The Association between Blood Indexes and Immune Cell Concentrations in the Primary Tumor Microenvironment Predicting Survival of Immunotherapy in Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
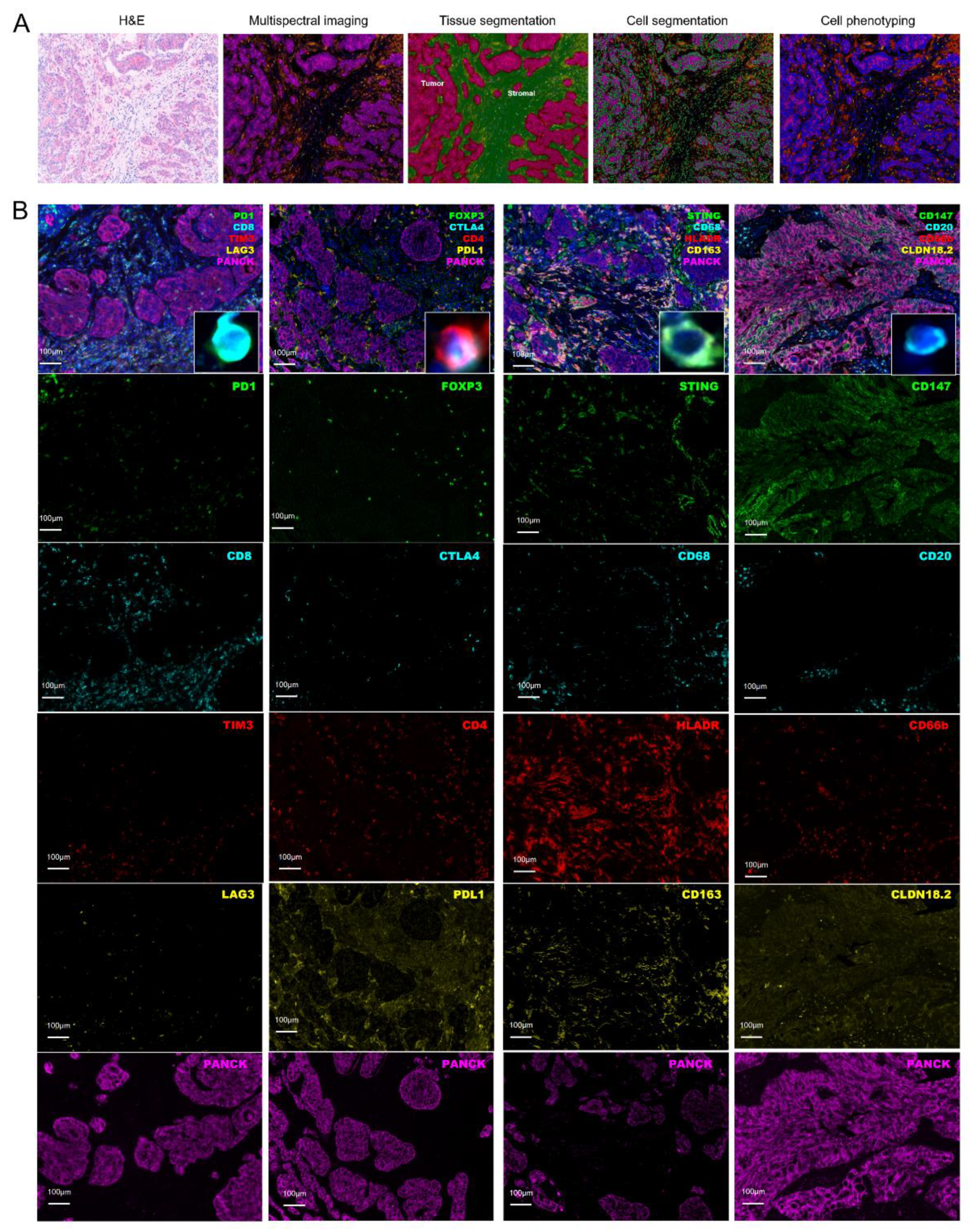
2.2. Assessment of Tumor Microenvironment
2.3. Assessment of Hematological Parameters
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Features of Gastric Cancer Patients
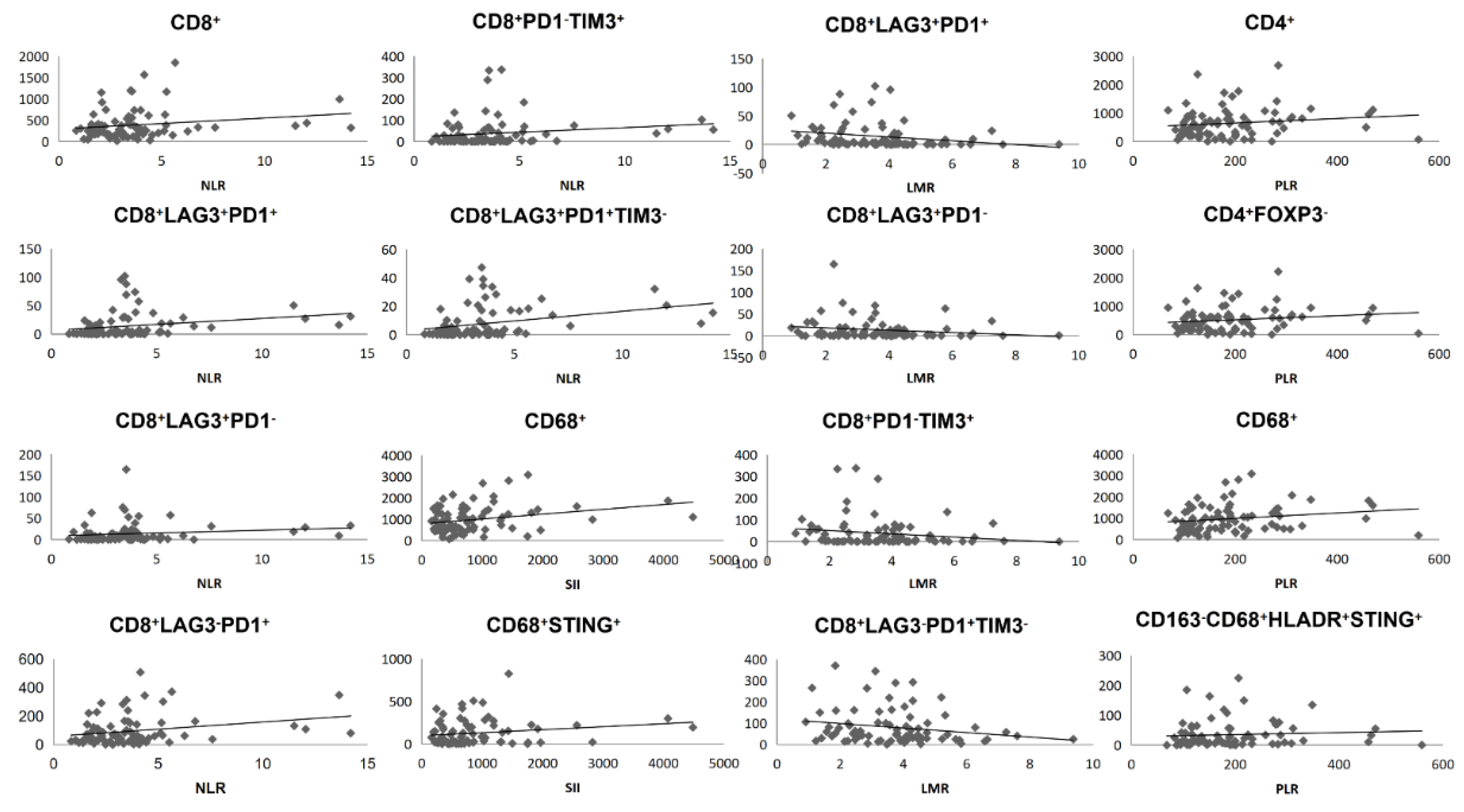
3.2. Blood Indexes Were Associated with Immune Cell Concentrations in Primary Tumor
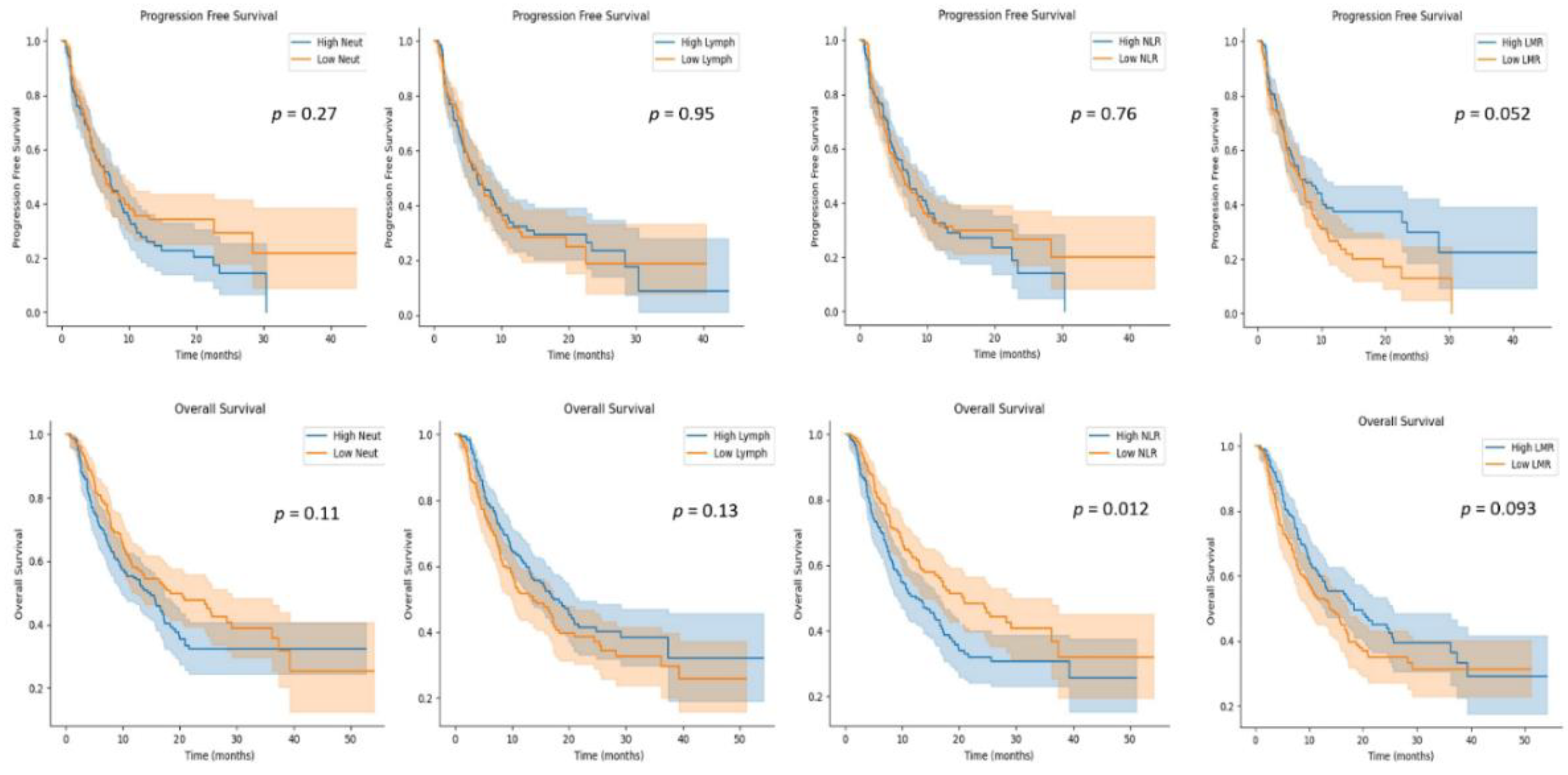
3.3. Blood Indexes Predicting Survival in GC Patients Receiving Anti-PD-1/PD-L1 Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.; Zhang, X.; Qi, C.; Shen, L.; Peng, Z. Appropriate PD-L1 Cutoff Value for Gastric Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 646355. [Google Scholar] [CrossRef] [PubMed]
- Bagaev, A.; Kotlov, N.; Nomie, K.; Svekolkin, V.; Gafurov, A.; Isaeva, O.; Osokin, N.; Kozlov, I.; Frenkel, F.; Gancharova, O.; et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021, 39, 845–865 e847. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Xing, D.; Luan, L.; Xu, H.; Sharma, R.B.; Popovic, A.; Pawlik, T.M.; Kim, A.K.; Zhu, Q.; Jaffee, E.M.; et al. Characterization of the Immune Microenvironment in Hepatocellular Carcinoma. Clin. Cancer Res. 2017, 23, 7333–7339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O'Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.S.; Tang, T.; Jia, J.L.; Xie, B.C.; Wu, T.L.; Sheng, Y.Y.; Xue, Y.Z.; Tang, H.M. CD137 agonist induces gastric cancer cell apoptosis by enhancing the functions of CD8(+) T cells via NF-kappaB signaling. Cancer Cell. Int. 2020, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gu, Y.; Zhang, H.; Fei, Y.; Cao, Y.; Fang, H.; Wang, J.; Lin, C.; Zhang, H.; Li, H.; et al. Immune inactivation by APOBEC3B enrichment predicts response to chemotherapy and survival in gastric cancer. Oncoimmunology 2021, 10, 1975386. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wang, Y.; Li, S.; Jiao, X.; Zou, J.; Wang, Z.; Qi, C.; Zhang, X.; Li, J.; Lu, Z.; et al. Early change in peripheral CD4(+) T cells associated with clinical outcomes of immunotherapy in gastrointestinal cancer. Immunotherapy 2021, 13, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Peng, Z.; Qi, C.; Gong, J.; Zhang, X.; Li, J.; Shen, L. Association of Lymphocyte-to-Monocyte Ratio With Survival in Advanced Gastric Cancer Patients Treated With Immune Checkpoint Inhibitor. Front. Oncol. 2021, 11, 589022. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8(+) T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, M. CD8(+) T cell exhaustion. Semin. Immunopathol. 2019, 41, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 2020, 43, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bassez, A.; Vos, H.; Van Dyck, L.; Floris, G.; Arijs, I.; Desmedt, C.; Boeckx, B.; Vanden Bempt, M.; Nevelsteen, I.; Lambein, K.; et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med. 2021, 27, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Ou, D.L.; Bai, L.Y.; Chen, C.W.; Lin, L.; Huang, S.F.; Cheng, A.L.; Jeng, Y.M.; Hsu, C. Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Liver Cancer 2021, 10, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Boor, P.P.C.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119 e1110. [Google Scholar] [CrossRef] [PubMed]
| Characteristic * | Total (Test Cohort) n = 80 | Total (Validation Cohort) n = 357 |
|---|---|---|
| Age | ||
| Median, IQR | 60 (54–66) | 59 (51–65) |
| Sex | ||
| Male | 61 (76.3%) | 255 (71.4%) |
| Female | 19 (23.7%) | 102 (28.6%) |
| ECOG PS | ||
| 0 | 49 (61.3%) | 166 (46.5%) |
| ≥1 | 31 (38.7%) | 191 (53.5%) |
| Location | ||
| GEJ | 24 (30.0%) | 112 (31.4%) |
| Non-GEJ | 56 (70.0%) | 245 (68.6%) |
| Differentiation | ||
| High | 0 (0%) | 1 (0.3%) |
| Moderate | 23 (28.8%) | 88 (24.6%) |
| Moderate-poor | 22 (27.5%) | 62 (17.4%) |
| Poor | 35 (43.7%) | 195 (54.6%) |
| NA | 0 (0%) | 11 (3.1%) |
| Lauren classification | ||
| Intestinal type | 38 (47.5%) | 138 (38.7%) |
| Diffused type | 18 (22.5%) | 89 (24.9%) |
| Mixed type | 24 (30.0%) | 79 (22.1%) |
| NA | 0 (0%) | 51 (14.3%) |
| Stage | ||
| I | 3 (3.8%) | 1 (0.3%) |
| II | 9 (11.3%) | 4 (1.1%) |
| III | 29 (36.2%) | 27 (7.6%) |
| IV | 39 (48.7%) | 324 (90.7%) |
| NA | 0 (0%) | 1 (0.3%) |
| HER2 expression | ||
| Positive | 22 (27.5%) | 75 (21.0%) |
| Negative | 58 (72.5%) | 268 (75.1%) |
| NA | 0 (0%) | 14 (3.9%) |
| PD-L1 expression (CPS) | ||
| ≥10 | 36 (45.0%) | 88 (24.6%) |
| 5-10 | 10 (12.5%) | 31 (8.7%) |
| 1-5 | 17 (21.25%) | 16 (4.5%) |
| <1 | 17 (21.25%) | 96 (26.9%) |
| NA | 0 (0%) | 126 (35.3%) |
| MMR status | ||
| pMMR | 69 (86.25%) | 266 (74.5%) |
| dMMR | 11 (13.75%) | 45 (12.6%) |
| NA | 0 (0%) | 46 (12.9%) |
| EBV status | ||
| Positive | 10 (12.5%) | 26 (7.3%) |
| Negative | 70 (87.5%) | 285 (79.8%) |
| NA | 0 (0%) | 46 (12.9%) |
| Variables | Progression-Free Survival | Progression-Free Survival | ||
|---|---|---|---|---|
| Hazard Ratio | p | Hazard Ratio | p | |
| Multivariate analysis with LMR | Multivariate analysis with Monocytes | |||
| Lauren | 0.013 | 0.0056 | ||
| Intestinal | 1 | 1 | ||
| Diffuse | 1.65 (1.11–2.45) | 1.77 (1.18–2.64) | ||
| Peritoneal | 0.69 | 0.84 | ||
| Absent | 1 | 1 | ||
| Present | 1.09 (0.71–1.68) | 0.96 (0.62–1.47) | ||
| Line | 0.026 | 0.0024 | ||
| First Line | 1 | 1 | ||
| Others | 1.55 (1.05–2.28) | 1.86 (1.25–2.78) | ||
| LMR | 0.034 | |||
| Low | 1 | |||
| High | 0.66 (0.45–0.97) | |||
| Monocytes | 0.0034 | |||
| Low | 1 | |||
| High | 1.82 (1.22–2.71) | |||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio | p | Hazard Ratio | p | |
| Gender | 0.003 | 0.28 | ||
| Male | 1 | 1 | ||
| Female | 0.63 (0.47–0.86) | 0.85 (0.57–1.29) | ||
| Age | <0.001 | 0.0076 | ||
| <60 | 1 | 1 | ||
| ≥60 | 0.68 (0.51–0.9) | 0.60 (0.41–0.88) | ||
| Differentiation | 0.023 | 0.25 | ||
| Moderate | 1 | 1 | ||
| Poor | 1.4 (1–1.9) | 0.62 (0.32–1.20) | ||
| HER2 | 0.029 | 0.12 | ||
| Negative | 1 | 1 | ||
| Positive | 0.65 (0.44–0.96) | 0.61 (0.36–1.03) | ||
| Peritoneal | 0.00021 | 0.32 | ||
| Absent | 1 | 1 | ||
| Present | 1.6 (1.3–2.1) | 0.85 (0.57–1.28) | ||
| Stage | 0.0023 | 0.0091 | ||
| I/II/III | 1 | 1 | ||
| IV | 3 (1.5–5.9) | 3.41 (1.32–8.79) | ||
| Line | <0.001 | <0.001 | ||
| First Line | 1 | 1 | ||
| Others | 2.6 (1.9–3.5) | 2.60 (1.65–4.09) | ||
| NLR | 0.013 | 0.17 | ||
| Low | 1 | 1 | ||
| High | 1.4 (1.1–1.9) | 1.30 (0.90–1.89) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Zhao, X.; Li, Y.; Yao, Q.; Jiang, L.; Feng, X.; Shen, L.; Li, Y.; Chen, Y. The Association between Blood Indexes and Immune Cell Concentrations in the Primary Tumor Microenvironment Predicting Survival of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 3608. https://doi.org/10.3390/cancers14153608
Yuan J, Zhao X, Li Y, Yao Q, Jiang L, Feng X, Shen L, Li Y, Chen Y. The Association between Blood Indexes and Immune Cell Concentrations in the Primary Tumor Microenvironment Predicting Survival of Immunotherapy in Gastric Cancer. Cancers. 2022; 14(15):3608. https://doi.org/10.3390/cancers14153608
Chicago/Turabian StyleYuan, Jiajia, Xingwang Zhao, Yanyan Li, Qian Yao, Lei Jiang, Xujiao Feng, Lin Shen, Yilin Li, and Yang Chen. 2022. "The Association between Blood Indexes and Immune Cell Concentrations in the Primary Tumor Microenvironment Predicting Survival of Immunotherapy in Gastric Cancer" Cancers 14, no. 15: 3608. https://doi.org/10.3390/cancers14153608
APA StyleYuan, J., Zhao, X., Li, Y., Yao, Q., Jiang, L., Feng, X., Shen, L., Li, Y., & Chen, Y. (2022). The Association between Blood Indexes and Immune Cell Concentrations in the Primary Tumor Microenvironment Predicting Survival of Immunotherapy in Gastric Cancer. Cancers, 14(15), 3608. https://doi.org/10.3390/cancers14153608






