LncRNA HOXC-AS1 Sponges miR-99a-3p and Upregulates MMP8, Ultimately Promoting Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Online Tools
2.2. Cell Culture
2.3. Cell Transfection
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Luciferase Reporter Assays
2.6. CCK-8 Assay
2.7. Colony Formation Assay
2.8. Western Blot
2.9. Statistical Analysis
3. Results
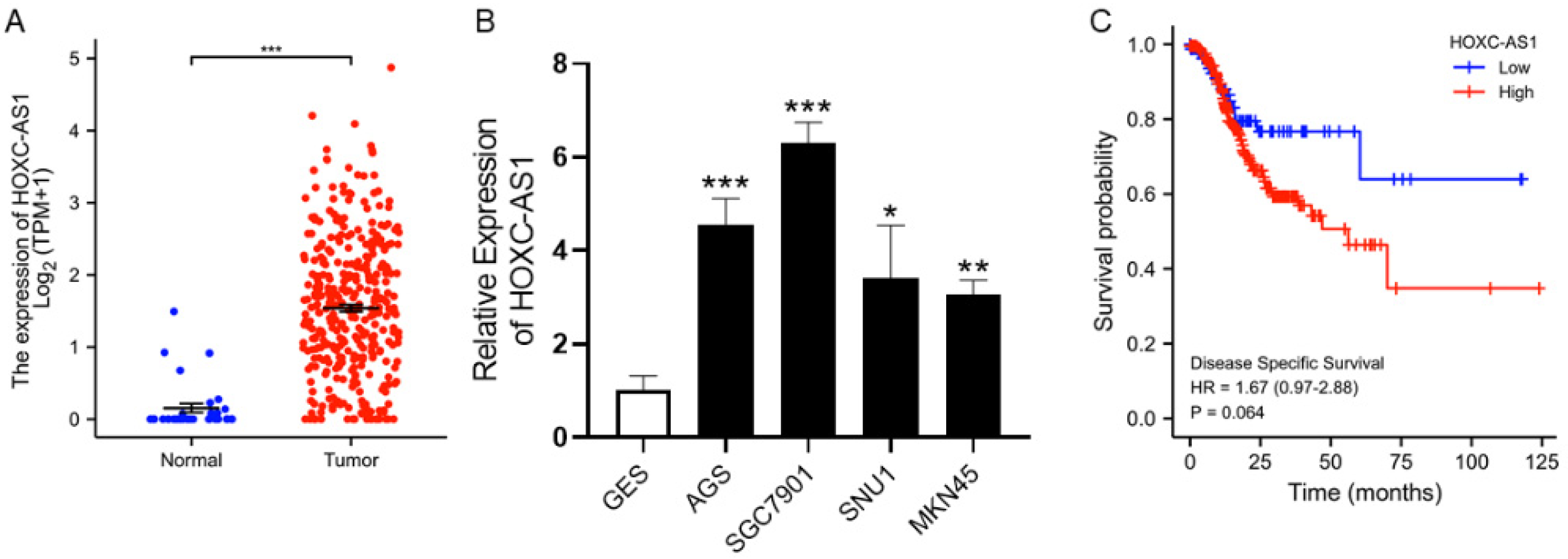
3.1. HOXC-AS1 Was Overexpressed in GC with Poor Prognosis
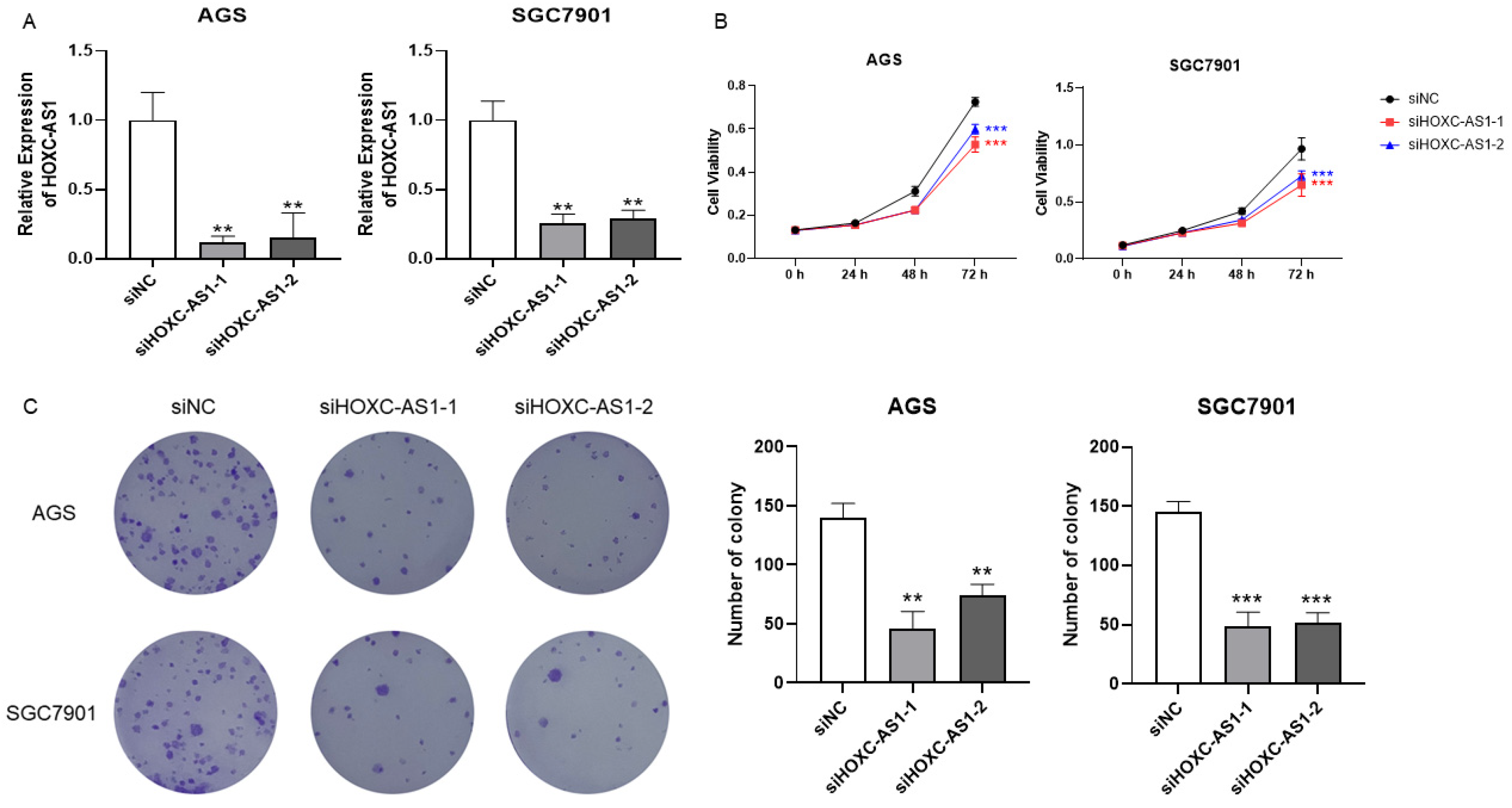
3.2. Downregulation of HOXC-AS1 Inhibited GC Cell Proliferation
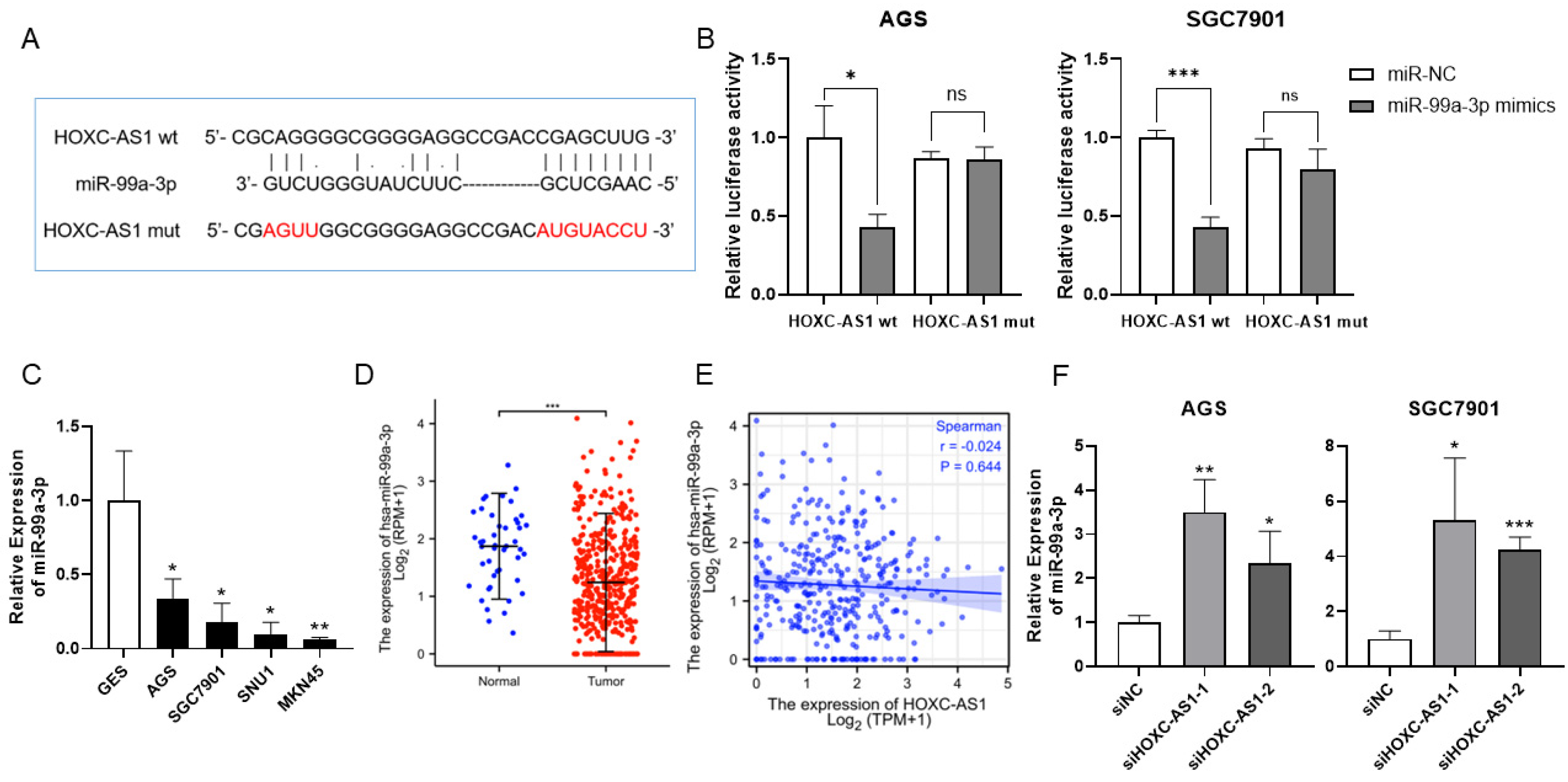
3.3. HOXC-AS1 Sponged miR-99a-3p in GC
3.4. MiR-99a-3p Regulated MMP8 in GC
3.5. HOXC-AS1 Promoted GC by Managing miR-99a-3p/MMP8 Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Roselló, S.; Roda, D.; Rodríguez-Braun, E. The treatment of advanced gastric cancer: Current strategies and future perspectives. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, v103–v107. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Badgwell, B. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Kopp, F.; Mendell, J. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Chang, H. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Xu, Z.; Ruan, S.; Mo, S.; Qin, J.; Cheng, X. Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 2020, 19, 96. [Google Scholar] [CrossRef]
- Liu, S.; Dang, H.; Lim, D.; Feng, F.; Maher, C. Long noncoding RNAs in cancer metastasis. Nat. Rev. Cancer 2021, 21, 446–460. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Y.; Deng, M.; Zhu, L.; Wu, X.; Li, G.; Shi, N.; Wen, C.; Huang, W.; Duan, Y.; et al. c-Myc-activated intronic miR-210 and lncRNA MIR210HG synergistically promote the metastasis of gastric cancer. Cancer Lett. 2022, 526, 322–334. [Google Scholar] [CrossRef]
- Piao, H.; Liu, Y.; Kang, Y.; Wang, Y.; Meng, X.; Yang, D.; Zhang, J. Hypoxia associated lncRNA HYPAL promotes proliferation of gastric cancer as ceRNA by sponging miR-431-5p to upregulate CDK14. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2022, 25, 44–63. [Google Scholar] [CrossRef]
- Xin, L.; Zhou, L.; Liu, C.; Zeng, F.; Yuan, Y.; Zhou, Q.; Li, S.; Wu, Y.; Wang, J.; Wu, D.; et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 2021, 22, e52124. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Zhao, Z.; Sun, Q.; Shao, G.; Huang, J.; Zhao, W.; Kuang, Y. LncRNA FEZF1-AS1 Promotes Multi-Drug Resistance of Gastric Cancer Cells via Upregulating ATG5. Front. Cell Dev. Biol. 2021, 9, 749129. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, B.; Gao, J.; Huang, Y.; Wang, C. viaLncRNA SLCO4A1-AS1 Accelerates Growth and Metastasis of Gastric Cancer Regulation of the miR-149/XIAP Axis. Front. Oncol. 2021, 11, 683256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zhang, T.; Su, L.; Liu, B.; Zhu, Z.; Li, C. LncRNA MALAT1 promotes gastric cancer progression via inhibiting autophagic flux and inducing fibroblast activation. Cell Death Dis. 2021, 12, 368. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, X.; Li, J.; Qiu, S.; Zhao, H.; Wang, Z.; Zheng, Y. LncRNA HOXC-AS1 promotes nasopharyngeal carcinoma (NPC) progression by sponging miR-4651 and subsequently upregulating FOXO6. J. Pharmacol. Sci. 2021, 147, 284–293. [Google Scholar] [CrossRef]
- Takayama, K.; Fujimura, T.; Suzuki, Y.; Inoue, S. Identification of long non-coding RNAs in advanced prostate cancer associated with androgen receptor splicing factors. Commun. Biol. 2020, 3, 393. [Google Scholar] [CrossRef]
- Zhou, C.; An, N.; Cao, C.; Wang, G. lncRNA HOXC-AS1 promotes gastric cancer via binding eIF4AIII by activating Wnt/β-catenin signaling. J. Gene Med. 2020, 22, e3202. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Lin, Z.; Zou, W.; Liu, Y.; Qian, H.; Jia, J. HOXC-AS1-MYC regulatory loop contributes to the growth and metastasis in gastric cancer. J. Exp. Clin. Cancer Res. CR 2019, 38, 502. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Dou, H.; Wang, P.; Zhao, S.; Wang, T.; Gong, W.; Zhao, J.; Li, E.; Tan, R.; Hou, Y. A novel small-molecule compound diaporine A inhibits non-small cell lung cancer growth by regulating miR-99a/mTOR signaling. Cancer Biol. Ther. 2014, 15, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Goodall, G.; Wickramasinghe, V. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y.; Wang, H.; Wu, J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988–2012) and Predictions to 2030. Gastroenterology 2021, 161, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.; Kuipers, E.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, P.; Xi, H.; Wei, B.; Chen, L.; Tang, Y. Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: A Literature Review. Front. Med. 2021, 8, 744839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cheng, J.; Li, B.; Nie, D.; Li, C.; Gui, S.; Wang, H.; Zhang, Y. Up-regulation of the expressions of MiR-149-5p and MiR-99a-3p in exosome inhibits the progress of pituitary adenomas. Cell Biol. Toxicol. 2021, 37, 633–651. [Google Scholar] [CrossRef]
- Arai, T.; Okato, A.; Yamada, Y.; Sugawara, S.; Kurozumi, A.; Kojima, S.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Regulation of NCAPG by miR-99a-3p (passenger strand) inhibits cancer cell aggressiveness and is involved in CRPC. Cancer Med. 2018, 7, 1988–2002. [Google Scholar] [CrossRef]
- Molina-Pinelo, S.; Carnero, A.; Rivera, F.; Estevez-Garcia, P.; Bozada, J.; Limon, M.; Benavent, M.; Gomez, J.; Pastor, M.; Chaves, M.; et al. MiR-107 and miR-99a-3p predict chemotherapy response in patients with advanced colorectal cancer. BMC Cancer 2014, 14, 656. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Pan, Y.; Wang, T.; Yao, Y.; Yuan, W.; Zhu, X.; Wang, K. MicroRNA-99a-3p/GRP94 axis affects metastatic progression of human papillary thyroid carcinoma by regulating ITGA2 expression and localization. Acta Biochim. Biophys. Sin. 2021, 53, 1650–1661. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef]
- Juurikka, K.; Butler, G.; Salo, T.; Nyberg, P.; Åström, P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 4506. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Fernández, A.; Fueyo, A.; Folgueras, A.; Garabaya, C.; Pennington, C.; Pilgrim, S.; Edwards, D.; Holliday, D.; Jones, J.; Span, P.; et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008, 68, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Varani, J.; Hattori, Y.; Chi, Y.; Schmidt, T.; Perone, P.; Zeigler, M.; Fader, D.; Johnson, T. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: Comparison with normal skin. Br. J. Cancer 2000, 82, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Giambernardi, T.; Sakaguchi, A.; Gluhak, J.; Pavlin, D.; Troyer, D.; Das, G.; Rodeck, U.; Klebe, R. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2001, 20, 577–587. [Google Scholar] [CrossRef]
- Korpi, J.T.; Kervinen, V.; Maklin, H.; Vaananen, A.; Lahtinen, M.; Laara, E.; Ristimaki, A.; Thomas, G.; Ylipalosaari, M.; Astrom, P.; et al. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br. J. Cancer 2008, 98, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Luo, M.; Chen, J.; Dang, Y.; Chen, G.; Li, L.; Zeng, J.; Lu, Y.; Yang, J. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 2016, 374, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, A.; Hagström, J.; Mustonen, H.; Kokkola, A.; Tervahartiala, T.; Sorsa, T.; Böckelman, C.; Haglund, C. Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2018, 40, 1010428318799266. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, J.; Jin, L.; Jiang, Y. Polymorphisms in matrix metalloproteinases 2, 3, and 8 increase recurrence and mortality risk by regulating enzyme activity in gastric adenocarcinoma. Oncotarget 2017, 8, 105971–105983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuijper, S.; Turner, C.J.; Adams, R.H. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc. Med. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Tanaka, M.; Sasaki, K.; Kamata, R.; Sakai, R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 2007, 120, 2179–2189. [Google Scholar] [CrossRef] [Green Version]
- Gharbavi, M.; Johari, B.; Rismani, E.; Mousazadeh, N.; Taromchi, A.; Sharafi, A. NANOG Decoy Oligodeoxynucleotide-Encapsulated Niosomes Nanocarriers: A Promising Approach to Suppress the Metastatic Properties of U87 Human Glioblastoma Multiforme Cells. ACS Chem. Neurosci. 2020, 11, 4499–4515. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, X.; Yang, Y.; Luo, J.; Ren, X.; Yuan, J.; Tong, Q. LncRNA HOXC-AS1 Sponges miR-99a-3p and Upregulates MMP8, Ultimately Promoting Gastric Cancer. Cancers 2022, 14, 3534. https://doi.org/10.3390/cancers14143534
Jiang Y, Li X, Yang Y, Luo J, Ren X, Yuan J, Tong Q. LncRNA HOXC-AS1 Sponges miR-99a-3p and Upregulates MMP8, Ultimately Promoting Gastric Cancer. Cancers. 2022; 14(14):3534. https://doi.org/10.3390/cancers14143534
Chicago/Turabian StyleJiang, Yue, Xiangpan Li, Yu Yang, Jiajun Luo, Xunshan Ren, Jingwen Yuan, and Qiang Tong. 2022. "LncRNA HOXC-AS1 Sponges miR-99a-3p and Upregulates MMP8, Ultimately Promoting Gastric Cancer" Cancers 14, no. 14: 3534. https://doi.org/10.3390/cancers14143534





