A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Animal Ethical Consideration and Limit Points
2.3. Cells and Cell Culture
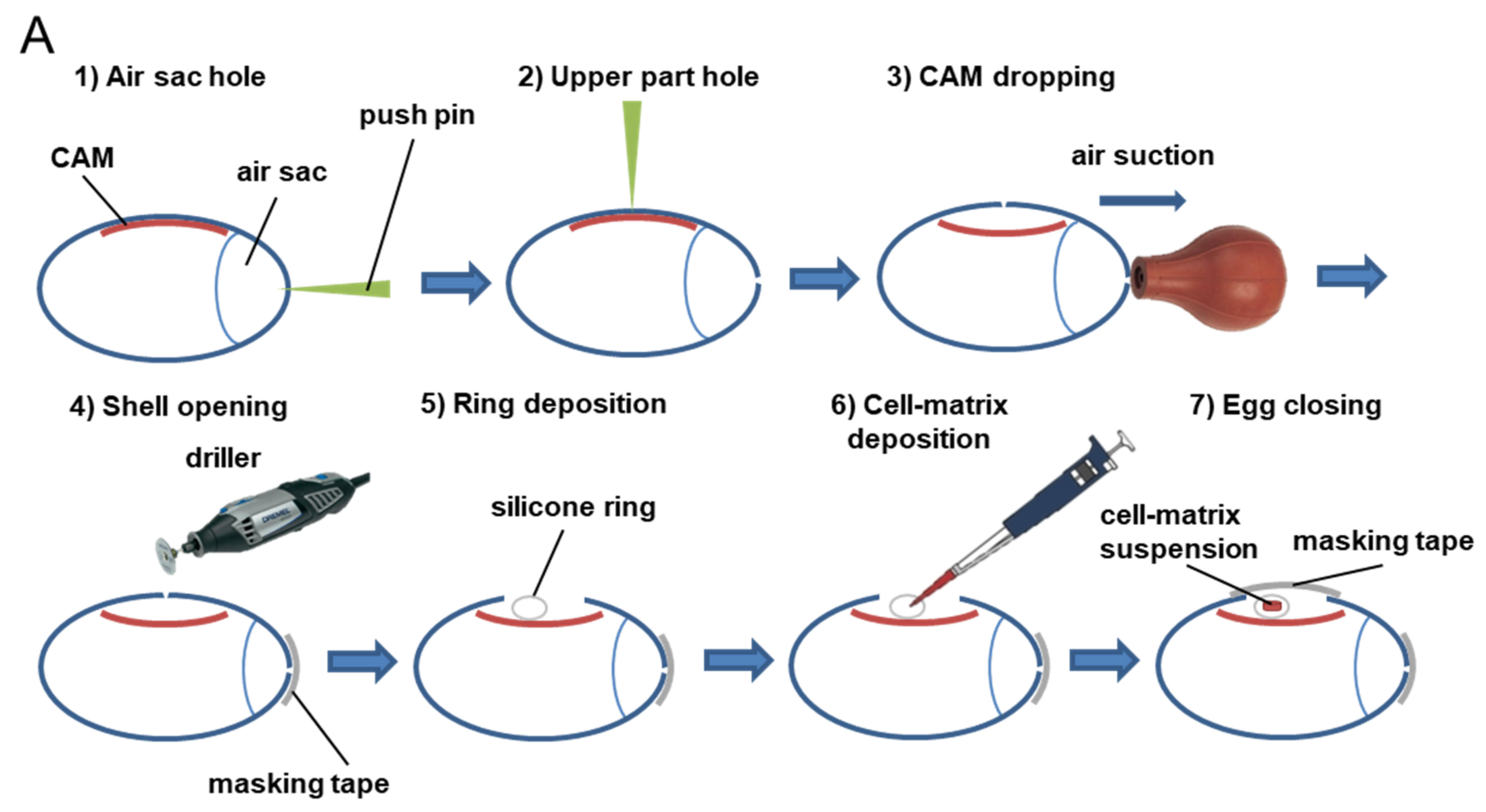
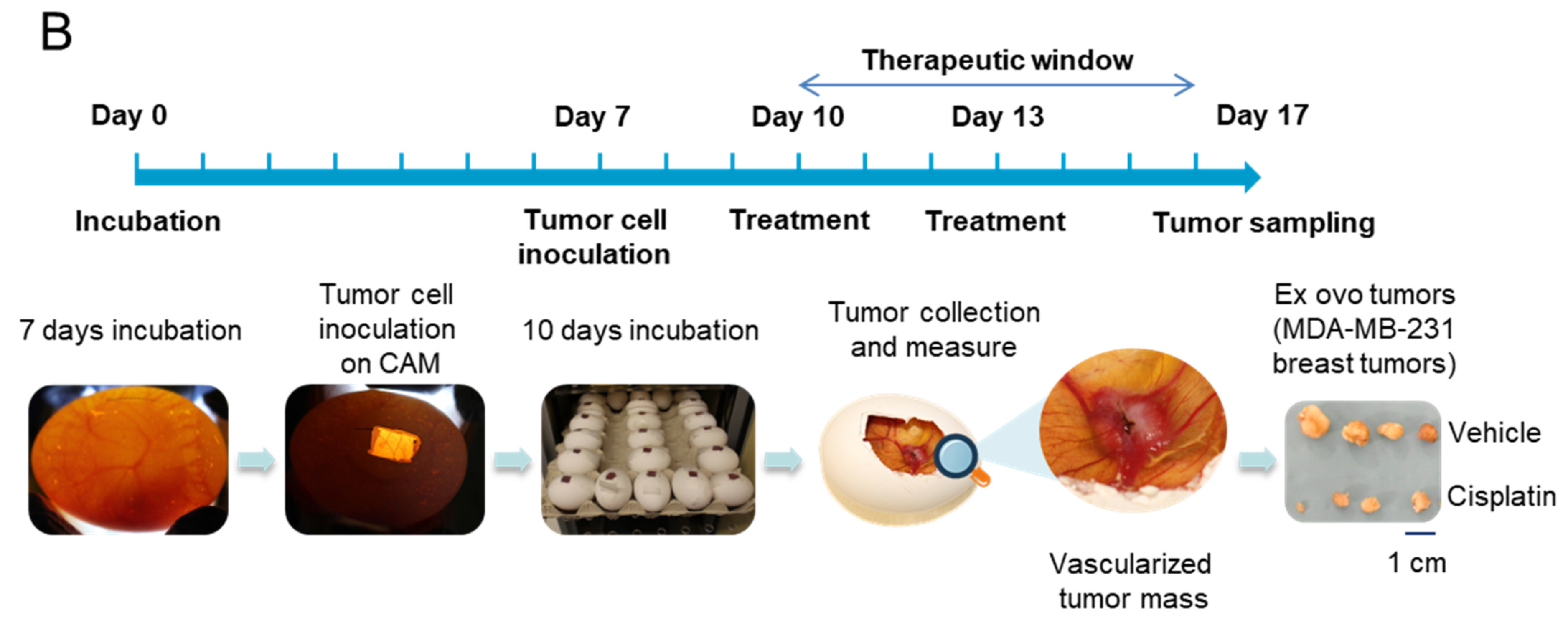
2.4. Tumor CAM Assays
2.5. Subcutaneous Graft Murine Models
2.6. Treatments
2.7. Statistics
3. Results
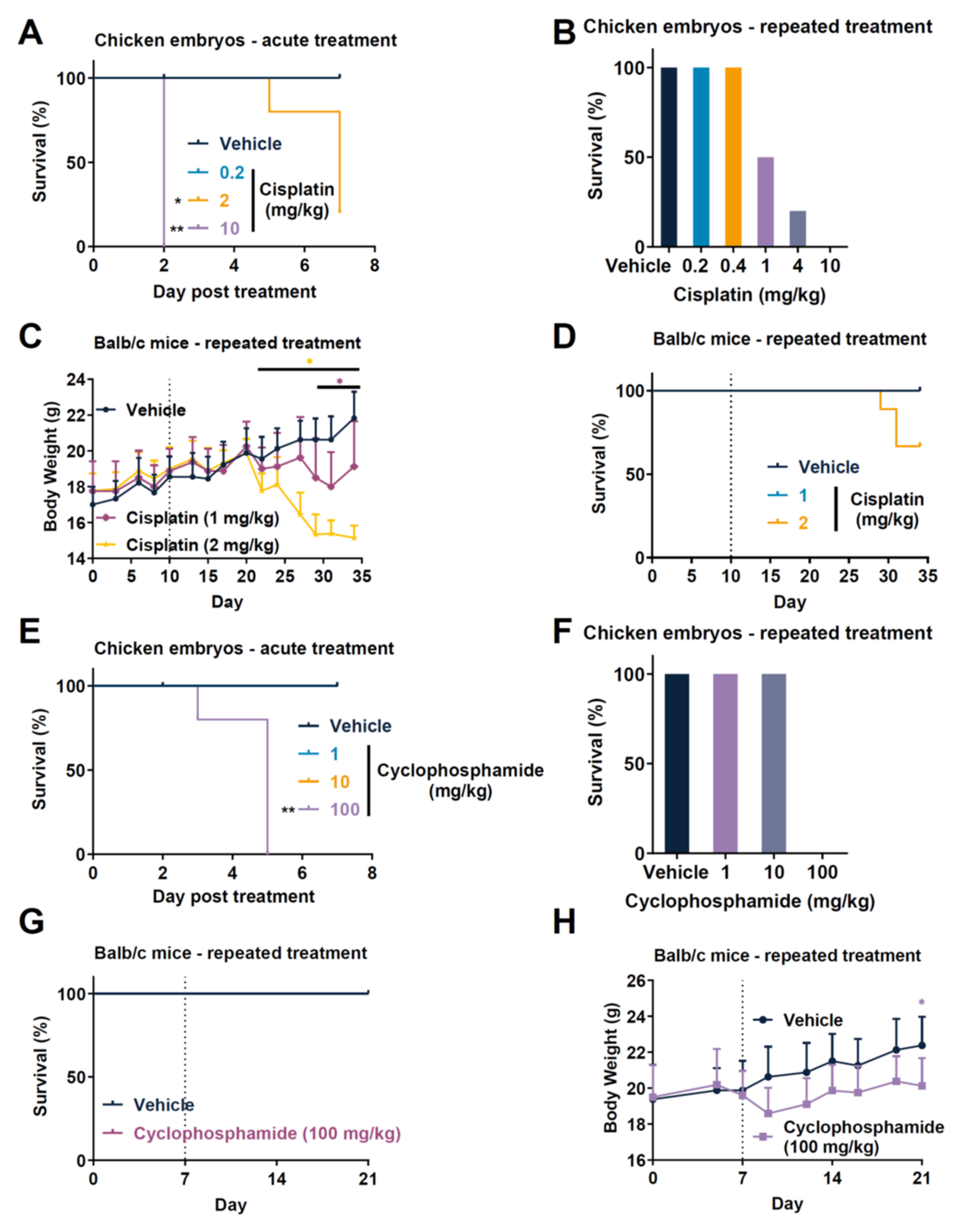
3.1. Comparison of Toxicity of Anticancer Drugs in Rodent Tumor Graft and TCAM Model
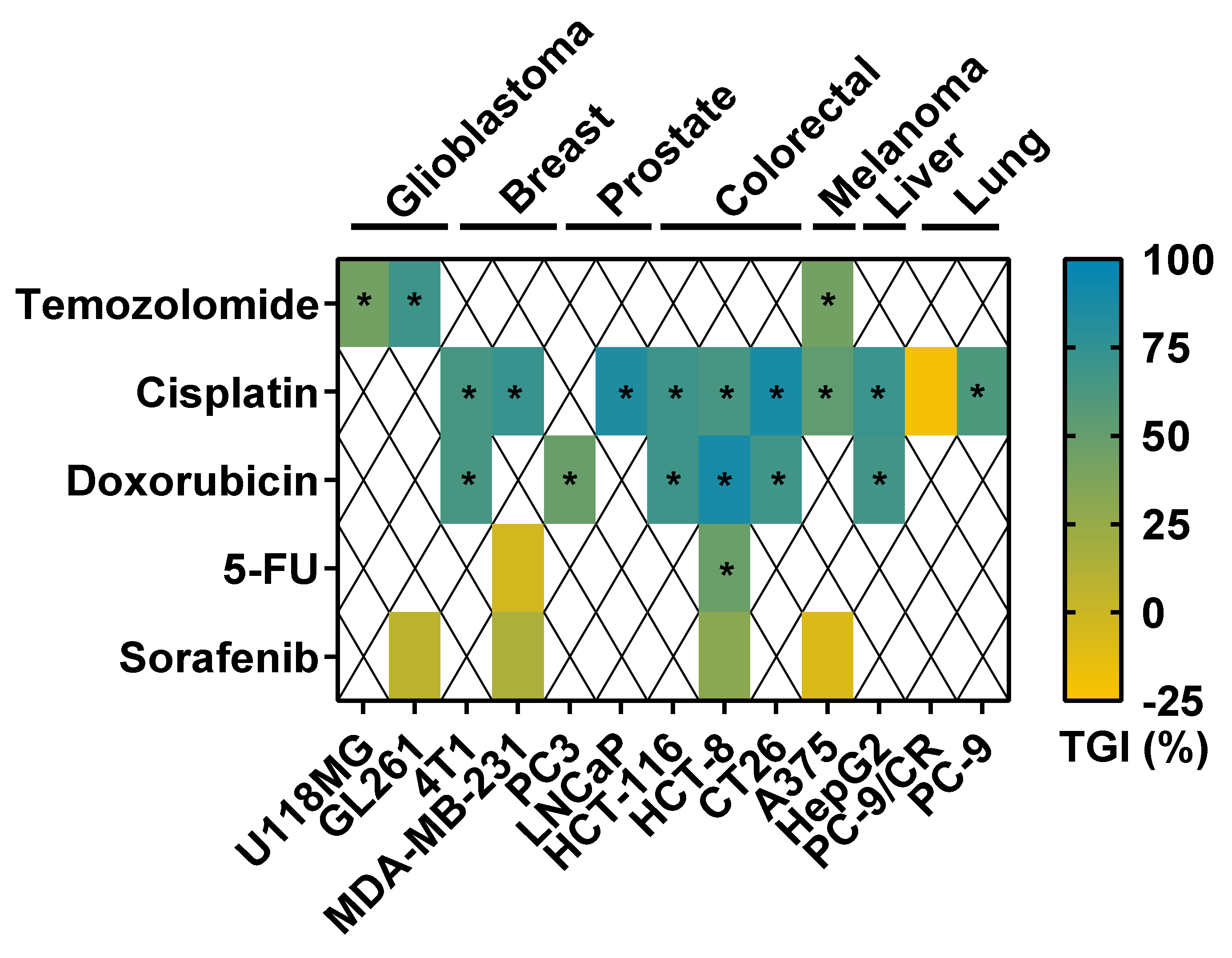
3.2. Tumor Response to Anticancer Drugs in TCAM Assay
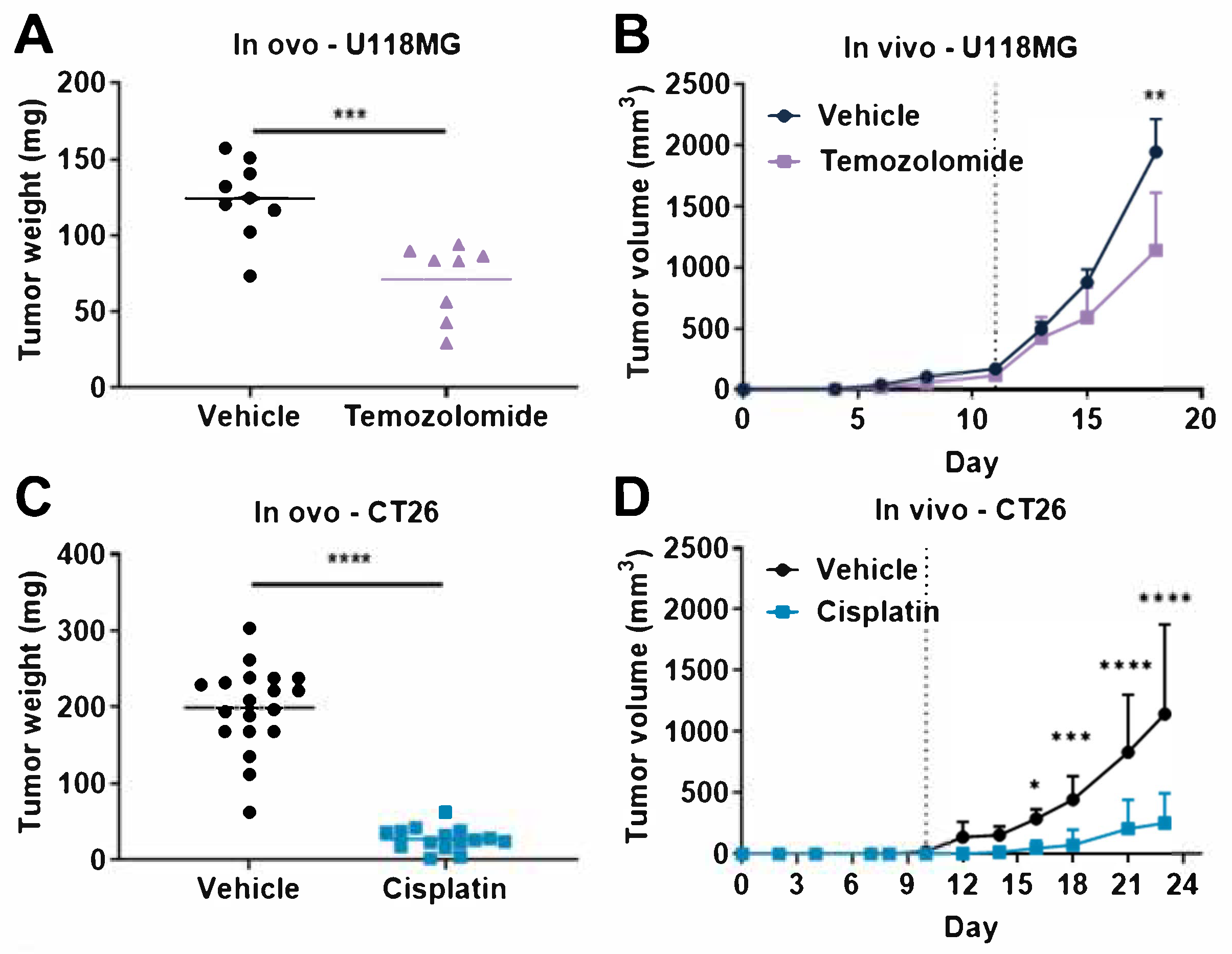
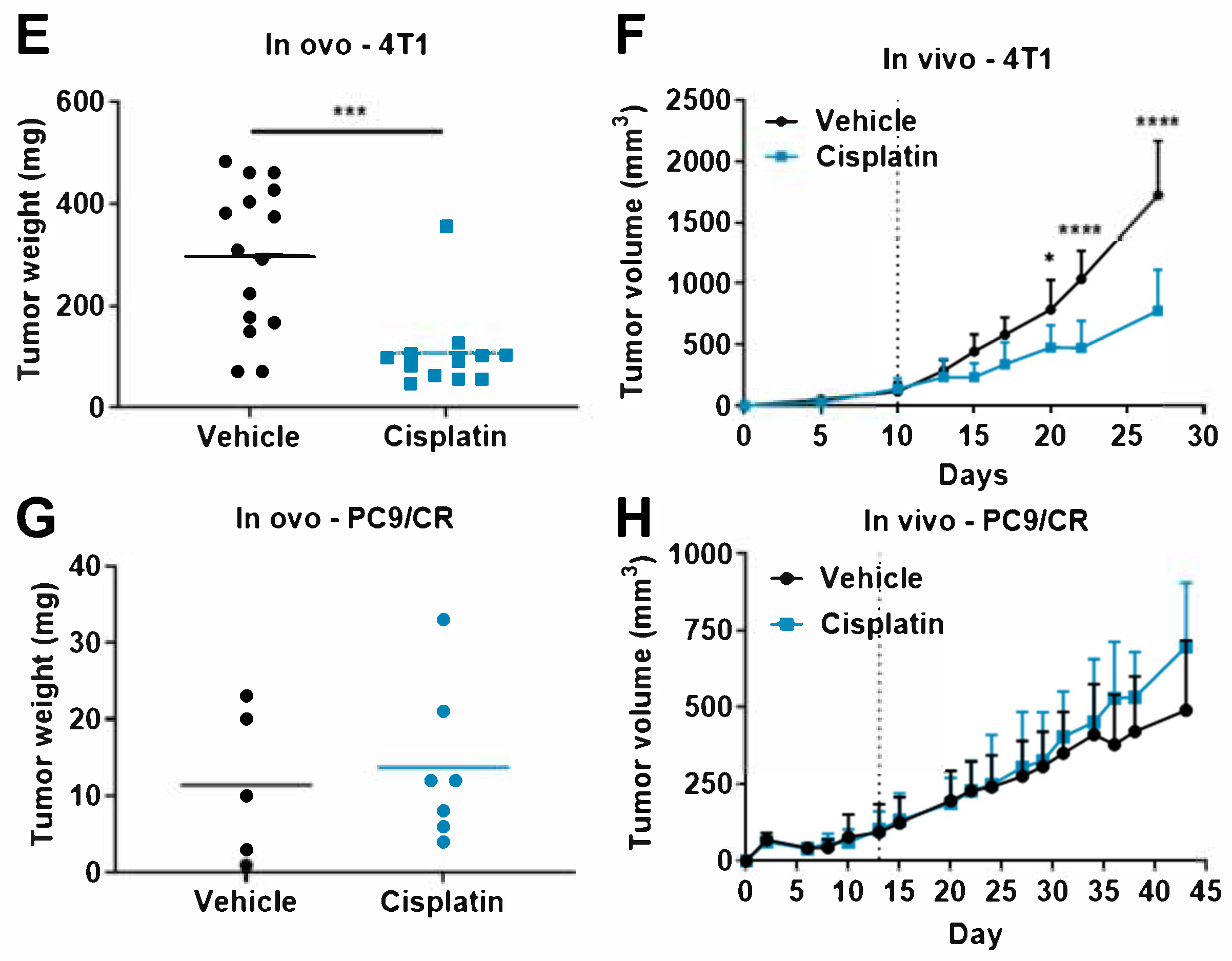
3.3. Comparison of Tumor Response to Anticancer Drugs in Rodent Tumor Graft and TCAM Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| CAM | chorioallantoic membrane |
| CR | cisplatin-resistant |
| ED | embryonic day |
| GBM | glioblastoma |
| SOC | standard of care |
| TNBC | triple-negative breast cancer |
| TCAM | tumor chorioallantoic membrane |
| TME | tumor microenvironment |
| TMZ | temozolomide |
References
- Tufan, A.C.; Satiroglu-Tufan, N.L. The Chick Embryo Chorioallantoic Membrane as a Model System for the Study of Tumor Angiogenesis, Invasion and Development of Anti-Angiogenic Agents. Curr. Cancer Drug Targets 2005, 5, 249–266. [Google Scholar] [CrossRef]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Eckrich, J.; Kugler, P.; Buhr, C.R.; Ernst, B.P.; Mendler, S.; Baumgart, J.; Brieger, J.; Wiesmann, N. Monitoring of Tumor Growth and Vascularization with Repetitive Ultrasonography in the Chicken Chorioallantoic-Membrane-Assay. Sci. Rep. 2020, 10, 18585. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique, Methuen & Co. Limited: Boston, MA, USA, 1959.
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken Embryo Development: Metabolic and Morphological Basis for in Ovo Feeding Technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- AVMA. AVMA Guidelines for the Euthanasia of Animals: American Veterinary Medical Association Edition; AVMA: Schaumburg, IL, USA, 2020. [Google Scholar]
- Dupertuis, Y.M.; Delie, F.; Cohen, M.; Pichard, C. In Ovo Method for Evaluating the Effect of Nutritional Therapies on Tumor Development, Growth and Vascularization. Clin. Nutr. Exp. 2015, 2, 9–17. [Google Scholar] [CrossRef]
- Murdamoothoo, D.; Schwenzer, A.; Kant, J.; Rupp, T.; Marzeda, A.; Midwood, K.; Orend, G. Investigating Cell-Type Specific Functions of Tenascin-C. Methods Cell Biol. 2018, 143, 401–428. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Presta, M. The Gelatin Sponge-Chorioallantoic Membrane Assay. Nat. Protoc. 2006, 1, 85–91. [Google Scholar] [CrossRef]
- Klingenberg, M.; Becker, J.; Eberth, S.; Kube, D.; Wilting, J. The Chick Chorioallantoic Membrane as an in Vivo Xenograft Model for Burkitt Lymphoma. BMC Cancer 2014, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick Embryo Chorioallantoic Membrane (CAM): An Alternative Predictive Model in Acute Toxicological Studies for Anti-Cancer Drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pathak, R.R.; Lopez-Rivera, E.; Friedman, S.L.; Aguirre-Ghiso, J.A.; Sikora, A.G. The In Ovo Chick Chorioallantoic Membrane (CAM) Assay as an Efficient Xenograft Model of Hepatocellular Carcinoma. J. Vis. Exp. 2015, 104, e52411. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Liu, M.; Scanlon, C.S.; Banerjee, R.; D’Silva, N.J. The Chick Chorioallantoic Membrane In Vivo Model to Assess Perineural Invasion in Head and Neck Cancer. J. Vis. Exp. 2019, 148, e59296. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-Y.; Koh, A.P.-F.; Antony, J.; Huang, R.Y.-J. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the Chick Embryo Chorioallantoic Membrane (CAM) as a Platform for Anti-Metastatic Drug Testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Aleksandrowicz, E.; Herr, I. Ethical Euthanasia and Short-Term Anesthesia of the Chick Embryo. ALTEX 2015, 32, 143–147. [Google Scholar] [CrossRef]
- Kishore, A.S.; Surekha, P.A.; Sekhar, P.V.R.; Srinivas, A.; Murthy, P.B. Hen Egg Chorioallantoic Membrane Bioassay: An In Vitro Alternative to Draize Eye Irritation Test for Pesticide Screening. Int. J. Toxicol. 2008, 27, 449–453. [Google Scholar] [CrossRef]
- Rupp, T.; Pelouin, O.; Genest, L.; Legrand, C.; Froget, G.; Castagné, V. Therapeutic Potential of Fingolimod in Triple Negative Breast Cancer Preclinical Models. Transl. Oncol. 2021, 14, 100926. [Google Scholar] [CrossRef]
- Rupp, T.; Genest, L.; Babin, D.; Legrand, C.; Hunault, M.; Froget, G.; Castagné, V. Anti-CTLA-4 and Anti-PD-1 Immunotherapies Repress Tumor Progression in Preclinical Breast and Colon Model with Independent Regulatory T Cells Response. Transl. Oncol. 2022, 20, 101405. [Google Scholar] [CrossRef]
- Chen, Y.; Han, F.; Cao, L.; Li, C.; Wang, J.; Li, Q.; Zheng, W.; Guo, Z.; Li, A.; Zhou, J. Dose-Response Relationship in Cisplatin-Treated Breast Cancer Xenografts Monitored with Dynamic Contrast-Enhanced Ultrasound. BMC Cancer 2015, 15, 136. [Google Scholar] [CrossRef][Green Version]
- Liang, Z.; Yoon, Y.; Votaw, J.; Goodman, M.M.; Williams, L.; Shim, H. Silencing of CXCR4 Blocks Breast Cancer Metastasis. Cancer Res. 2005, 65, 967–971. [Google Scholar] [PubMed]
- Kleijn, A.; van den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; de Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8+T Cell Anti-Tumor Activity. Mol. Ther. Oncolytics 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-T.; Chen, R.; Wang, H.; Song, H.; Zhang, Q.; Chen, L.-Y.; Lappin, H.; Vasconcelos, G.; Lita, A.; Maric, D.; et al. Novel Targeting of Transcription and Metabolism in Glioblastoma. Clin. Cancer Res. 2018, 24, 1124–1137. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Singh, R.K.; Fidler, I.J.; Raz, A. Murine Models to Evaluate Novel and Conventional Therapeutic Strategies for Cancer. Am. J. Pathol. 2007, 170, 793–804. [Google Scholar] [CrossRef]
- Michael, M.; Doherty, M.M. Tumoral Drug Metabolism: Overview and Its Implications for Cancer Therapy. JCO 2005, 23, 205–229. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Yu, Y.; Xu, C.; Xu, H.; Qin, J. Assessment of Metabolism-Dependent Drug Efficacy and Toxicity on a Multilayer Organs-on-a-Chip. Integr. Biol. 2016, 8, 1022–1029. [Google Scholar] [CrossRef]
- Schmidt, G.S.; Figueiredo, E.A.P.; Saatkamp, M.G.; Bomm, E.R. Effect of Storage Period and Egg Weight on Embryo Development and Incubation Results. Braz. J. Poult. Sci. 2009, 11, 1–5. [Google Scholar] [CrossRef]
- Quirbt, I.; Verma, S.; Petrella, T.; Bak, K.; Charette, M. Temozolomide for the Treatment of Metastatic Melanoma. Curr. Oncol. 2007, 14, 27–33. [Google Scholar]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA Torsion, and Chromatin Dynamics. Biochim. Et Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Gong, L.; Giacomini, M.M.; Giacomini, C.; Maitland, M.L.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Sorafenib Pathways. Pharm. Genom. 2017, 27, 240–246. [Google Scholar] [CrossRef]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and Development of Sorafenib: A Multikinase Inhibitor for Treating Cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Strojnik, T.; Kavalar, R.; Barone, T.A.; Plunkett, R.J. Experimental Model and Immunohistochemical Comparison of U87 Human Glioblastoma Cell Xenografts on the Chicken Chorioallantoic Membrane and in Rat Brains. Anticancer Res. 2010, 30, 4851–4860. [Google Scholar]
- Aguirre-Ghiso, J.A.; Ossowski, L.; Rosenbaum, S.K. Green Fluorescent Protein Tagging of Extracellular Signal-Regulated Kinase and P38 Pathways Reveals Novel Dynamics of Pathway Activation during Primary and Metastatic Growth. Cancer Res. 2004, 64, 7336–7345. [Google Scholar] [CrossRef]
- Lyu, M.A.; Choi, Y.K.; Park, B.N.; Kim, B.J.; Park, I.K.; Hyun, B.H.; Kook, Y.H. Over-Expression of Urokinase Receptor in Human Epidermoid-Carcinoma Cell Line (HEp3) Increases Tumorigenicity on Chorio-Allantoic Membrane and in Severe-Combined-Immunodeficient Mice. Int. J. Cancer 1998, 77, 257–263. [Google Scholar] [CrossRef]
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In Vivo Anti-Tumor Activity of the Organometallic Ruthenium(II)-Arene Complex [Ru(H6-p-Cymene)Cl2(Pta)] (RAPTA-C) in Human Ovarian and Colorectal Carcinomas. Chem. Sci. 2014, 5, 4742–4748. [Google Scholar] [CrossRef]
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schlingemann, R.O.; et al. Rapid Optimization of Drug Combinations for the Optimal Angiostatic Treatment of Cancer. Angiogenesis 2015, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Arita, M.; Watanabe, S.; Aoki, N.; Kuwahara, S.; Suzuki, R.; Goto, S.; Abe, Y.; Takahashi, M.; Sato, M.; Hokari, S.; et al. Combination Therapy of Cisplatin with Cilastatin Enables an Increased Dose of Cisplatin, Enhancing Its Antitumor Effect by Suppression of Nephrotoxicity. Sci.Rep. 2021, 11, 750. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic Death of Colon Cancer Cells Treated with Oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef]
- Hill, D.P.; Harper, A.; Malcolm, J.; McAndrews, M.S.; Mockus, S.M.; Patterson, S.E.; Reynolds, T.; Baker, E.J.; Bult, C.J.; Chesler, E.J.; et al. Cisplatin-Resistant Triple-Negative Breast Cancer Subtypes: Multiple Mechanisms of Resistance. BMC Cancer 2019, 19, 1039. [Google Scholar] [CrossRef]
- Hu, X.-C.; Zhang, J.; Xu, B.-H.; Cai, L.; Ragaz, J.; Wang, Z.-H.; Wang, B.-Y.; Teng, Y.-E.; Tong, Z.-S.; Pan, Y.-Y.; et al. Cisplatin plus Gemcitabine versus Paclitaxel plus Gemcitabine as First-Line Therapy for Metastatic Triple-Negative Breast Cancer (CBCSG006): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 436–446. [Google Scholar] [CrossRef]
- Köberle, B.; Schoch, S. Platinum Complexes in Colorectal Cancer and Other Solid Tumors. Cancers 2021, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Lee, K.W.; Oh, D.Y.; Kim, J.H.; Im, S.A.; Kim, T.Y.; Bang, Y.J. Combination Chemotherapy with Capecitabine and Cisplatin for Patients with Metastatic Hepatocellular Carcinoma. Ann. Oncol. 2009, 20, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Osaki, A.; Suda, T.; Kamimura, K.; Tsuchiya, A.; Tamura, Y.; Takamura, M.; Igarashi, M.; Kawai, H.; Yamagiwa, S.; Aoyagi, Y. A Safe and Effective Dose of Cisplatin in Hepatic Arterial Infusion Chemotherapy for Hepatocellular Carcinoma. Cancer Med. 2013, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Beteta-Göbel, R.; Fernández-Díaz, J.; Arbona-González, L.; Rodríguez-Lorca, R.; Torres, M.; Busquets, X.; Fernández-García, P.; Escribá, P.V.; Lladó, V. The Novel Antitumor Compound HCA Promotes Glioma Cell Death by Inducing Endoplasmic Reticulum Stress and Autophagy. Cancers 2021, 13, 4290. [Google Scholar] [CrossRef] [PubMed]
- Bjørnstad, S.; Austdal, L.P.E.; Roald, B.; Glover, J.C.; Paulsen, R.E. Cracking the Egg: Potential of the Developing Chicken as a Model System for Nonclinical Safety Studies of Pharmaceuticals. J. Pharmacol. Exp. Ther. 2015, 355, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Neumann, A.; Kersting, C.; Agelopoulos, K.; Gebert, C.; Gosheger, G.; Buerger, H.; Hagedorn, M. Morphologic Characterization of Osteosarcoma Growth on the Chick Chorioallantoic Membrane. BMC Res. Notes 2010, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick Ex Ovo Culture and Ex Ovo CAM Assay: How It Really Works. J. Vis. Exp. 2009, 33, e1620. [Google Scholar] [CrossRef]
- Sys, G.M.L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The In Ovo CAM-Assay as a Xenograft Model for Sarcoma. J. Vis. Exp. 2013, 77, e50522. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Scanlon, C.S.; Banerjee, R.; Russo, N.; Inglehart, R.C.; Willis, A.L.; Weiss, S.J.; D’Silva, N.J. The Histone Methyltransferase EZH2 Mediates Tumor Progression on the Chick Chorioallantoic Membrane Assay, a Novel Model of Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2013, 6, 273–281. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Zumbrink, L.; Brenig, B.; Foerster, A.; Hurlin, J.; von Wenzlawowicz, M. Electrical Anaesthesia of Male Chicken Embryos in the Second Third of the Incubation Period in Compliance with Animal Welfare. Europ. Poult. Sci. 2020, 84, 1–11. [Google Scholar] [CrossRef]
- Baker, S.D.; Wirth, M.; Statkevich, P.; Reidenberg, P.; Alton, K.; Sartorius, S.E.; Dugan, M.; Cutler, D.; Batra, V.; Grochow, L.B.; et al. Absorption, Metabolism, and Excretion of 14C-Temozolomide Following Oral Administration to Patients with Advanced Cancer. Clin Cancer Res. 1999, 5, 309–317. [Google Scholar]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Le-Hoang Doan, T.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick Chorioallantoic Membrane Assay as an in Vivo Model to Study the Effect of Nanoparticle-Based Anticancer Drugs in Ovarian Cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef] [PubMed]
- Zosen, D.; Hadera, M.G.; Lumor, J.S.; Andersen, J.M.; Paulsen, R.E. Chicken Embryo as Animal Model to Study Drug Distribution to the Developing Brain. J. Pharmacol. Toxicol. Methods 2021, 112, 107105. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Korol, E.E.; Kayaniyil, S.; Varol, N.; Ebner, T.; Goring, S.M. Efficacy and Safety of First-Line Carboplatin-versus Cisplatin-Based Chemotherapy for Non-Small Cell Lung Cancer: A Meta-Analysis. Lung Cancer 2019, 135, 196–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupp, T.; Legrand, C.; Hunault, M.; Genest, L.; Babin, D.; Froget, G.; Castagné, V. A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity. Cancers 2022, 14, 3548. https://doi.org/10.3390/cancers14143548
Rupp T, Legrand C, Hunault M, Genest L, Babin D, Froget G, Castagné V. A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity. Cancers. 2022; 14(14):3548. https://doi.org/10.3390/cancers14143548
Chicago/Turabian StyleRupp, Tristan, Christophe Legrand, Marion Hunault, Laurie Genest, David Babin, Guillaume Froget, and Vincent Castagné. 2022. "A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity" Cancers 14, no. 14: 3548. https://doi.org/10.3390/cancers14143548
APA StyleRupp, T., Legrand, C., Hunault, M., Genest, L., Babin, D., Froget, G., & Castagné, V. (2022). A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity. Cancers, 14(14), 3548. https://doi.org/10.3390/cancers14143548





