic prostate cancer. Nuklearmedizin 2017, 56, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.T.; Jochumsen, M.R.; Klingenberg, S.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. Evaluation of Predictors of Biochemical Recurrence in Prostate Cancer Patients, as Detected by 68Ga-PSMA PET/CT. Diagnostics 2022, 12, 195. [Google Scholar] [CrossRef]
- Ferraro, D.A.; Rüschoff, J.H.; Muehlematter, U.J.; Kranzbühler, B.; Müller, J.; Messerli, M.; Husmann, L.; Hermanns, T.; Eberli, D.; Rupp, N.J.; et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with 68Ga-PSMA-11-PET. Theranostics 2020, 10, 6082–6094. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 42, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Hegemann, N.-S.; Fendler, W.P.; Buchner, A.; Stief, C.; Rogowski, P.; Niyazi, M.; Eze, C.; Li, M.; Bartenstein, P.; Belka, C.; et al. Detection level and pattern of positive lesions using PSMA PET/CT for staging prior to radiation therapy. Radiat. Oncol. 2017, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Crocerossa, F.; Marchioni, M.; Novara, G.; Carbonara, U.; Ferro, M.; Russo, G.I.; Porpiglia, F.; Di Nicola, M.; Damiano, R.; Autorino, R.; et al. Detection Rate of Prostate Specific Membrane Antigen Tracers for Positron Emission Tomography/Computerized Tomography in Prostate Cancer Biochemical Recurrence: A Systematic Review and Network Meta-Analysis. J. Urol. 2021, 205, 356–369. [Google Scholar] [CrossRef]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Debus, N.; Uhrig, M.; Hope, T.A.; Evans, M.J.; Holland-Letz, T.; Giesel, F.L.; Kopka, K.; Hadaschik, B.; Kratochwil, C.; et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2045–2054. [Google Scholar] [CrossRef] [Green Version]
- Onal, C.; Guler, O.C.; Torun, N.; Reyhan, M.; Yapar, A.F. The effect of androgen deprivation therapy on 68Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, L.Y.; Fulton, M.D.; Johnson, J.M.; Berkman, C.E. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int. J. Oncol. 2012, 41, 2087–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronsert, P.; Reichel, K.; Ruf, J. Loss of PSMA Expression in Non-neuroendocrine Dedifferentiated Acinar Prostate Cancer. Clin. Nucl. Med. 2018, 43, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to PSMA PET? Ther. Adv. Med. Oncol. 2019, 11, 1758835919876828. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-P.; Lv, J.; Li, M.-Z.; Xie, L.-J.; Li, J.-P.; Li, J.-F.; Cheng, M.-H. Biphasic GA 68-labeled prostate specific membrane antigen-11 positron emission tomography/computed tomography scans in the differential diagnosis and risk stratification of initial primary prostate cancer. Quant. Imaging Med. Surg. 2021, 11, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lindenberg, L.; Mena, E.; Turkbey, B.; Seidel, J.; Ton, A.; McKinney, Y.; Eclarinal, P.; Merino, M.; Pinto, P.; et al. A Pilot Study of Dynamic 18F-DCFPyL PET/CT Imaging of Prostate Adenocarcinoma in High-Risk Primary Prostate Cancer Patients. Mol. Imaging Biol. 2022, 24, 444–452. [Google Scholar] [CrossRef]
- Sahlmann, C.-O.; Meller, B.; Bouter, C.; Ritter, C.O.; Ströbel, P.; Lotz, J.; Trojan, L.; Meller, J.; Hijazi, S. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 898–905. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Bögemann, M.; Wagner, S.; Schäfers, M.; Stegger, L.; Weckesser, M. 18F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: Biodistribution, tumour detection and activity kinetics. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1329–1334. [Google Scholar] [CrossRef]
- Werner, R.A.; Sheikhbahaei, S.; Jones, K.M.; Javadi, M.S.; Solnes, L.B.; Ross, A.E.; Allaf, M.E.; Pienta, K.J.; Lapa, C.; Buck, A.K.; et al. Patterns of uptake of prostate-specific membrane antigen (PSMA)-targeted 18F-DCFPyL in peripheral ganglia. Ann. Nucl. Med. 2017, 31, 696–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.A.; Buchholz, H.-G.; Wieler, H.J.; Rosar, F.; Miederer, M.; Fischer, N.; Schreckenberger, M. Dual-Time Point [68Ga]Ga-PSMA-11 PET/CT Hybrid Imaging for Staging and Restaging of Prostate Cancer. Cancers 2020, 12, 2788. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Fragoso Costa, P.; Eiber, M.; Klose, J.M.; Wosniack, J.; Reis, H.; Szarvas, T.; Hadaschik, B.; Lückerath, K.; Herrmann, K.; et al. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 7431. [Google Scholar] [CrossRef]
- Leitsmann, C.; Thelen, P.; Schmid, M.; Meller, J.; Sahlmann, C.-O.; Meller, B.; Trojan, L.; Strauss, A. Enhancing PSMA-uptake with androgen deprivation therapy—A new way to detect prostate cancer metastases? Int. Braz. J. Urol. 2019, 45, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, I.; Niklas-Hünermund, J.; Sachpekidis, C.; Zacho, H.D.; Mingels, C.; Dijkstra, L.; Bohn, K.P.; Läppchen, T.; Gourni, E.; Rominger, A.; et al. Combination of Forced Diuresis with Additional Late Imaging in 68Ga-PSMA-11 PET/CT: Effects on Lesion Visibility and Radiotracer Uptake. J. Nucl. Med. 2021, 62, 1252–1257. [Google Scholar] [CrossRef]
- Uprimny, C.; Bayerschmidt, S.; Kroiss, A.S.; Fritz, J.; Nilica, B.; Svirydenka, A.; Decristoforo, C.; Di Santo, G.; von Guggenberg, E.; Horninger, W.; et al. Impact of forced diuresis with furosemide and hydration on the halo artefact and intensity of tracer accumulation in the urinary bladder and kidneys on [68Ga]Ga-PSMA-11-PET/CT in the evaluation of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 123–133. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. Available online: https://uroweb.org/eau-guidelines/citing-usage-republication (accessed on 20 June 2022).
- Sonni, I.; Felker, E.R.; Lenis, A.T.; Sisk, A.E.; Bahri, S.; Allen-Auerbach, M.S.; Armstrong, W.R.; Suvannarerg, V.; Tubtawee, T.; Grogan, T.; et al. Head-to-Head Comparison of 68Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J. Nucl. Med. 2021, 63, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Annabattula, C.; Lewis, J.; Shetty, D.V.; Kam, J.; MacLean, F.; Arianayagam, M.; Canagasingham, B.; Ferguson, R.; Khadra, M.; et al. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: Correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018, 21, 204–211. [Google Scholar] [CrossRef]
- Kalapara, A.A.; Nzenza, T.; Pan, H.Y.C.; Ballok, Z.; Ramdave, S.; O’Sullivan, R.; Ryan, A.; Cherk, M.; Hofman, M.S.; Konety, B.R.; et al. Detection and localisation of primary prostate cancer using 68 gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. 2019, 126, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Q.; Zhang, C.; Zhao, X.; Marra, G.; Gao, J.; Lv, X.; Zhang, B.; Fu, Y.; Wang, F.; et al. Combination of 68Ga-PSMA PET/CT and Multiparametric MRI Improves the Detection of Clinically Significant Prostate Cancer: A Lesion-by-Lesion Analysis. J. Nucl. Med. 2019, 60, 944–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, P.; Roberts, M.J.; Morton, A.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; Yaxley, J. Improved specificity with 68Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: A single institution comparative analysis with radical prostatectomy histology. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Privé, B.M.; Israël, B.; Schilham, M.G.M.; Muselaers, C.H.J.; Zámecnik, P.; Mulders, P.F.A.; Witjes, J.A.; Sedelaar, M.; Mehra, N.; Verzijlbergen, F.; et al. Evaluating F-18-PSMA-1007-PET in primary prostate cancer and comparing it to multi-parametric MRI and histopathology. Prostate Cancer Prostatic Dis. 2021, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Laudicella, R.; Skawran, S.; Ferraro, D.A.; Mühlematter, U.J.; Maurer, A.; Grünig, H.; Rüschoff, H.J.; Rupp, N.; Donati, O.; Eberli, D.; et al. Quantitative imaging parameters to predict the local staging of prostate cancer in intermediate- to high-risk patients. Insights Imaging 2022, 13, 75. [Google Scholar] [CrossRef]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, T.; Zhang, Z.; Zhang, N.; Du, P.; Yang, Y.; Liu, Y.; Yu, W.; Li, N.; Gorin, M.A.; et al. 68Ga-PSMA PET/CT Combined with PET/Ultrasound-Guided Prostate Biopsy Can Diagnose Clinically Significant Prostate Cancer in Men with Previous Negative Biopsy Results. J. Nucl. Med. 2020, 61, 1314–1319. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Li, W.-C.; Xu, Z.; Jiang, N.; Zang, S.-M.; Xu, L.-W.; Huang, W.-B.; Wang, F.; Sun, H.-B. 68Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Laudicella, R.; Zeimpekis, K.; Mebert, I.; Müller, J.; Maurer, A.; Grünig, H.; Donati, O.; Sapienza, M.T.; Rueschoff, J.H.; et al. Hot needles can confirm accurate lesion sampling intraoperatively using [18F]PSMA-1007 PET/CT-guided biopsy in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1721–1730. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wen, Q.; Zhang, H.; Ji, B. Head-to-Head Comparison of 68Ga-PSMA-11 PET/CT and Multiparametric MRI for Pelvic Lymph Node Staging Prior to Radical Prostatectomy in Patients With Intermediate to High-Risk Prostate Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 737989. [Google Scholar] [CrossRef] [PubMed]
- Luiting, H.B.; van Leeuwen, P.J.; Busstra, M.B.; Brabander, T.; van der Poel, H.G.; Donswijk, M.L.; Vis, A.N.; Emmett, L.; Stricker, P.D.; Roobol, M.J. Use of Gallium-68 Prostate-Specific Membrane Antigen Positron-Emission Tomography for Detecting Lymph Node Metastases in Primary and Recurrent Prostate Cancer and Location of Recurrence after Radical Prostatectomy: An Overview of the Current Literature: Use of 68Ga-PSMA PET/CT after RP: Review. BJU Int. 2020, 125, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.; Pellegrino, A.; Mazzone, E.; Cannoletta, D.; de Angelis, M.; Barletta, F.; Scuderi, S.; Cucchiara, V.; Gandaglia, G.; Raggi, D.; et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur. Urol. Oncol. 2021, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Zhang, C.; Liu, Z.; Shen, G.; Wu, X.; Nie, L.; Chang, T.; Xu, H.; Bao, Y.; Yang, L.; et al. The Role of 68Ga-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Intermediate/High Risk Patients with Prostate Cancer: A Diagnostic Meta-Analysis. Front. Oncol. 2020, 10, 1365. [Google Scholar] [CrossRef]
- Hope, T.A.; Goodman, J.Z.; Allen, I.E.; Calais, J.; Fendler, W.P.; Carroll, P.R. Metaanalysis of 68Ga-PSMA-11 PET Accuracy for the Detection of Prostate Cancer Validated by Histopathology. J. Nucl. Med. 2019, 60, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Weckesser, M.; Ahmadzadehfar, H.; Schäfers, M.; Stegger, L.; Bögemann, M. Advantage of 18F-PSMA-1007 over 68Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Sprute, K.; Kramer, V.; Koerber, S.A.; Meneses, M.; Fernandez, R.; Soza-Ried, C.; Eiber, M.; Weber, W.A.; Rauscher, I.; Rahbar, K.; et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J. Nucl. Med. 2021, 62, 208–213. [Google Scholar] [CrossRef]
- Malaspina, S.; Anttinen, M.; Taimen, P.; Jambor, I.; Sandell, M.; Rinta-Kiikka, I.; Kajander, S.; Schildt, J.; Saukko, E.; Noponen, T.; et al. Prospective comparison of 18F-PSMA-1007 PET/CT, whole-body MRI and CT in primary nodal staging of unfavourable intermediate- and high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, M.; Wurzer, A.; Schwamborn, K.; Ulbrich, L.; Jooß, L.; Maurer, T.; Horn, T.; Rauscher, I.; Haller, B.; Herz, M.; et al. Histologically Confirmed Diagnostic Efficacy of 18F-rhPSMA-7 PET for N-Staging of Patients with Primary High-Risk Prostate Cancer. J. Nucl. Med. 2020, 61, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual Comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, B.H.E.; Bodar, Y.J.L.; Zwezerijnen, G.J.C.; Meijer, D.; van der Voorn, J.P.; Nieuwenhuijzen, J.A.; Wondergem, M.; Roeleveld, T.A.; Boellaard, R.; Hoekstra, O.S.; et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer—The SALT trial. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.-J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic Efficacy of 68 Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.-D.; et al. Distribution of Metastatic Sites in Patients with Prostate Cancer: A Population-Based Analysis: Sites of Metastases in PCa Patients. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef]
- Pinto, F.; Totaro, A.; Palermo, G.; Calarco, A.; Sacco, E.; D’Addessi, A.; Racioppi, M.; Valentini, A.; Gui, B.; Bassi, P. Imaging in Prostate Cancer Staging: Present Role and Future Perspectives. Urol. Int. 2012, 88, 125–136. [Google Scholar] [CrossRef]
- Makarov, D.V.; Loeb, S.; Ulmert, D.; Drevin, L.; Lambe, M.; Stattin, P. Prostate Cancer Imaging Trends After a Nationwide Effort to Discourage Inappropriate Prostate Cancer Imaging. JNCI J. Natl. Cancer Inst. 2013, 105, 1306–1313. [Google Scholar] [CrossRef]
- Shen, G.; Deng, H.; Hu, S.; Jia, Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skelet. Radiol. 2014, 43, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Helyar, V.; Mohan, H.K.; Barwick, T.; Livieratos, L.; Gnanasegaran, G.; Clarke, S.E.M.; Fogelman, I. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 706–713. [Google Scholar] [CrossRef]
- Strobel, K.; Burger, C.; Seifert, B.; Husarik, D.B.; Soyka, J.D.; Hany, T.F. Characterization of Focal Bone Lesions in the Axial Skeleton: Performance of Planar Bone Scintigraphy Compared with SPECT and SPECT Fused with CT. Am. J. Roentgenol. 2007, 188, W467–W474. [Google Scholar] [CrossRef] [PubMed]
- Tabotta, F.; Jreige, M.; Schaefer, N.; Becce, F.; Prior, J.O.; Nicod Lalonde, M. Quantitative bone SPECT/CT: High specificity for identification of prostate cancer bone metastases. BMC Musculoskelet. Disord. 2019, 20, 619. [Google Scholar] [CrossRef] [Green Version]
- Pomykala, K.L.; Czernin, J.; Grogan, T.R.; Armstrong, W.R.; Willliams, J.; Calais, J. Total-Body 68Ga-PSMA-11 PET/CT for Bone Metastasis Detection in Prostate Cancer Patients: Potential Impact on Bone Scan Guidelines. J. Nucl. Med. 2020, 61, 405–411. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Zacho, H.D.; Nielsen, J.B.; Haberkorn, U.; Stenholt, L.; Petersen, L.J. 68Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: A systematic review of the published literature. Clin. Physiol. Funct. Imaging 2018, 38, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gou, Z.; Wu, R.; Yuan, Y.; Yu, G.; Zhao, Y. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skelet. Radiol. 2019, 48, 1915–1924. [Google Scholar] [CrossRef]
- Moreira, D.M.; Presti, J.C.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Freedland, S.J. Natural History of Persistently Elevated Prostate Specific Antigen After Radical Prostatectomy: Results From the SEARCH Database. J. Urol. 2009, 182, 2250–2256. [Google Scholar] [CrossRef]
- Spratt, D.E.; Yousefi, K.; Deheshi, S.; Ross, A.E.; Den, R.B.; Schaeffer, E.M.; Trock, B.J.; Zhang, J.; Glass, A.G.; Dicker, A.P.; et al. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. J. Clin. Oncol. 2017, 35, 1991–1998. [Google Scholar] [CrossRef]
- Wiegel, T.; Bartkowiak, D.; Bottke, D.; Thamm, R.; Hinke, A.; Stöckle, M.; Rübe, C.; Semjonow, A.; Wirth, M.; Störkel, S.; et al. Prostate-Specific Antigen Persistence After Radical Prostatectomy as a Predictive Factor of Clinical Relapse-Free Survival and Overall Survival: 10-Year Data of the ARO 96-02 Trial. Int. J. Radiat. Oncol. 2015, 91, 288–294. [Google Scholar] [CrossRef]
- Sood, A.; Keeley, J.; Palma-Zamora, I.; Arora, S.; Dalela, D.; Olson, P.; Hanna, R.; Cotter, D.; Jeong, W.; Elshaikh, M.; et al. Ten-year disease progression and mortality rates in men who experience biochemical recurrence versus persistence after radical prostatectomy and undergo salvage radiation therapy: A post-hoc analysis of RTOG 9601 trial data. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 599.e1–599.e8. [Google Scholar] [CrossRef]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. 68 Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/mL. Efficacy and impact on treatment strategy. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 11–19. [Google Scholar] [CrossRef]
- Ceci, F.; Castellucci, P.; Graziani, T.; Farolfi, A.; Fonti, C.; Lodi, F.; Fanti, S. 68Ga-PSMA-11 PET/CT in recurrent prostate cancer: Efficacy in different clinical stages of PSA failure after radical therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Düwel, C.; Haller, B.; Rischpler, C.; Heck, M.M.; Gschwend, J.E.; Schwaiger, M.; Maurer, T.; Eiber, M. Efficacy, Predictive Factors, and Prediction Nomograms for 68Ga-labeled Prostate-specific Membrane Antigen–ligand Positron-emission Tomography/Computed Tomography in Early Biochemical Recurrent Prostate Cancer After Radical Prostatectomy. Eur. Urol. 2018, 73, 656–661. [Google Scholar] [CrossRef]
- Mena, E.; Lindenberg, M.L.; Shih, J.H.; Adler, S.; Harmon, S.; Bergvall, E.; Citrin, D.; Dahut, W.; Ton, A.T.; McKinney, Y.; et al. Clinical impact of PSMA-based 18F–DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 4–11. [Google Scholar] [CrossRef]
- Wondergem, M.; Jansen, B.H.E.; van der Zant, F.M.; van der Sluis, T.M.; Knol, R.J.J.; van Kalmthout, L.W.M.; Hoekstra, O.S.; van Moorselaar, R.J.A.; Oprea-Lager, D.E.; Vis, A.N. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1911–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, D.; Donswijk, M.L.; Bodar, Y.J.L.; Van Leeuwen, P.J.; Van Der Poel, H.G.; Vogel, W.V.; Nieuwenhuijzen, J.A.; Hendrikse, N.H.; Oprea-Lager, D.E.; Vis, A.N. Biochemical Persistence of Prostate-specific Antigen after Robot-assisted Laparoscopic Radical Prostatectomy: Tumor localizations using PSMA PET/CT imaging. J. Nucl. Med. 2021, 62, 961–967. [Google Scholar] [CrossRef]
- Farolfi, A.; Gafita, A.; Calais, J.; Eiber, M.; Afshar-Oromieh, A.; Spohn, F.; Barbato, F.; Weber, M.; Ilhan, H.; Cervati, V.; et al. 68Ga-PSMA-11 Positron Emission Tomography Detects Residual Prostate Cancer after Prostatectomy in a Multicenter Retrospective Study. J. Urol. 2019, 202, 1174–1181. [Google Scholar] [CrossRef]
- Hijazi, S.; Meller, B.; Leitsmann, C.; Strauss, A.; Ritter, C.; Lotz, J.; Meller, J.; Trojan, L.; Sahlmann, C.-O. See the Unseen: Mesorectal Lymph Node Metastases in Prostate Cancer: Mesorectal Lymph Node Metastases in Prostate Cancer. Prostate 2016, 76, 776–780. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.-S.; Fendler, W.P.; Ilhan, H.; Herlemann, A.; Buchner, A.; Stief, C.; Eze, C.; Rogowski, P.; Li, M.; Bartenstein, P.; et al. Outcome after PSMA PET/CT based radiotherapy in patients with biochemical persistence or recurrence after radical prostatectomy. Radiat. Oncol. 2018, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Shikanov, S.; Kocherginsky, M.; Shalhav, A.L.; Eggener, S.E. Cause-specific mortality following radical prostatectomy. Prostate Cancer Prostatic Dis. 2012, 15, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative Nomogram Predicting the 10-Year Probability of Prostate Cancer Recurrence After Radical Prostatectomy. JNCI J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef] [Green Version]
- Pfitzenmaier, J.; Pahernik, S.; Tremmel, T.; Haferkamp, A.; Buse, S.; Hohenfellner, M. Positive surgical margins after radical prostatectomy: Do they have an impact on biochemical or clinical progression? BJU Int. 2008, 102, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.L.; Pitroda, S.P.; Eggener, S.E.; Stadler, W.M.; Pelizzari, C.A.; Vannier, M.W.; Oto, A. Evaluation of the Prostate Bed for Local Recurrence After Radical Prostatectomy Using Endorectal Magnetic Resonance Imaging. Int. J. Radiat. Oncol. 2013, 85, 378–384. [Google Scholar] [CrossRef]
- Linder, B.J.; Kawashima, A.; Woodrum, D.A.; Tollefson, M.K.; Karnes, J.; Davis, B.J.; Rangel, L.J.; King, B.F.; Mynderse, L.A. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can. J. Urol. 2014, 21, 7283–7289. [Google Scholar]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Freitag, M.T.; Radtke, J.P.; Afshar-Oromieh, A.; Roethke, M.C.; Hadaschik, B.A.; Gleave, M.; Bonekamp, D.; Kopka, K.; Eder, M.; Heusser, T.; et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: Comparison with mpMRI integrated in simultaneous PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 776–787. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Beer, A.J.; Graner, F.-P.; Haller, B.; Weirich, G.; Doherty, A.; Gschwend, J.E.; Schwaiger, M.; Eiber, M. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J. Nucl. Med. 2016, 57, 1713–1719. [Google Scholar] [CrossRef] [Green Version]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Pereira Mestre, R.; Treglia, G.; Ferrari, M.; Pascale, M.; Mazzara, C.; Azinwi, N.C.; Llado’, A.; Stathis, A.; Giovanella, L.; Roggero, E. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13063. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer With Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef]
- Morawitz, J.; Kirchner, J.; Lakes, J.; Bruckmann, N.M.; Mamlins, E.; Hiester, A.; Aissa, J.; Loberg, C.; Schimmöller, L.; Arsov, C.; et al. PSMA PET/CT vs. CT alone in newly diagnosed biochemical recurrence of prostate cancer after radical prostatectomy: Comparison of detection rates and therapeutic implications. Eur. J. Radiol. 2021, 136, 109556. [Google Scholar] [CrossRef]
- Tan, N.; Bavadian, N.; Calais, J.; Oyoyo, U.; Kim, J.; Turkbey, I.B.; Mena, E.; Davenport, M.S. Imaging of Prostate Specific Membrane Antigen Targeted Radiotracers for the Detection of Prostate Cancer Biochemical Recurrence after Definitive Therapy: A Systematic Review and Meta-Analysis. J. Urol. 2019, 202, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Annunziata, S.; Pizzuto, D.A.; Giovanella, L.; Prior, J.O.; Ceriani, L. Detection Rate of 18F-Labeled PSMA PET/CT in Biochemical Recurrent Prostate Cancer: A Systematic Review and a Meta-Analysis. Cancers 2019, 11, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Abufaraj, M.; Janisch, F.; Iwata, T.; Parizi, M.K.; Foerster, B.; Fossati, N.; Briganti, A.; Egawa, S.; Hartenbach, M.; et al. Performance of [68Ga] Ga-PSMA 11 PET for detecting prostate cancer in the lymph nodes before salvage lymph node dissection: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2020, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, A.; Kulanthaivelu, R.; Bauman, G.; Ortega, C.; Veit-Haibach, P.; Metser, U. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Wondergem, M.; Van Der Zant, F.M.; Broos, W.A.M.; Knol, R.J.J. Clinical impact of PSMA PET in biochemically recurrent prostate cancer; a review of the literature. Tijdschr. Urol. 2020, 10, 109–121. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Steuber, T.; Jilg, C.; Tennstedt, P.; De Bruycker, A.; Tilki, D.; Decaestecker, K.; Zilli, T.; Jereczek-Fossa, B.A.; Wetterauer, U.; Grosu, A.L.; et al. Standard of Care Versus Metastases-directed Therapy for PET-detected Nodal Oligorecurrent Prostate Cancer Following Multimodality Treatment: A Multi-institutional Case-control Study. Eur. Urol. Focus 2018, 5, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Lilleby, O.; Hernes, E.; Lilleby, W. Metastatic-directed therapy using PSMA-PET/CT at PSA relapse. Urol. Case Rep. 2019, 27, 100992. [Google Scholar] [CrossRef]
- Mazzola, R.; Francolini, G.; Triggiani, L.; Napoli, G.; Cuccia, F.; Nicosia, L.; Livi, L.; Magrini, S.M.; Salgarello, M.; Alongi, F. Metastasis-directed Therapy (SBRT) Guided by PET-CT 18F-CHOLINE Versus PET-CT 68Ga-PSMA in Castration-sensitive Oligorecurrent Prostate Cancer: A Comparative Analysis of Effectiveness. Clin. Genitourin. Cancer 2021, 19, 230–236. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Fendler, W.P.; Elashoff, D.; Nickols, N.G. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019, 19, 18. [Google Scholar] [CrossRef]
- Mjaess, G.; Vierasu, I.; Lacroix, S.; Aoun, F.; Goldman, S.; Roumeguère, T.; Albisinni, S. Is there a role for repeating PSMA PET/CT after a negative scan in biochemical recurrent prostate cancer? Acta Oncol. 2020, 59, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Shi, Y.; Zhu, Y.; Xu, L.; Huang, G.; Liu, J. Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2970–2977. [Google Scholar] [CrossRef]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; Oikonomou, E.; De Dosso, S.; Jermini, F.; Prior, J.O.; Roggero, E.; et al. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: A meta-analysis. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 127–139. [Google Scholar] [PubMed]
- Emmett, L.; van Leeuwen, P.J.; Nandurkar, R.; Scheltema, M.J.; Cusick, T.; Hruby, G.; Kneebone, A.; Eade, T.; Fogarty, G.; Jagavkar, R.; et al. Treatment Outcomes from 68Ga-PSMA PET/CT–Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J. Nucl. Med. 2017, 58, 1972–1976. [Google Scholar] [CrossRef] [Green Version]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Lanzafame, H.; Farolfi, A.; Mapelli, P.; Picchio, M.; Burger, I.A.; Iagaru, A.; Minutoli, F.; Evangelista, L. PSMA and Choline PET for the Assessment of Response to Therapy and Survival Outcomes in Prostate Cancer Patients: A Systematic Review from the Literature. Cancers 2022, 14, 1770. [Google Scholar] [CrossRef]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2020, 61, 678–682. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Weber, M.; Hadaschik, B.; Wang, H.; Armstrong, W.R.; Tauber, R.; Grogan, T.R.; Czernin, J.; Rettig, M.B.; et al. Novel framework for treatment response evaluation using PSMA-PET/CT in patients with metastatic castration-resistant prostate cancer (RECIP 1.0): An international multicenter study. J. Nucl. Med. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Fendler, W.P.; Murthy, V.; Hui, W.; Armstrong, W.R.; Herrmann, K.; Weber, W.A.; Calais, J.; Eiber, M.; et al. Measuring response in metastatic castration-resistant prostate cancer using PSMA PET/CT: Comparison of RECIST 1.1, aPCWG3, aPERCIST, PPP, and RECIP 1.0 criteria. 2022Eur. J. Nucl. Med. Mol. Imaging, 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Grubmüller, B.; Senn, D.; Kramer, G.; Baltzer, P.; D’Andrea, D.; Grubmüller, K.H.; Mitterhauser, M.; Eidherr, H.; Haug, A.R.; Wadsak, W.; et al. Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosar, F.; Wenner, F.; Khreish, F.; Dewes, S.; Wagenpfeil, G.; Hoffmann, M.A.; Schreckenberger, M.; Bartholomä, M.; Ezziddin, S. Early molecular imaging response assessment based on determination of total viable tumor burden in [68Ga]Ga-PSMA-11 PET/CT independently predicts overall survival in [177Lu]Lu-PSMA-617 radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
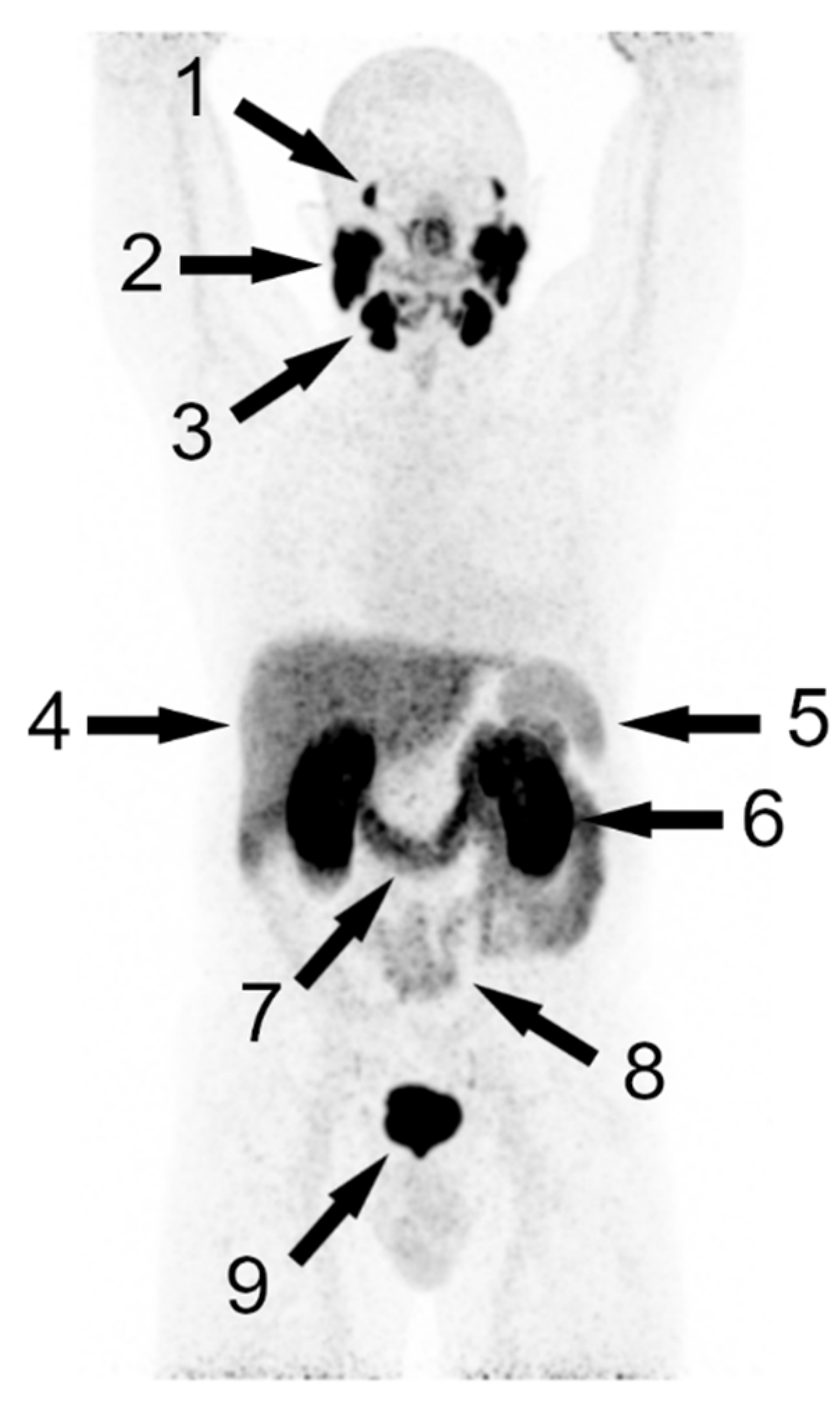

| Physiological PSMA Expression |
|---|
| Submandibular glands |
| Normal prostate epithelium |
| Duodenum |
| Colon |
| Proximal tubules of the kidney |
| Sympathetic ganglia |
| Normal transitional epithelium of the bladder |
| Normal breast parenchymal elements |
| Hepatocytes |
| Testis |
| Esophagus |
| Stomach |
| Small intestine |
| Fallopian tube epithelium |
| Pathological PSMA Expression (Primary Tumor and Metastases) |
|---|
| Prostate carcinoma |
| Renal carcinoma * |
| Bladder carcinoma * |
| Brain tumors * |
| Thyroid tumors * |
| Hepatocellular carcinoma * |
| Lung carcinoma * |
| Squamous cell carcinomas of oral cavity * |
| Adenoid cystic carcinoma * |
| Salivary duct carcinoma * |
| Adrenocortical carcinoma * |
| Gynecologic malignancies * |
| High-grade sarcomas * |
| Pancreatic carcinoma * |
| Colorectal carcinoma * |
| Gastric carcinoma * |
| Intestinal adenocarcinoma (*) |
| Pathological Tracer Uptake (Benign Tumors) |
|---|
| Elastofibroma dorsi |
| Dermatofibroma |
| Acrochordon |
| Fibromatosis desmoid tumor |
| Intramuscular myxoma |
| Pseudoangiomatous stromal hyperplasia of the breast |
| Thymoma |
| Thyroid adenoma |
| Parathyroid adenoma |
| Adrenal adenoma |
| Meningioma |
| Schwannoma |
| Peripheral nerve sheath tumors |
| Neurofibroma |
| Hemangioma |
| Angiolipoma |
| Hemangiopericytoma |
| Pathological Tracer Uptake (Granulomatous or Inflammatory Diseases) |
|---|
| Pulmonary sarcoidosis |
| Wegener’s granulomatosis |
| Bronchiectasis |
| Anthracosilicosis |
| Berylliosis |
| Pulmonary histoplasmosis |
| Tuberculosis |
| Asbestosis |
| Perianal fistula |
| Renal abscess |
| Post-operative inflammatory processes |
| Crohn’s disease |
| Pathological Tracer Uptake (Bone Diseases) |
|---|
| Fracture * |
| Osteophyte |
| Osteoarthritis |
| Paget’s disease + |
| Osteomyelitis |
| Fibrous dysplasia |
| Hemangioma |
| Pathological Tracer Uptake (Various Diseases/Findings) |
|---|
| Lymphoma |
| Testicular tumors |
| Thymic carcinoma |
| Polycythemia vera (diffuse bone marrow uptake) |
| Atelectasis |
| Amyloidosis of the seminal vesicles |
| Gynecomastia |
| Barrett’s esophagus |
| Tracers | Nuclides | Class |
|---|---|---|
| PSMA-11 | 68Ga | Diagnostics |
| PSMA-617 | 68Ga, 177Lu, 225Ac | Theranostics |
| PSMA-I&T | 68Ga, 177Lu, 225Ac | Theranostics |
| DCFPyL | 18F | Diagnostics |
| DCFBC | 18F | Diagnostics |
| PSMA-1007 | 18F | Diagnostics |
| Organ | Uptake |
|---|---|
| Kidney | +++ |
| Lacrimal glands | +++ |
| Parotid glands | +++ |
| Submandibular glands | +++ |
| Duodenum | ++ |
| Liver | + |
| Spleen | + |
| Small Intestine | + |
| Factor | Effect |
| Intrinsic (primary tumor, metastases) | Heterogeneity of PSMA expression |
| PSMA therapy | Loss of PSMA expression |
| Chemotherapy | Loss of PSMA expression |
| ADT—short term | Increase of PSMA expression |
| ADT—long term | Decrease of PSMA expression |
| Gleason score | PSMA expression primary tumor |
| Predictors | Prediction |
| Primary tumor % PSMA negativity | PSMA PET negativity |
| Primary tumor growth pattern | PSMA PET uptake |
| PSA-value | PSMA PET positivity |
| PSA doubling time | PSMA PET positivity |
| Authors (Year) | Number of Studies Included (Patients) | Tracers | Overall Positivity | Change in Patient Management |
|---|---|---|---|---|
| Tan et al. (2019) [132] | 43 (5113) | [18F]DCFPyL, [18F]DCFBC, [68Ga]Ga-PSMA-11, [18F]PSMA-1007, [68Ga]Ga-PSMA I&T | 70.2% | - |
| Pozdnyakov et al. (2022) [136] | 34 (3680) | 68Ga-PSMA and 18F-labeled PSMA tracers | 68.2% | 56.4% (95% CI: 48.0–63.9%) |
| Perera et al. (2020) [125] | 30 (4476) | 68Ga-PSMA tracers | 28% prostate bed 38% pelvic lymph nodes 13% extrapelvic lymph nodes 22% bone 5% distant viscera | - |
| Wondergem et al. (2020) [137] | 16 (1899) | [68Ga]Ga-PSMA-11, [18F]DCFPyL, [18F]PSMA-1007, [68Ga]Ga-THP-PSMA | - | 45% |
| Treglia et al. (2019) [133] | 6 (645) | [18F]PSMA-1007, [18F]DCFPyL, [18F]DCFBC | 81% (95% CI: 71–88%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisney, A.R.; Leitsmann, C.; Strauß, A.; Meller, B.; Bucerius, J.A.; Sahlmann, C.-O. The Role of PSMA PET/CT in the Primary Diagnosis and Follow-Up of Prostate Cancer—A Practical Clinical Review. Cancers 2022, 14, 3638. https://doi.org/10.3390/cancers14153638
Lisney AR, Leitsmann C, Strauß A, Meller B, Bucerius JA, Sahlmann C-O. The Role of PSMA PET/CT in the Primary Diagnosis and Follow-Up of Prostate Cancer—A Practical Clinical Review. Cancers. 2022; 14(15):3638. https://doi.org/10.3390/cancers14153638
Chicago/Turabian StyleLisney, Anna Rebecca, Conrad Leitsmann, Arne Strauß, Birgit Meller, Jan Alexander Bucerius, and Carsten-Oliver Sahlmann. 2022. "The Role of PSMA PET/CT in the Primary Diagnosis and Follow-Up of Prostate Cancer—A Practical Clinical Review" Cancers 14, no. 15: 3638. https://doi.org/10.3390/cancers14153638
APA StyleLisney, A. R., Leitsmann, C., Strauß, A., Meller, B., Bucerius, J. A., & Sahlmann, C.-O. (2022). The Role of PSMA PET/CT in the Primary Diagnosis and Follow-Up of Prostate Cancer—A Practical Clinical Review. Cancers, 14(15), 3638. https://doi.org/10.3390/cancers14153638






