Satisfaction with Long-Term Aesthetic and 10 Years Oncologic Outcome following Risk-Reducing Mastectomy and Implant-Based Breast Reconstruction with or without Nipple Preservation
Abstract
:Simple Summary
Abstract
1. Introduction
- Objectively compare nipple symmetry and projection following NSM with SSM and nipple reconstruction in women undergoing bilateral RRM with implant-based reconstruction;
- 2.
- Compare participant satisfaction with the decision to preserve or sacrifice the nipples;
- 3.
- Compare participant satisfaction with the appearance of the nipples;
- 4.
- Compare oncological outcomes between NSM and SSM.
2. Materials and Methods
2.1. Power Calculation
2.2. Recruitment to Study
2.3. Operative Technique
2.4. Participant Demographics and Surgical Outcomes
2.5. Objective Outcome of Nipple Symmetry
- Good symmetry: When all three parameters are <1 cm each;
- Moderate symmetry: If any one parameter is between 1 and 2 cm difference;
- Poor symmetry: If any one parameter is >2 cm different.
2.6. Participant Satisfaction Questionnaire
2.7. Oncological Outcomes
3. Results
3.1. Participant Demographics and Surgical Outcomes
3.2. Objective Outcome of Nipple Symmetry
3.3. Participant Satisfaction Questionnaire
3.4. Oncological Outcome
4. Discussion
4.1. Objective Symmetry
4.2. Participant Satisfaction
4.3. Nipple-Related Complications
4.4. Oncological Outcome
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liede, A.; Cai, M.; Crouter, T.F.; Niepel, D.; Callaghan, F.; Evans, D.G. Risk-reducing mastectomy rates in the US: A closer examination of the Angelina Jolie effect. Breast Cancer Res. Treat. 2018, 171, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.G.; Wisely, J.; Clancy, T.; Lalloo, F.; Wilson, M.; Johnson, R.; Duncan, J.; Barr, L.; Gandhi, A.; Howell, A. Longer term effects of the Angelina Jolie effect: Increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res. 2015, 17, 143. [Google Scholar] [CrossRef] [Green Version]
- Neuburger, J.; Macneill, F.; Jeevan, R.; van der Meulen, J.H.; Cromwell, D.A. Trends in the use of bilateral mastectomy in England from 2002 to 2011: Retrospective analysis of hospital episode statistics. BMJ Open 2013, 3, e003179. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.M.; Ryu, J.M.; Park, H.S.; Park, J.S.; Kang, E.; Lee, S.; Lee, H.B.; Youn, H.J.; Yoo, T.K.; Kim, J.; et al. Trends in Risk-Reducing Mastectomy and Risk-Reducing Salpingo-Oophorectomy in Korean Carriers of the BRCA1/2 Mutation. J. Breast Cancer 2020, 23, 647–655. [Google Scholar] [CrossRef]
- Al-Ghazal, S.K.; Fallowfield, L.; Blamey, R.W. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef]
- Toth, B.A.; Lappert, P. Modified skin incisions for mastectomy: The need for plastic surgical input in preoperative planning. Plast. Reconstr. Surg. 1991, 87, 1048–1053. [Google Scholar] [CrossRef]
- Carlson, G.W.; Styblo, T.M.; Lyles, R.H.; Jones, G.; Murray, D.R.; Staley, C.A.; Wood, W.C. The use of skin sparing mastectomy in the treatment of breast cancer: The Emory experience. Surg. Oncol. 2003, 12, 265–269. [Google Scholar] [CrossRef]
- Greenway, R.M.; Schlossberg, L.; Dooley, W.C. Fifteen-year series of skin-sparing mastectomy for stage 0 to 2 breast cancer. Am. J. Surg. 2005, 190, 918–922. [Google Scholar] [CrossRef]
- Spiegel, A.J.; Butler, C.E. Recurrence following treatment of ductal carcinoma in situ with skin-sparing mastectomy and immediate breast reconstruction. Plast. Reconstr. Surg. 2003, 111, 706–711. [Google Scholar] [CrossRef] [Green Version]
- Medina-Franco, H.; Vasconez, L.O.; Fix, R.J.; Heslin, M.J.; Beenken, S.W.; Bland, K.I.; Urist, M.M. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann. Surg. 2002, 235, 814–819. [Google Scholar] [CrossRef]
- Meretoja, T.J.; Rasia, S.; von Smitten, K.A.; Asko-Seljavaara, S.L.; Kuokkanen, H.O.; Jahkola, T.A. Late results of skin-sparing mastectomy followed by immediate breast reconstruction. Br. J. Surg. 2007, 94, 1220–1225. [Google Scholar] [CrossRef]
- Vaughan, A.; Dietz, J.R.; Aft, R.; Gillanders, W.E.; Eberlein, T.J.; Freer, P.; Margenthaler, J.A. Scientific Presentation Award. Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am. J. Surg. 2007, 194, 438–443. [Google Scholar] [CrossRef]
- Lanitis, S.; Tekkis, P.P.; Sgourakis, G.; Dimopoulos, N.; Al Mufti, R.; Hadjiminas, D.J. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: A meta-analysis of observational studies. Ann. Surg. 2010, 251, 632–639. [Google Scholar] [CrossRef]
- Kissin, M.W.; Kark, A.E. Nipple preservation during mastectomy. Br. J. Surg. 1987, 74, 58–61. [Google Scholar] [CrossRef]
- Petit, J.Y.; Veronesi, U.; Orecchia, R.; Rey, P.; Didier, F.; Giraldo, A.; Luini, A.; De Lorenzi, F.; Rietjens, M.; Garusi, C.; et al. The nipple-sparing mastectomy: Early results of a feasibility study of a new application of perioperative radiotherapy (ELIOT) in the treatment of breast cancer when mastectomy is indicated. Tumori 2003, 89, 288–291. [Google Scholar] [CrossRef] [Green Version]
- Blanckaert, M.; Vranckx, J. Oncological safety of therapeutic ‘nipple-sparing mastectomy’ followed by reconstruction: A systematic review. Acta Chir. Belg. 2021, 121, 155–163. [Google Scholar] [CrossRef]
- Galimberti, V.; Morigi, C.; Bagnardi, V.; Corso, G.; Vicini, E.; Fontana, S.K.R.; Naninato, P.; Ratini, S.; Magnoni, F.; Toesca, A.; et al. Oncological Outcomes of Nipple-Sparing Mastectomy: A Single-Center Experience of 1989 Patients. Ann. Surg. Oncol. 2018, 25, 3849–3857. [Google Scholar] [CrossRef]
- Muller, T.; Baratte, A.; Bruant-Rodier, C.; Bodin, F.; Mathelin, C. Oncological safety of nipple-sparing prophylactic mastectomy: A review of the literature on 3716 cases. Ann. De Chir. Plast. Esthétique 2018, 63, e6–e13. [Google Scholar] [CrossRef] [Green Version]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef]
- Wellisch, D.K.; Schain, W.S.; Noone, R.B.; Little, J.W., 3rd. The psychological contribution of nipple addition in breast reconstruction. Plast. Reconstr. Surg. 1987, 80, 699–704. [Google Scholar] [CrossRef]
- Jabor, M.A.; Shayani, P.; Collins, D.R., Jr.; Karas, T.; Cohen, B.E. Nipple-areola reconstruction: Satisfaction and clinical determinants. Plast. Reconstr. Surg. 2002, 110, 457–463, 457–463; discussion 464–455. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Didier, F.; Radice, D.; Gandini, S.; Bedolis, R.; Rotmensz, N.; Maldifassi, A.; Santillo, B.; Luini, A.; Galimberti, V.; Scaffidi, E.; et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res. Treat. 2009, 118, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Sacchini, V.; Pinotti, J.A.; Barros, A.C.; Luini, A.; Pluchinotta, A.; Pinotti, M.; Boratto, M.G.; Ricci, M.D.; Ruiz, C.A.; Nisida, A.C.; et al. Nipple-sparing mastectomy for breast cancer and risk reduction: Oncologic or technical problem? J. Am. Coll. Surg. 2006, 203, 704–714. [Google Scholar] [CrossRef]
- Manning, A.T.; Sacchini, V.S. Conservative mastectomies for breast cancer and risk-reducing surgery: The Memorial Sloan Kettering Cancer Center experience. Gland Surg. 2016, 5, 55–62. [Google Scholar] [CrossRef]
- Mitchell, S.B.P. Chapter 23 The American Society of Breast Surgeons Nipple-Sparing Mastectomy Registry; Operative Approcahes to Nipple-sparing mastectomy; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hopwood, P.; Lee, A.; Shenton, A.; Baildam, A.; Brain, A.; Lalloo, F.; Evans, G.; Howell, A. Clinical follow-up after bilateral risk reducing (‘prophylactic’) mastectomy: Mental health and body image outcomes. Psychooncology 2000, 9, 462–472. [Google Scholar] [CrossRef]
- Frost, M.H.; Schaid, D.J.; Sellers, T.A.; Slezak, J.M.; Arnold, P.G.; Woods, J.E.; Petty, P.M.; Johnson, J.L.; Sitta, D.L.; McDonnell, S.K.; et al. Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA 2000, 284, 319–324. [Google Scholar] [CrossRef]
- Payne, D.K.; Biggs, C.; Tran, K.N.; Borgen, P.I.; Massie, M.J. Women’s regrets after bilateral prophylactic mastectomy. Ann. Surg. Oncol. 2000, 7, 150–154. [Google Scholar] [CrossRef]
- Braude, L.; Kirsten, L.; Gilchrist, J.; Juraskova, I. A systematic review of women’s satisfaction and regret following risk-reducing mastectomy. Patient Educ. Couns. 2017, 100, 2182–2189. [Google Scholar] [CrossRef]
- Choi, M.; Frey, J.D.; Salibian, A.A.; Karp, N.S. Nipple-Areola Complex Malposition in Nipple-Sparing Mastectomy: A Review of Risk Factors and Corrective Techniques from Greater than 1000 Reconstructions. Plast. Reconstr. Surg. 2017, 140, 247e–257e. [Google Scholar] [CrossRef]
- Gahm, J.; Jurell, G.; Edsander-Nord, A.; Wickman, M. Patient satisfaction with aesthetic outcome after bilateral prophylactic mastectomy and immediate reconstruction with implants. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Razdan, S.N.; Patel, V.; Jewell, S.; McCarthy, C.M. Quality of life among patients after bilateral prophylactic mastectomy: A systematic review of patient-reported outcomes. Qual. Life Res. 2016, 25, 1409–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Verschuer, V.M.; Mureau, M.A.; Gopie, J.P.; Vos, E.L.; Verhoef, C.; Menke-Pluijmers, M.B.; Koppert, L.B. Patient Satisfaction and Nipple-Areola Sensitivity After Bilateral Prophylactic Mastectomy and Immediate Implant Breast Reconstruction in a High Breast Cancer Risk Population: Nipple-Sparing Mastectomy Versus Skin-Sparing Mastectomy. Ann. Plast. Surg. 2016, 77, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.W.; Amara, D.; Piper, M.L.; Klassen, A.F.; Tsangaris, E.; Pusic, A.L. Development and Validation of a Nipple-Specific Scale for the BREAST-Q to Assess Patient-Reported Outcomes following Nipple-Sparing Mastectomy. Plast. Reconstr. Surg. 2019, 143, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.N.; Faulkner, H.R.; Smith, B.L.; Korotkin, J.E.; Lanahan, C.R.; Brown, C.; Gadd, M.A.; Specht, M.C.; Hughes, K.S.; Oseni, T.S.; et al. Nipple-Sparing Mastectomy versus Skin-Sparing Mastectomy: Does Saving the Nipple Impact Short- and Long-Term Patient Satisfaction? Ann. Surg. Oncol. 2022, 29, 1033–1040. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, J.; Sollazzo, V.; Mohammed, K.; Gui, G. Sensory change of the reconstructed breast envelope after skin-sparing mastectomy. Eur. J. Surg. Oncol. 2016, 42, 973–979. [Google Scholar] [CrossRef]
- Benediktsson, K.P.; Perbeck, L.; Geigant, E.; Solders, G. Touch sensibility in the breast after subcutaneous mastectomy and immediate reconstruction with a prosthesis. Br. J. Plast. Surg. 1997, 50, 443–449. [Google Scholar] [CrossRef]
- Petit, J.Y.; Veronesi, U.; Orecchia, R.; Rey, P.; Martella, S.; Didier, F.; Viale, G.; Veronesi, P.; Luini, A.; Galimberti, V.; et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: One thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res. Treat. 2009, 117, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Tsangaris, E.; Klassen, A.F.; Kaur, M.N.; Voineskos, S.; Bordeleau, L.; Zhong, T.; Broyles, J.; Pusic, A.L. Development and Psychometric Validation of the BREAST-Q Sensation Module for Women Undergoing Post-Mastectomy Breast Reconstruction. Ann. Surg. Oncol. 2021, 28, 7842–7853. [Google Scholar] [CrossRef]
- Wagner, J.L.; Fearmonti, R.; Hunt, K.K.; Hwang, R.F.; Meric-Bernstam, F.; Kuerer, H.M.; Bedrosian, I.; Crosby, M.A.; Baumann, D.P.; Ross, M.I.; et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann. Surg. Oncol. 2012, 19, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Rawlani, V.; Fiuk, J.; Johnson, S.A.; Buck, D.W., 2nd; Hirsch, E.; Hansen, N.; Khan, S.; Fine, N.A.; Kim, J.Y. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. Can. J. Plast. Surg. 2011, 19, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbine, N.E.; Lostumbo, L.; Wallace, J.; Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 2018, 4, Cd002748. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Sellers, T.A.; Schaid, D.J.; Frank, T.S.; Soderberg, C.L.; Sitta, D.L.; Frost, M.H.; Grant, C.S.; Donohue, J.H.; Woods, J.E.; et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J. Natl. Cancer Inst. 2001, 93, 1633–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijers-Heijboer, H.; van Geel, B.; van Putten, W.L.; Henzen-Logmans, S.C.; Seynaeve, C.; Menke-Pluymers, M.B.; Bartels, C.C.; Verhoog, L.C.; van den Ouweland, A.M.; Niermeijer, M.F.; et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2001, 345, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Arver, B.; Isaksson, K.; Atterhem, H.; Baan, A.; Bergkvist, L.; Brandberg, Y.; Ehrencrona, H.; Emanuelsson, M.; Hellborg, H.; Henriksson, K.; et al. Bilateral prophylactic mastectomy in Swedish women at high risk of breast cancer: A national survey. Ann. Surg. 2011, 253, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Klijn, J.G.M.; Geel, B.; Meijers-Heijboer, H.; Tilanus-Linthorst, M.; Bartels, K.; Crepin, E.; Seynaeve, C.; Menke-Pluymers, M.; Putten, W.; Brekelmans, C. Results of the Extended Series on Prophylactic Mastectomy versus Surveillance in BRCA1/2 Mutation Carriers in Rotterdam; Springer: New York, NY, USA, 2004; p. S10. [Google Scholar]
- Skytte, A.B.; Crüger, D.; Gerster, M.; Laenkholm, A.V.; Lang, C.; Brøndum-Nielsen, K.; Andersen, M.K.; Sunde, L.; Kølvraa, S.; Gerdes, A.M. Breast cancer after bilateral risk-reducing mastectomy. Clin. Genet. 2011, 79, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Frasson, A.L.; Lichtenfels, M.; de Souza, A.A.B.; Vollbrecht, B.; Falcone, A.B.; Frasson, M.; Barbosa, F. Risk-reducing mastectomy: A case series of 124 procedures in Brazilian patients. Breast Cancer Res. Treat. 2020, 181, 69–75. [Google Scholar] [CrossRef]
- Cohen, W.A.; Mundy, L.R.; Ballard, T.N.; Klassen, A.; Cano, S.J.; Browne, J.; Pusic, A.L. The BREAST-Q in surgical research: A review of the literature 2009-2015. J. Plast. Reconstr. Aesthetic Surg. 2015, 69, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Spindler, N.; Ebel, F.; Briest, S.; Wallochny, S.; Langer, S. Quality of Life After Bilateral Risk-Reducing Mastectomy and Simultaneous Reconstruction Using Pre-Pectoral Silicone Implants. Patient Prefer. Adherence 2021, 15, 741–750. [Google Scholar] [CrossRef]
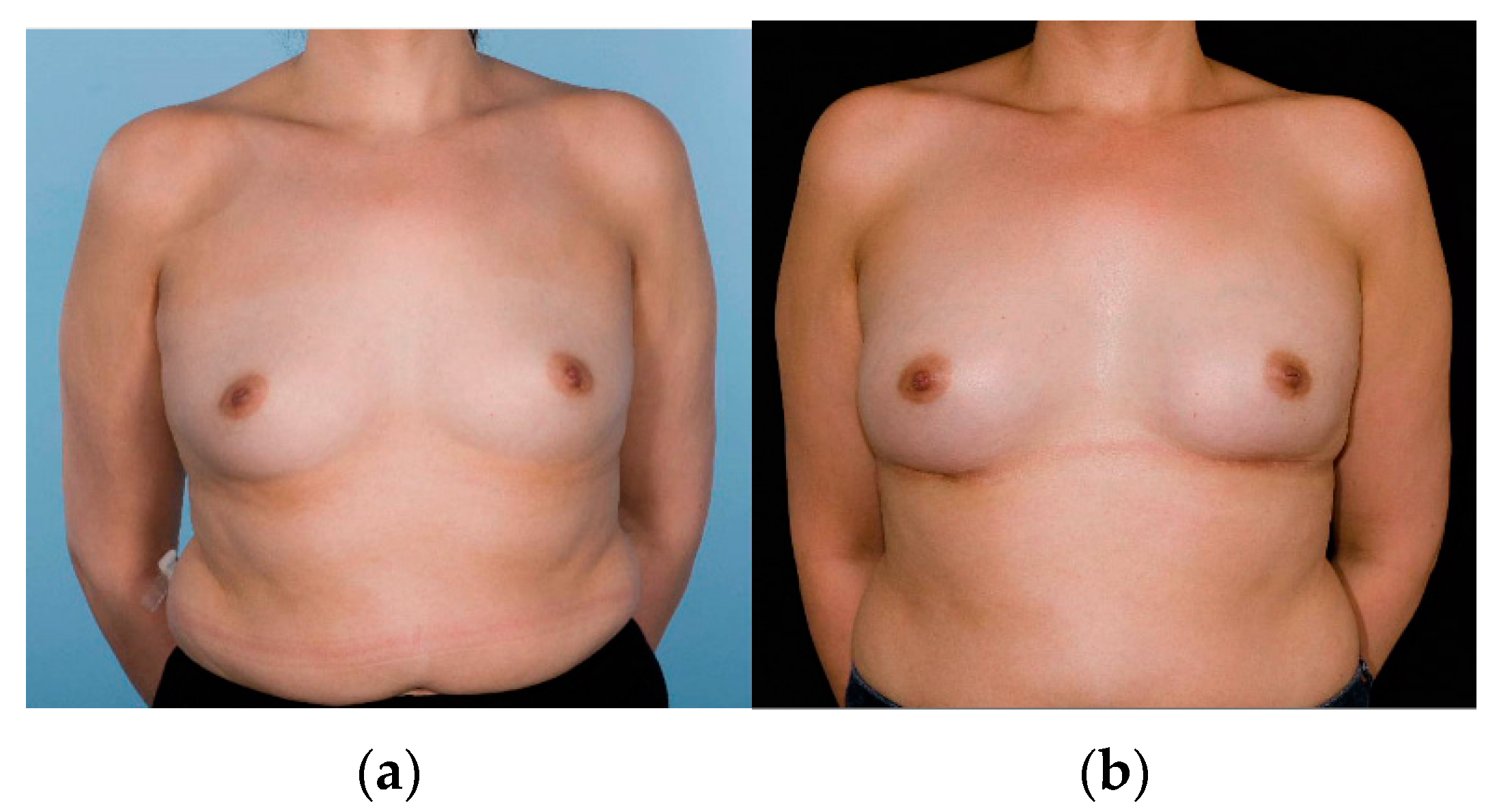
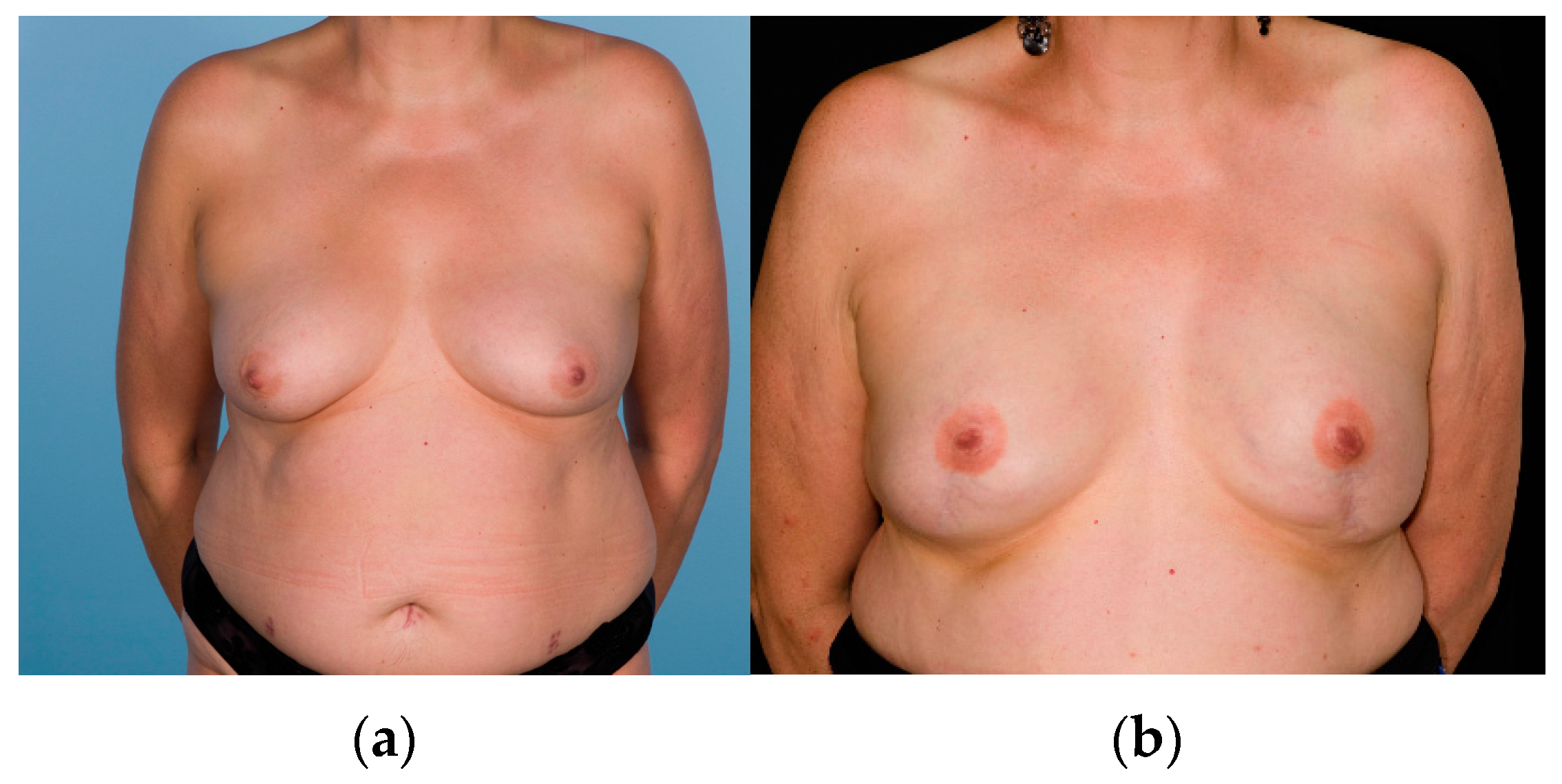
| Items | Nipple-Sparing Mastectomy (NSM) (n = 60 Participants) | Nipple-Sacrificing Skin-Sparing Mastectomy (SSM) (n = 33 Participants) | p-Value |
|---|---|---|---|
| Participants demographics | Median, (IQR) | Median, (IQR) | |
| Time from mastectomy and reconstruction to participation in study (months) | 85.5 (50.4–138.2) | 116.2 (79.0–148.3) | 0.136 |
| Age at time of surgery (years) | 37 (33–41) | 41 (34–44) | 0.068 |
| BMI at the time of surgery (kg/m2) | 23.0 (21.2–24.9) | 24.4 (22.3–26.4) | 0.033 |
| Participantsgenetic status | n = 60, (%) participants | n = 33, (%) participants | |
| BRCA1 | 23 (38.3) | 11 (33.3) | 0.240 |
| BRCA 2 | 14 (23.3) | 11 (33.3) | |
| TP53 | 1 (1.7) | 0 | |
| Negative test results | 16 (26.7) | 4 (12.1) | |
| Unknown (not tested) | 6 (10) | 7 (21.1) | |
| Participantssurgery type | n = 60, (%) participants | n = 33, (%) participants | |
| Reconstruction type | <0.001 | ||
| LD + Implant | 0 | 11 (33) | |
| Sub-muscular implant | 60 (100) | 22 (67) | |
| Implant used: | 0.258 | ||
| Tissue expander | 6 (10) | 4 (12) | |
| Permanent expander implant | 44 (73) | 19 (58) | |
| Direct to permanent fixed volume implant | 10 (17) | 10 (30) | |
| Per breast complications | n = 120, (%) breasts | n = 66, (%) breasts | |
| Haematoma | 3 (2.5) | 0 | 0.553 |
| Wound infection | 12(10) | 4 (6.1) | 0.424 |
| Nipple necrosis partial | 11 (9.1) | - | - |
| Nipple necrosis full thickness | 5 (4.2) | - | - |
| Delayed nipple reconstruction | |||
| No | - | 10 (30) | - |
| Yes | - | 23 (70) |
| Distance Measurements for Left and Right Breasts (cm) | Nipple-Sparing Mastectomy (NSM) n = 114 | Nipple-Sacrificing Skin-Sparing Mastectomy (SSM) with Nipple Reconstruction n = 46 | p-Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Sternal notch to nipple | 20.73 (2.03) | 22.5 (3.10) | <0.001 |
| Nipple to infra-mammary fold | 7.93 (1.62) | 8.40 (1.81) | 0.107 |
| Transverse base width | 13.68 (1.69) | 13.95 (1.51) | 0.354 |
| Midline to nipple | 10.82 (1.54) | 10.97 (1.95) | 0.614 |
| Nipple diameter | 1.08 (0.26) | 1.26 (0.5) | 0.003 |
| Nipple projection | 0.57 (0.26) | 0.38 (0.23) | <0.001 |
| Absolute distance difference between left and right side per participant (cm) | Median (IQR) | Median (IQR) | |
| Sternal notch to nipple | 0.5 (0–1.0) | 0.5 (0–1.0) | 0.705 |
| Nipple to infra-mammary fold | 0.5 (0–1.0) | 0.5 (0–1.0) | 0.367 |
| Transverse base width | 0 (0–0.5) | 0 (0–0.5) | 0.629 |
| Midline to nipple | 0.5 (0–1.0) | 0.5 (0–1.0) | 0.827 |
| Nipple diameter | 0 (0–0.1) | 0 (0–0.1) | 0.802 |
| Nipple projection | 0 (0–0.1) | 0 (0–0.1) | 0.799 |
| Symmetry | Nipple-Sparing Mastectomy (NSM) n = 57 | Nipple-Sacrificing Skin-Sparing Mastectomy (SSM) with Nipple Reconstruction n = 23 | Total n = 80 | p-Value |
|---|---|---|---|---|
| Good symmetry | 20 (35) | 6 (26) | 26 (33) | 0.670 |
| Moderate symmetry | 34 (60) | 15 (65) | 49 (61) | |
| Poor symmetry | 3 (5) | 2 (9) | 5 (6) |
| Items | Nipple-Sparing (n = 58) | Nipple-Sacrificing Skin-Sparing Mastectomy (SSM) (n = 33) | p-Value |
|---|---|---|---|
| n (%) | n (%) | ||
| Did you participate in the decision about nipple preservation or removal? | |||
| No | 0 | 2 (6) | |
| Yes | 58 (100) | 31 (94) | 0.129 |
| If yes, are you satisfied with the decision you made? | 0.257 | ||
| Very Much | 50 (86) | 24 (77) | |
| Quite a bit | 6 (10) | 4 (13) | |
| A little | 0 | 2 (6) | |
| Not at all | 2 (3) | 1 (3) | |
| Did you undergo a nipple reconstruction? | - | - | |
| No | 10 (30.3) | ||
| Yes | 23 (69.7) | ||
| n = 58 | n = 23 (Questions for participants who underwent nipple reconstruction) | ||
| Overall, how satisfied are you with the nipples? | 0.176 | ||
| Very Much | 41 (71) | 11 (48) | |
| Quite a bit | 14 (24) | 8 (35) | |
| A little | 2 (3) | 3 (13) | |
| Not at all | 1 (2) | 1 (4) | |
| Is the position of the nipples the same as before the operation? | 0.020 | ||
| Very Much | 24 (41) | 18 (78) | |
| Quite a bit | 21 (36) | 3 (13) | |
| A little | 7 (12) | 2 (9) | |
| Not at all | 6 (10) | 0 | |
| Is the projection of the nipples the same as before the operation? | 0.186 | ||
| Yes | 34 (59) | 14 (61) | |
| Too prominent | 11 (19) | 1 (4) | |
| Too flat | 13 (22) | 8 (3) | |
| How is the nipple sensation compared to before the operation? | <0.001 | ||
| Yes, same as before | 3 (5) | 0 | |
| Less than before | 26 (45) | 0 | |
| A little | 0 | 7 (30) | |
| None | 29 (50) | 16 (70) | |
| How would you describe the colour of the areola compared to before surgery (nipple preservation participants only)? | - | ||
| Same | 53 (89) | ||
| Darker | 0 | ||
| Lighter | 6 (10) | ||
| Does your nipple respond to cold or touch (nipple preservation participants only)? | |||
| No | 14 (24) | ||
| Yes | 45 (76) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, R.L.; Tasoulis, M.K.; Hristova, E.; Teoh, V.; Agusti, A.; Ward, A.; Montgomery, C.; Mohammed, K.; Self, J.; Rusby, J.E.; et al. Satisfaction with Long-Term Aesthetic and 10 Years Oncologic Outcome following Risk-Reducing Mastectomy and Implant-Based Breast Reconstruction with or without Nipple Preservation. Cancers 2022, 14, 3607. https://doi.org/10.3390/cancers14153607
O’Connell RL, Tasoulis MK, Hristova E, Teoh V, Agusti A, Ward A, Montgomery C, Mohammed K, Self J, Rusby JE, et al. Satisfaction with Long-Term Aesthetic and 10 Years Oncologic Outcome following Risk-Reducing Mastectomy and Implant-Based Breast Reconstruction with or without Nipple Preservation. Cancers. 2022; 14(15):3607. https://doi.org/10.3390/cancers14153607
Chicago/Turabian StyleO’Connell, Rachel Louise, Marios Konstantinos Tasoulis, Evguenia Hristova, Victoria Teoh, Ana Agusti, Ann Ward, Catherine Montgomery, Kabir Mohammed, Janet Self, Jennifer E. Rusby, and et al. 2022. "Satisfaction with Long-Term Aesthetic and 10 Years Oncologic Outcome following Risk-Reducing Mastectomy and Implant-Based Breast Reconstruction with or without Nipple Preservation" Cancers 14, no. 15: 3607. https://doi.org/10.3390/cancers14153607
APA StyleO’Connell, R. L., Tasoulis, M. K., Hristova, E., Teoh, V., Agusti, A., Ward, A., Montgomery, C., Mohammed, K., Self, J., Rusby, J. E., & Gui, G. (2022). Satisfaction with Long-Term Aesthetic and 10 Years Oncologic Outcome following Risk-Reducing Mastectomy and Implant-Based Breast Reconstruction with or without Nipple Preservation. Cancers, 14(15), 3607. https://doi.org/10.3390/cancers14153607






