Photochemical Internalization of siRNA for Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction on Cancer and Treatments
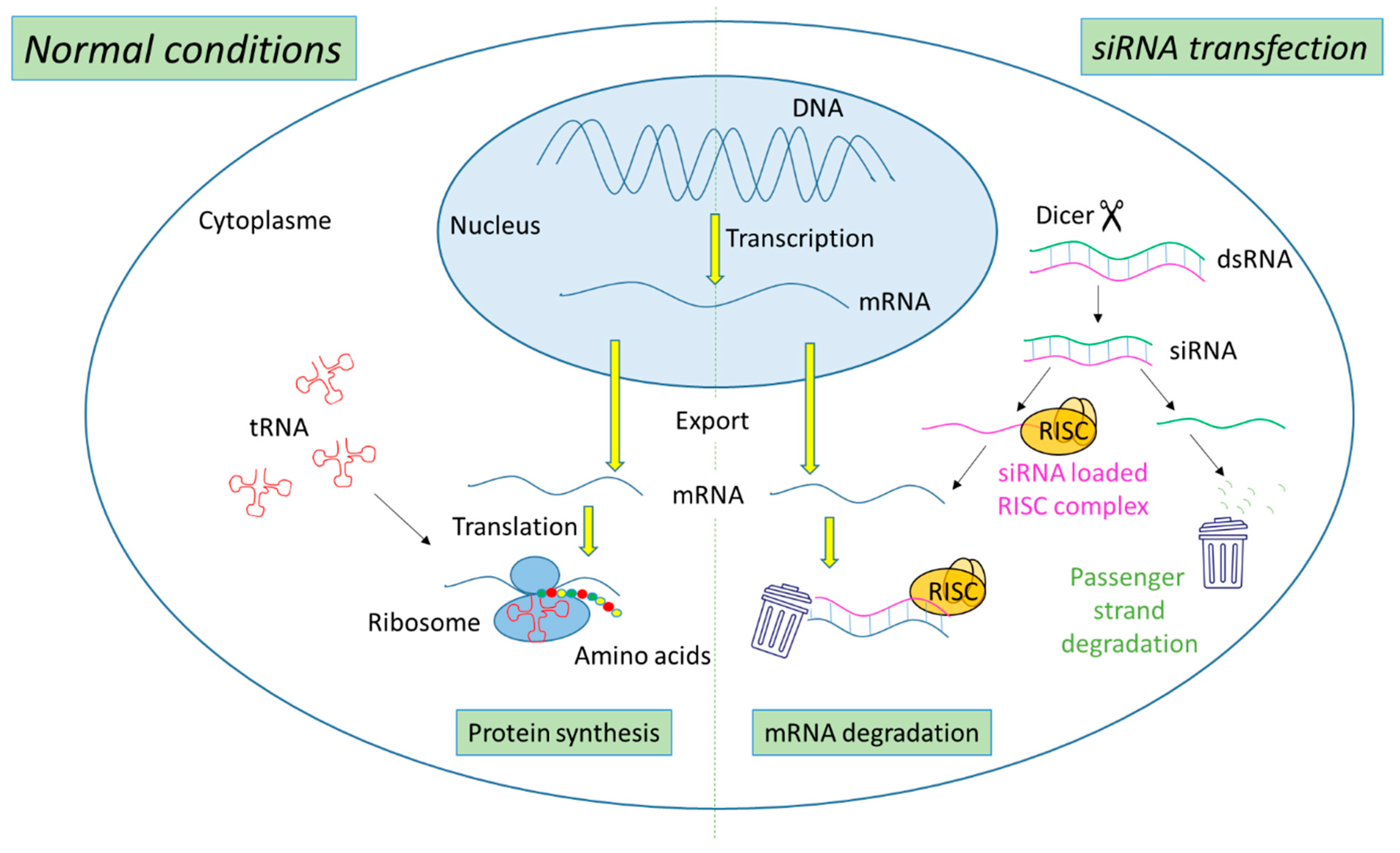
2. Ribonucleic Acid Interference (RNAi) Technology
2.1. Mechanism of Action of siRNA
2.2. siRNA-Based Cancer Therapies
2.3. Hurdles to siRNA Delivery
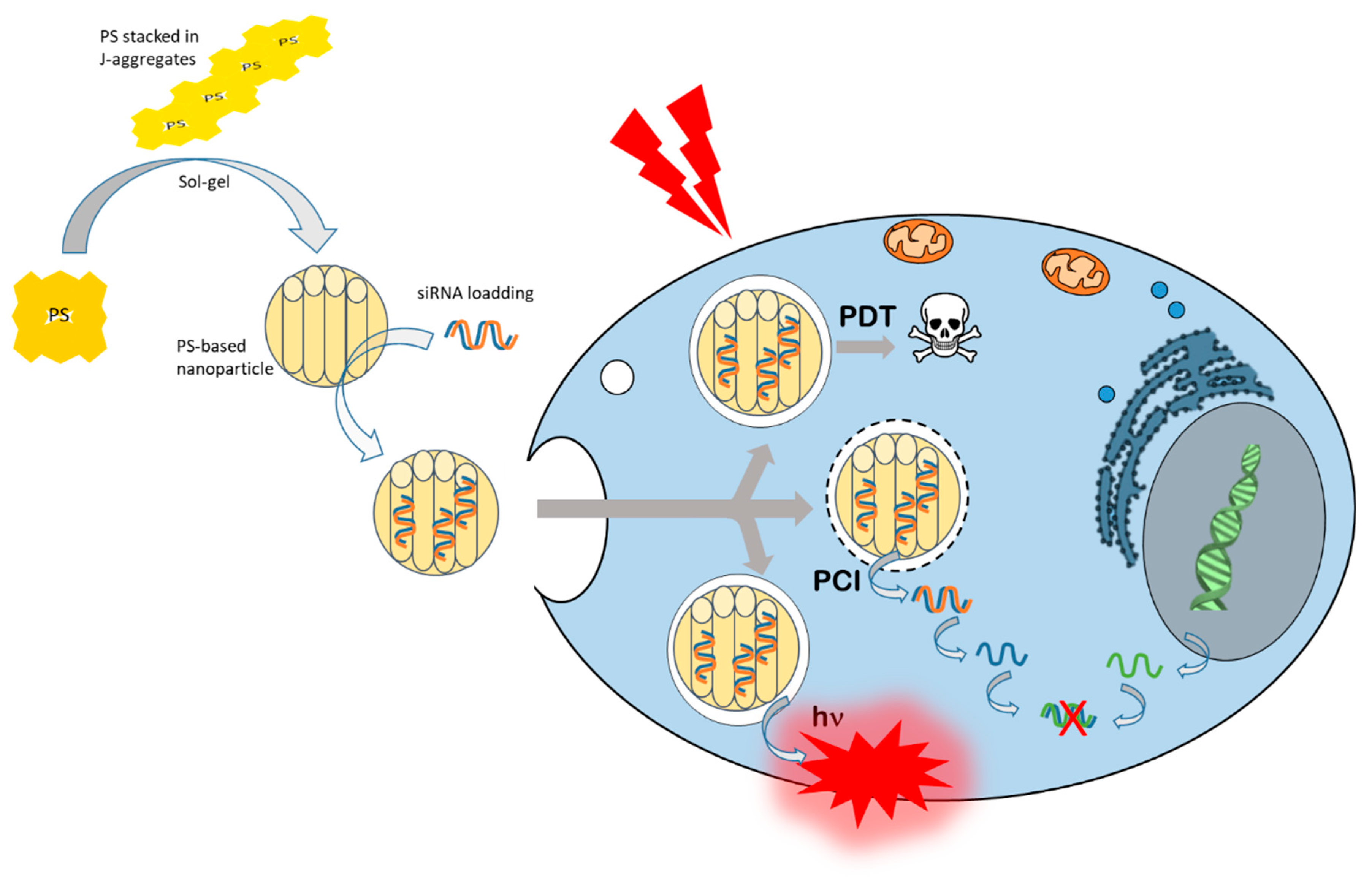
3. Photochemical Internalization (PCI) Mechanism
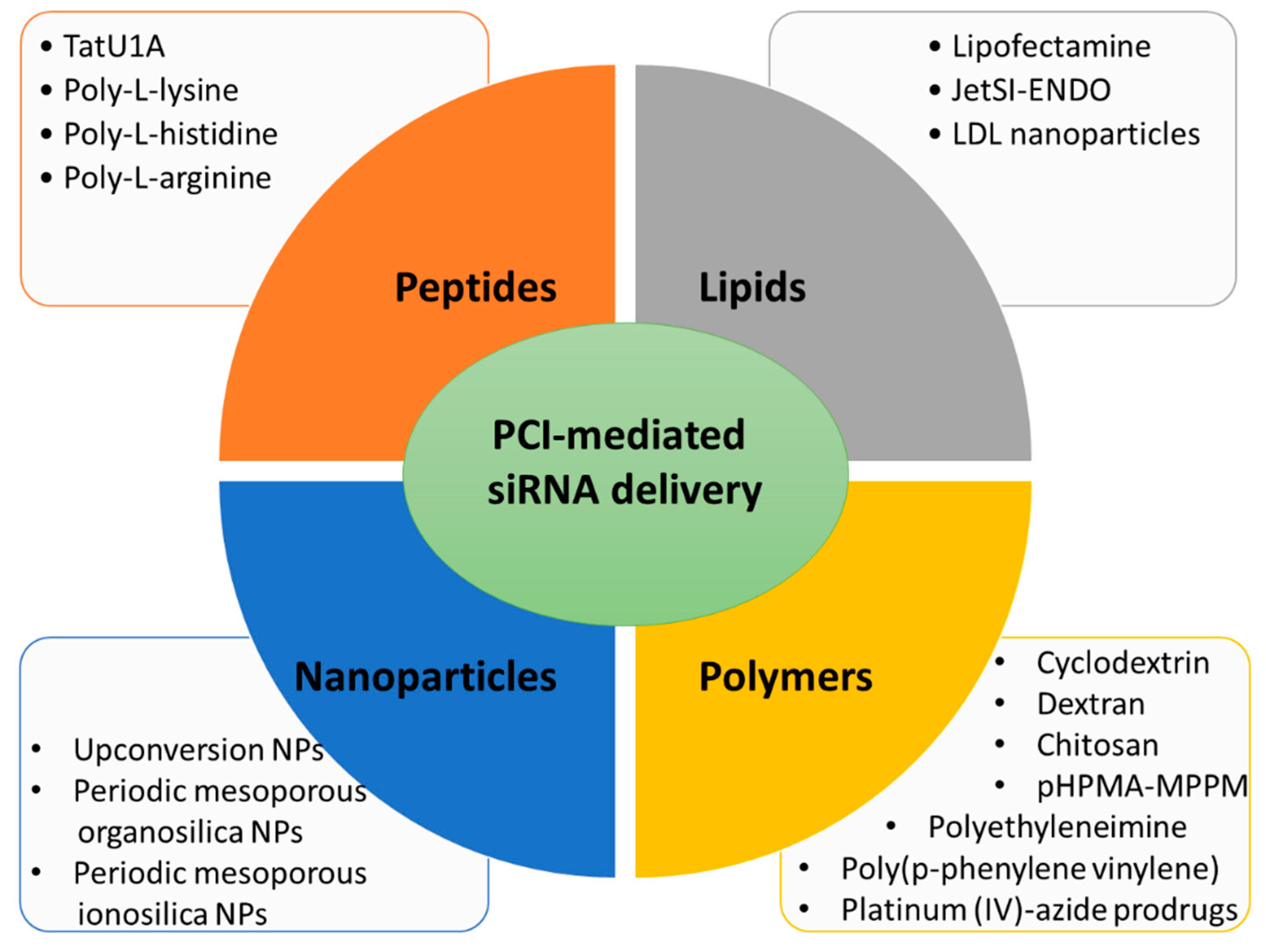
3.1. Lipid Carriers for PCI-Mediated siRNA Delivery
3.2. Peptide Carriers for PCI-Mediated siRNA Delivery
3.3. Polymer Carriers for PCI-Mediated siRNA Delivery
3.4. Nanoparticles for PCI-Mediated siRNA Delivery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yahya, E.B.; Alqadhi, A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Farooq, M.A.; Aquib, M.; Mily, B.J.M.; Murtaza, G. Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.M.A. Toxicity Studies of Polymer Based Supermagnetic Iron Oxide Nanoparticles. Ph.D. Thesis, Universidad Zaragoza, Zaragoza, Spain, 2014. [Google Scholar]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Shotorbani, S.S.; Baradaran, B. RNA Interference and its Role in Cancer Therapy. Adv. Pharm. Bull. 2014, 4, 313–321. [Google Scholar]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Tan, F.L.; Yin, J.Q. RNAi, a new therapeutic strategy against viral infection. Cell Res. 2004, 14, 460–466. [Google Scholar] [CrossRef]
- Holoch, D.; Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015, 16, 71–84. [Google Scholar] [CrossRef]
- Hammond, S.M.; Caudy, A.A.; Hannon, G.J. Post-transcriptional gene silencing by double-stranded RNA. Nat. Rev. Genet. 2001, 2, 110–119. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, J.Y.; Lee, S.N.; Choi, J.H.; Choi, J.W. RNA interference (RNAi)-based plasmonic nanomaterials for cancer diagnosis and therapy. J. Control. Release 2022, 342, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Lin, Y.Y.; Macaes, B.; Meneshian, A.; Hung, C.F.; Wu, T.C. Prospects of RNA interference therapy for cancer. Gene Ther. 2006, 13, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Pengnam, S.; Plianwong, S.; Yingyongnarongkul, B.-k.; Patrojanasophon, P.; Opanasopit, P. Delivery of small interfering RNAs by nanovesicles for cancer therapy. Drug Metab. Pharmacokinet. 2022, 42, 100425. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Di Fabrizio, E.; Ferretti, E.; Tomao, S.; Gulino, A. Nanoparticle-based delivery of small interfering RNA: Challenges for cancer therapy. Int. J. Nanomed. 2012, 7, 3637–3657. [Google Scholar]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef]
- Lares, M.R.; Rossi, J.J.; Ouellet, D.L. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010, 28, 570–579. [Google Scholar] [CrossRef]
- de Fougerolles, A.; Vornlocher, H.P.; Maraganore, J.; Lieberman, J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007, 6, 443–453. [Google Scholar] [CrossRef]
- Dönmez, Y.; Gündüz, U. Reversal of multidrug resistance by small interfering RNA (siRNA) in doxorubicin-resistant MCF-7 breast cancer cells. Biomed. Pharmacother. 2011, 65, 85–89. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 46, 274–283. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef]
- Golan, T.; Khvalevsky, E.Z.; Hubert, A.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; Ben David, E.; Raskin, S.; et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015, 6, 24560–24570. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.M.; Ang, C.; DiMaio, C.J.; Javle, M.M.; Gutierrez, M.; Yarom, N.; Stemmer, S.M.; Golan, T.; Geva, R.; Semenisty, V.; et al. A phase II study of siG12D-LODER in combination with chemotherapy in patients with locally advanced pancreatic cancer (PROTACT). J. Clin. Oncol. 2020, 38, TPS4672. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D.; et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 2015, 33, 11006. [Google Scholar] [CrossRef]
- A Study of NBF-006 in Non-Small Cell Lung, Pancreatic, or Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03819387 (accessed on 3 May 2022).
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-Human Phase I Study of the Liposomal RNA Interference Therapeutic Atu027 in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef]
- Schultheis, B.; Strumberg, D.; Kuhlmann, J.; Wolf, M.; Link, K.; Seufferlein, T.; Kaufmann, J.; Feist, M.; Gebhardt, F.; Khan, M.; et al. Safety, Efficacy and Pharcacokinetics of Targeted Therapy with The Liposomal RNA Interference Therapeutic Atu027 Combined with Gemcitabine in Patients with Pancreatic Adenocarcinoma. A Randomized Phase Ib/IIa Study. Cancers 2020, 12, 3130. [Google Scholar] [CrossRef]
- Oner, E.; Kotmakci, M.; Baird, A.M.; Gray, S.G.; Butuner, B.D.; Bozkurt, E.; Kantarci, A.G.; Finn, S.P. Development of EphA2 siRNA-loaded lipid nanoparticles and combination with a small-molecule histone demethylase inhibitor in prostate cancer cells and tumor spheroids. J. Nanobiotechnol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kang, I.; Yu, K.-R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.-B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef]
- Liu, F.; Shollenberger, L.; Conwell, C.C.; Yuan, X.; Huang, L. Mechanism of naked DNA clearance after intravenous injection. J. Gene Med. 2007, 9, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.L.; A Whitehead, K.; Vegas, A.J.; Anderson, D.G. Action and Reaction: The Biological Response to siRNA and Its Delivery Vehicles. Mol. Ther. 2012, 20, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sharma, R.K.; Singh, S.K. Antisense oligonucleotides: Modifications and clinical trials. MedChemComm 2014, 5, 1454–1471. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’In, A.V.; Milstein, S.; et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Gore, K.R. Advances in siRNA therapeutics and synergistic effect on siRNA activity using emerging dual ribose modifications. RNA Biol. 2022, 19, 452–467. [Google Scholar] [CrossRef]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.; Erande, N.; Matsuda, S.; Kel’in, A.; Woods, L.B.; Chickering, T.; Pallan, P.S.; Schlegel, M.K.; Zlatev, I.; Egli, M.; et al. Synthesis, chirality-dependent conformational and biological properties of siRNAs containing 5′-(R)- and 5′-(S)-C-methyl-guanosine. Nucleic Acids Res. 2020, 48, 10101–10124. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Guo, S.; Huang, L. Nanoparticles Escaping RES and Endosome: Challenges for siRNA Delivery for Cancer Therapy. J. Nanomater. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Qi, L.; O’Regan, R.M.; Nie, S.; Gao, X. Proton-Sponge Coated Quantum Dots for siRNA Delivery and Intracellular Imaging. J. Am. Chem. Soc. 2008, 130, 9006–9012. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hersch, N.; Merkel, R.; Csiszar, A.; Hoffmann, B. Changing the Way of Entrance: Highly Efficient Transfer of mRNA and siRNA via Fusogenic Nano-Carriers. J. Biomed. Nanotechnol. 2019, 15, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Otterhaug, T.; Janetzki, S.; Welters, M.J.P.; Håkerud, M.; Nedberg, A.G.; Edwards, V.T.; Boekestijn, S.; Loof, N.M.; Selbo, P.K.; Olivecrona, H.; et al. Photochemical Internalization Enhanced Vaccination Is Safe, and Gives Promising Cellular Immune Responses to an HPV Peptide-Based Vaccine in a Phase I Clinical Study in Healthy Volunteers. Front. Immunol. 2021, 11, 576756. [Google Scholar] [CrossRef] [PubMed]
- Dechêne, A.; Kasper, S.; Olivecrona, H.; Schirra, J.; Trojan, J. Photochemical internalization and gemcitabine combined with first-line chemotherapy in perihilar cholangiocarcinoma: Observations in three patients. Endosc. Int. Open 2020, 8, E1878–E1883. [Google Scholar] [CrossRef] [PubMed]
- Ożog, L.; Aebisher, D. Singlet oxygen lifetime and diffusion measurements. Eur. J. Clin. Exp. Med. 2018, 16, 123–126. [Google Scholar] [CrossRef]
- Klaper, M.; Fudickar, W.; Linker, T. Role of Distance in Singlet Oxygen Applications: A Model System. J. Am. Chem. Soc. 2016, 138, 7024–7029. [Google Scholar] [CrossRef]
- Raemdonck, K.; Naeye, B.; Høgset, A.; Demeester, J.; De Smedt, S.C. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J. Control. Release 2010, 145, 281–288. [Google Scholar] [CrossRef]
- Berg, K.; Folini, M.; Prasmickaite, L.; Selbo, P.; Bonsted, A.; Engesaeter, B.; Zaffaroni, N.; Weyergang, A.; Dietzea, A.; Maelandsmo, G.; et al. Photochemical Internalization: A New Tool for Drug Delivery. Curr. Pharm. Biotechnol. 2007, 8, 362–372. [Google Scholar] [CrossRef]
- Berg, K.; Selbo, P.K.; Prasmickaite, L.; E Tjelle, T.; Sandvig, K.; Moan, J.; Gaudernack, G.; Fodstad, O.; Kjølsrud, S.; Anholt, H.; et al. Photochemical internalization: A novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999, 59, 1180–1183. [Google Scholar]
- Selbo, P.K.; Sivam, G.; Fodstad, O.; Sandvig, K.; Berg, K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int. J. Cancer 2001, 92, 761–766. [Google Scholar] [CrossRef]
- Berg, K.; Dietze, A.; Kaalhus, O.; Høgset, A. Site-Specific Drug Delivery by Photochemical Internalization Enhances the Antitumor Effect of Bleomycin. Clin. Cancer Res. 2005, 11, 8476–8485. [Google Scholar] [CrossRef]
- Sultan, A.; Jerjes, W.; Berg, K.; Høgset, A.; Mosse, C.A.; Hamoudi, R.; Hamdoon, Z.; Simeon, C.; Carnell, D.; Forster, M.; et al. Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: A phase 1, dose-escalation, first-in-man trial. Lancet Oncol. 2016, 17, 1217–1229. [Google Scholar] [CrossRef]
- Oliveira, S.; Fretz, M.M.; Høgset, A.; Storm, G.; Schiffelers, R.M. Photochemical internalization enhances silencing of epidermal growth factor receptor through improved endosomal escape of siRNA. Biochim. et Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kraja, I.; Bing, R.; Hiwatashi, N.; Rousseau, B.; Nalband, D.; Kirshenbaum, K.; Branski, R.C. Preliminary study of a novel transfection modality for in vivo siRNA delivery to vocal fold fibroblasts. Laryngoscope 2017, 127, E231–E237. [Google Scholar] [CrossRef] [PubMed]
- Bøe, S.; Longva, A.; Hovig, E. Photochemically Induced Gene Silencing Using Small Interfering RNA Molecules in Combination with Lipid Carriers. Oligonucleotides 2007, 17, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lovell, J.F.; Chen, J.; Lin, Q.; Ding, L.; Ng, K.K.; Pandey, R.K.; Manoharan, M.; Zhang, Z.; Zheng, G. Mechanistic Insights into LDL Nanoparticle-Mediated siRNA Delivery. Bioconjug. Chem. 2011, 23, 33–41. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In Vivo Protein Transduction: Delivery of a Biologically Active Protein into the Mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef]
- Del Gaizo, V.; Payne, R.M. A novel TAT-mitochondrial signal sequence fusion protein is processed, stays in mitochondria, and crosses the placenta. Mol. Ther. 2003, 7, 720–730. [Google Scholar] [CrossRef]
- Endoh, T.; Sisido, M.; Ohtsuki, T. Cellular siRNA Delivery Mediated by a Cell-Permeant RNA-Binding Protein and Photoinduced RNA Interference. Bioconjug. Chem. 2008, 19, 1017–1024. [Google Scholar] [CrossRef]
- Maiolo, J.R.; Ottinger, E.A.; Ferrer, M. Specific Redistribution of Cell-Penetrating Peptides from Endosomes to the Cytoplasm and Nucleus upon Laser Illumination. J. Am. Chem. Soc. 2004, 126, 15376–15377. [Google Scholar] [CrossRef]
- Matsushita, M.; Noguchi, H.; Lu, Y.-F.; Tomizawa, K.; Michiue, H.; Li, S.-T.; Hirose, K.; Bonner-Weir, S.; Matsui, H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett. 2004, 572, 221–226. [Google Scholar] [CrossRef]
- Jørgensen, J.A.L.; Longva, A.S.; Hovig, E.; Bøe, S.L. Evaluation of Biodegradable Peptide Carriers for Light-Directed Targeting. Nucleic Acid Ther. 2013, 23, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Soe, T.H.; Watanabe, K.; Ohtsuki, T. Photoinduced Endosomal Escape Mechanism: A View from Photochemical Internalization Mediated by CPP-Photosensitizer Conjugates. Molecules 2020, 26, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.-W.; Giuntini, F.; Eggleston, I.M.; Bown, S.G.; MacRobert, A.J. Photochemical internalisation of a macromolecular protein toxin using a cell penetrating peptide-photosensitiser conjugate. J. Control. Release 2012, 157, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Miki, S.; Kobayashi, S.; Haraguchi, T.; Nakata, E.; Hirakawa, K.; Sumita, K.; Watanabe, K.; Okazaki, S. The molecular mechanism of photochemical internalization of cell penetrating peptide-cargo-photosensitizer conjugates. Sci. Rep. 2015, 5, 18577. [Google Scholar] [CrossRef] [PubMed]
- Sarko, D.; Beijer, B.; Garcia Boy, R.; Nothelfer, E.-M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The Pharmacokinetics of Cell-Penetrating Peptides. Mol. Pharm. 2010, 7, 2224–2231. [Google Scholar] [CrossRef]
- Krämer, S.; Wunderli-Allenspach, H. No entry for TAT(44–57) into liposomes and intact MDCK cells: Novel approach to study membrane permeation of cell-penetrating peptides. Biochim. et Biophys. Acta (BBA)-Biomembr. 2002, 1609, 161–169. [Google Scholar] [CrossRef][Green Version]
- Mitchell, D.; Steinman, L.; Kim, D.; Fathman, C.; Rothbard, J. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, J.M.; Haque, S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie 2019, 160, 61–75. [Google Scholar] [CrossRef]
- Bøe, S.L.; Longva, A.S.; Hovig, E. Cyclodextrin-Containing Polymer Delivery System for Light-Directed siRNA Gene Silencing. Oligonucleotides 2010, 20, 175–182. [Google Scholar] [CrossRef]
- Jørgensen, J.A.L.; Hovig, E.; Bøe, S.L. Potent Gene Silencing In Vitro at Physiological pH Using Chitosan Polymers. Nucleic Acid Ther. 2012, 22, 96–102. [Google Scholar] [CrossRef]
- Raemdonck, K.; Naeye, B.; Buyens, K.; Vandenbroucke, R.E.; Høgset, A.; Demeester, J.; De Smedt, S.C. Biodegradable Dextran Nanogels for RNA Interference: Focusing on Endosomal Escape and Intracellular siRNA Delivery. Adv. Funct. Mater. 2009, 19, 1406–1415. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Lammers, T.; Schiffelers, R.; van Steenbergen, M.J.; Hennink, W.E.; Storm, G. Gene silencing activity of siRNA polyplexes based on biodegradable polymers. Eur. J. Pharm. Biopharm. 2011, 77, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Boe, S.; Longva, A.S.; Hovig, E. Evaluation of Various Polyethylenimine Formulations for Light-Controlled Gene Silencing using Small Interfering RNA Molecules. Oligonucleotides 2008, 18, 123–132. [Google Scholar] [CrossRef]
- Berg, K.; Nordstrand, S.; Selbo, P.K.; Tran, D.T.T.; Angell-Petersen, E.; Høgset, A. Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem. Photobiol. Sci. 2011, 10, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.-A.; Pillai, C.K.S. Biodegradable Polymers for Gene Delivery. Molecules 2019, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Mo, H.; Koo, H.; Park, J.-Y.; Cho, M.Y.; Jin, G.-W. Visualization of the Degradation of a Disulfide Polymer, Linear Poly(ethylenimine sulfide), for Gene Delivery. Bioconjug. Chem. 2006, 18, 13–18. [Google Scholar] [CrossRef]
- Jiang, G.; Park, K.; Kim, J.; Kim, K.S.; Oh, E.J.; Kang, H.; Han, S.-E.; Oh, Y.-K.; Park, T.G.; Hahn, S.K. Hyaluronic acid–polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers 2008, 89, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Viard, M.; Zakrevsky, P.; Zampino, S.; Chen, A.; Isemann, C.; Alvi, S.; Clogston, J.; Chitgupi, U.; Lovell, J.F.; et al. Photoactivation of sulfonated polyplexes enables localized gene silencing by DsiRNA in breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102176. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Chen, H.; Wang, X.; Zhang, P.; Lv, F.; Liu, L.; Wang, S. Cationic Poly(p-phenylene vinylene) Materials as a Multifunctional Platform for Light-Enhanced siRNA Delivery. Chem.-Asian J. 2016, 11, 2686–2689. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; He, S.; Lu, H.; Cheng, Y.; Zhou, D.; Huang, Y. Photoactivatable Prodrug-Backboned Polymeric Nanoparticles for Efficient Light-Controlled Gene Delivery and Synergistic Treatment of Platinum-Resistant Ovarian Cancer. Nano Lett. 2020, 20, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, M.K.G.; Bansal, A.; Huang, K.; Yao, R.; Li, B.N.; Zhang, Y. Near-Infrared-Light-Based Nano-Platform Boosts Endosomal Escape and Controls Gene Knockdown in Vivo. ACS Nano 2014, 8, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Teraphongphom, N.; Kong, C.S.; Warram, J.M.; Rosenthal, E.L. Specimen mapping in head and neck cancer using fluorescence imaging: Specimen Mapping in HNC. Laryngoscope Investig. Otolaryngol. 2017, 2, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 1–22. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horizons 2016, 1, 168–184. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, G.-Y.; Sun, L.-D.; Xiao, J.-W.; Zhou, J.-C.; Yan, C.-H. Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect. ACS Nano 2013, 7, 7200–7206. [Google Scholar] [CrossRef]
- Zhang, Z.; Jayakumar, M.K.G.; Zhen, Z.; Shikha, S.; Zhang, Y.; Bansal, A.; Poon, D.J.J.; Chu, P.L.; Yeo, E.L.L.; Chua, M.L.K.; et al. Upconversion superballs for programmable photoactivation of therapeutics. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Biswas, S.; Ahn, H.-Y.; Bondar, M.V.; Belfield, K.D. Two-Photon Absorption Enhancement of Polymer-Templated Porphyrin-Based J-Aggregates. Langmuir 2011, 28, 1515–1522. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Kondratuk, D.V.; Cnossen, A.; Anderson, H.L.; Drobizhev, M.; Rebane, A. Cooperative Enhancement of Two-Photon Absorption in Self-Assembled Zinc-Porphyrin Nanostructures. J. Phys. Chem. C 2016, 120, 11663–11670. [Google Scholar] [CrossRef]
- Jimenez, C.M.; Aggad, D.; Croissant, J.G.; Tresfield, K.; Laurencin, D.; Berthomieu, D.; Cubedo, N.; Rossel, M.; Alsaiari, S.; Anjum, D.H.; et al. Porous Porphyrin-Based Organosilica Nanoparticles for NIR Two-Photon Photodynamic Therapy and Gene Delivery in Zebrafish. Adv. Funct. Mater. 2018, 28, 1800235. [Google Scholar] [CrossRef]
- Ekineker, G.; Nguyen, C.; Bayır, S.; Dominguez Gil, S.; İşci, Ü.; Daurat, M.; Godefroy, A.; Raehm, L.; Charnay, C.; Oliviero, E.; et al. Phthalocyanine-based mesoporous organosilica nanoparticles: NIR photodynamic efficiency and siRNA photochemical internalization. Chem. Commun. 2019, 55, 11619–11622. [Google Scholar] [CrossRef] [PubMed]
- Mezghrani, B.; Ali, L.M.A.; Richeter, S.; Durand, J.-O.; Hesemann, P.; Bettache, N. Periodic Mesoporous Ionosilica Nanoparticles for Green Light Photodynamic Therapy and Photochemical Internalization of siRNA. ACS Appl. Mater. Interfaces 2021, 13, 29325–29339. [Google Scholar] [CrossRef] [PubMed]
| Name/Sponsor | Route of Administration | Delivery System | Targeting Moiety | Target Gene | Disease | Clinical Trail Number (ClinicalTrials.gov) | Phase/Status | Period | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CALAA-01/Calando Pharmaceuticals | i.v. | Cyclodextrin polymer-based nanoparticle | Transferrin | RRM2 | Solid tumors (Melanoma, gastrointestinal, prostate, etc.) | NCT00689065 | Phase I/Terminated | 2008–2012 | [21] |
| siG12D LODER/Silenseed Ltd. | Endoscopic intervention | Biodegradable Polymeric matrix | ----- | KRAS(G12D) and G12X mutations | Locally advanced pancreatic cancer | NCT01188785 | Phase I/Completed | 2011–2013 | [22] |
| siG12D-LODERs plus chemotherapy (Gemcitabine + nab-Paclitaxel or Folfirinox or modified Folfirinox) /Silenseed Ltd. | Endoscopic intervention | Biodegradable Polymeric matrix | ----- | KRAS(G12D) and G12X mutations | Locally advanced pancreatic cancer | NCT01676259 | Phase II/Recruiting | 2018–Est.2023 | [23] |
| ALN-VSP02/Alnylam Pharmaceuticals | i.v. | Lipid nanoparticle | ----- | VEGF KSP | Solid tumors with liver involvement. | NCT00882180 NCT01158079 | Phase I/Completed | 2009–2011 2010–2012 | [24] |
| TKM-PLK1 (TKM-080301)/National Cancer Institute (NCI) | Hepatic Intra-Arterial Administration | Lipid nanoparticle | ----- | PLK1 | Primary or secondary liver cancer. | NCT01437007 | Phase I/Completed | 2011–2012 | [25] |
| Arbutus Biopharma Corporation | i.v. | Cancer, neuroendocrine tumors, adrenocortical carcinoma | NCT01262235 | Phase I/II/Completed | 2010–2015 | ||||
| Arbutus Biopharma Corporation | i.v. | Hepatocellular Carcinoma | NCT02191878 | Phase I/II/Completed | 2014–2016 | ||||
| DCR-MYC/Dicerna Pharmaceuticals, Inc. | i.v. | EnCoreTM lipid nanoparticle | ----- | MYC | Solid tumors, multiple myeloma, lymphoma | NCT02110563 | Phase I/Terminated | 2014–2016 | [26] |
| NBF-006/Nitto BioPharma, Inc. | Lipid nanoparticle | GSTP | Non-Small cell lung, pancreatic and colorectal Cancers | NCT03819387 | Phase I/Recruiting | 2019–Est.2023 | [27] | ||
| Atu027/Silence Therapeutics GmbH | i.v. | Liposomes | ----- | PKN3 | Advanced Solid Cancer | NCT00938574 | Phase I/Completed | 2009–2012 | [28] |
| Atu027-I-02 (Atu027 plus gemcitabine)/Silence Therapeutics GmbH | i.v. | Liposomes | ----- | PKN3 | Advanced or Metastatic Pancreatic Cancer | NCT01808638 | Phase I/II/Completed | 2013/2016 | [29] |
| EphA2-targeting DOPC-encapsulated siRNA/M.D. Anderson Cancer Center | i.v. | Liposomes | ----- | EphA2 | Advanced or recurrent solid tumors | NCT01591356 | Phase I/Active, not recruiting | 2015–Est.2024 | [30] |
| Mesenchymal Stromal Cells-derived Exosomes with KRAS(G12D) siRNA/M.D. Anderson Cancer Center | MSC exosome | CD47 | KRAS(G12D) | Metastatic pancreatic ductal adenocarcinoma with KrasG12D mutation | NCT03608631 | Phase I/Recruiting | 2021–Est.2023 | [31] |
| Type of Carrier | Cell Line | PS | λex (nm) | Carrier | Knockdown (%) | siRNA | Ref | |
|---|---|---|---|---|---|---|---|---|
| (−) PCI | (+) PCI | |||||||
| Lipid | A431 | TPPS2a | 375–450 | Lipofectamine | 10 40 | 70 80 | EGFR | [54] |
| OHS | TPPS2a | 420 | JetSI-ENDO | 20 | 90 | S100A4 | [56] | |
| HepG2 | AlPCS2a | 660 | LDL nanoparticles | 38 | 78 | ApoB | [57] | |
| Peptides | CHO | AlexaFluor 546 | 540 | TatU1A | 0 | ~70 | dEGFP | [60] |
| OHS | TPPS2a | 420 | PLL PLH PLA | ~10 ~10 ~15 | ~80 ~45 ~90 | S100A4 | [63] | |
| SK-MEL-28 | TPPS2a | 420 | PLA | 0 | ~85 | MEK1 MEK2 | [63] | |
| Poymers | OHS | TPPS2a | 420 | β-6CDP | 10 | ~90 | S100A4 | [71] |
| OHS | TPPS2a | 420 | Chitosan | ~50 | ~40 | S100A4 | [72] | |
| HuH-7 Luc | TPPS2a | 375–450 | Dextran nanogel | ~30 | ~80 | Luciferase | [73] | |
| HuH-7-EGFP | TPPS2a | 375–450 | Dextran nanogel (25µg/mL) | ~60 (day6) | (PCI t2) ~80 (day6) | EGFP | [48] | |
| H1299 | TPPS2a | 375–450 | pHPMA-MPPM or TMC | 30–40 | 70–80 | Luciferase | [74] | |
| OHS | TPPS2a | 420 | PEI | ~10 | ~90 | S100A4 | [75] | |
| A375-GFP | TPCS2a | 652 | PEI | n/a | n/a | EGFP | [76] | |
| MDA-MB-231/GFP | pyropheophorbide-α | 661 | Sulfonated PEI | n/a | n/a | GFP | [80] | |
| Hela-Luc | PPV | 400–800 | PPV | ~80 | ~85 | Luciferase | [82] | |
| A2780 A2780DDP | Pt(IV) | 430 | Pt(IV) | ~32 ~26 | ~52 ~63 | c-fos | [83] | |
| Nanoparticles | B16F0 | TPPS2a | 980 | UCNPs Coated with MSN | n/a | +30 | STAT3 Morpholino | [84] |
| Hela Cal27 | ZnPc | 980 | UCNPs | ~70 ~60 | ~90 ~80 | SOD1 | [90] | |
| MCF-7-LUC | Porphyrin | 800 | PMO | 0 | ~50 | Luciferase | [93] | |
| MCF-7-LUC | ZnPc | 810 | PMO | 0 | 64 | Luciferase | [94] | |
| MDA-MB-231 | Porphyrin | 545 | PMINPs | 17 | 83 | Luciferase | [95] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, L.M.A.; Gary-Bobo, M. Photochemical Internalization of siRNA for Cancer Therapy. Cancers 2022, 14, 3597. https://doi.org/10.3390/cancers14153597
Ali LMA, Gary-Bobo M. Photochemical Internalization of siRNA for Cancer Therapy. Cancers. 2022; 14(15):3597. https://doi.org/10.3390/cancers14153597
Chicago/Turabian StyleAli, Lamiaa Mohamed Ahmed, and Magali Gary-Bobo. 2022. "Photochemical Internalization of siRNA for Cancer Therapy" Cancers 14, no. 15: 3597. https://doi.org/10.3390/cancers14153597
APA StyleAli, L. M. A., & Gary-Bobo, M. (2022). Photochemical Internalization of siRNA for Cancer Therapy. Cancers, 14(15), 3597. https://doi.org/10.3390/cancers14153597






