Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy
Abstract
Simple Summary
Abstract
1. Cellular Metabolism of Normal and Cancerous Cells
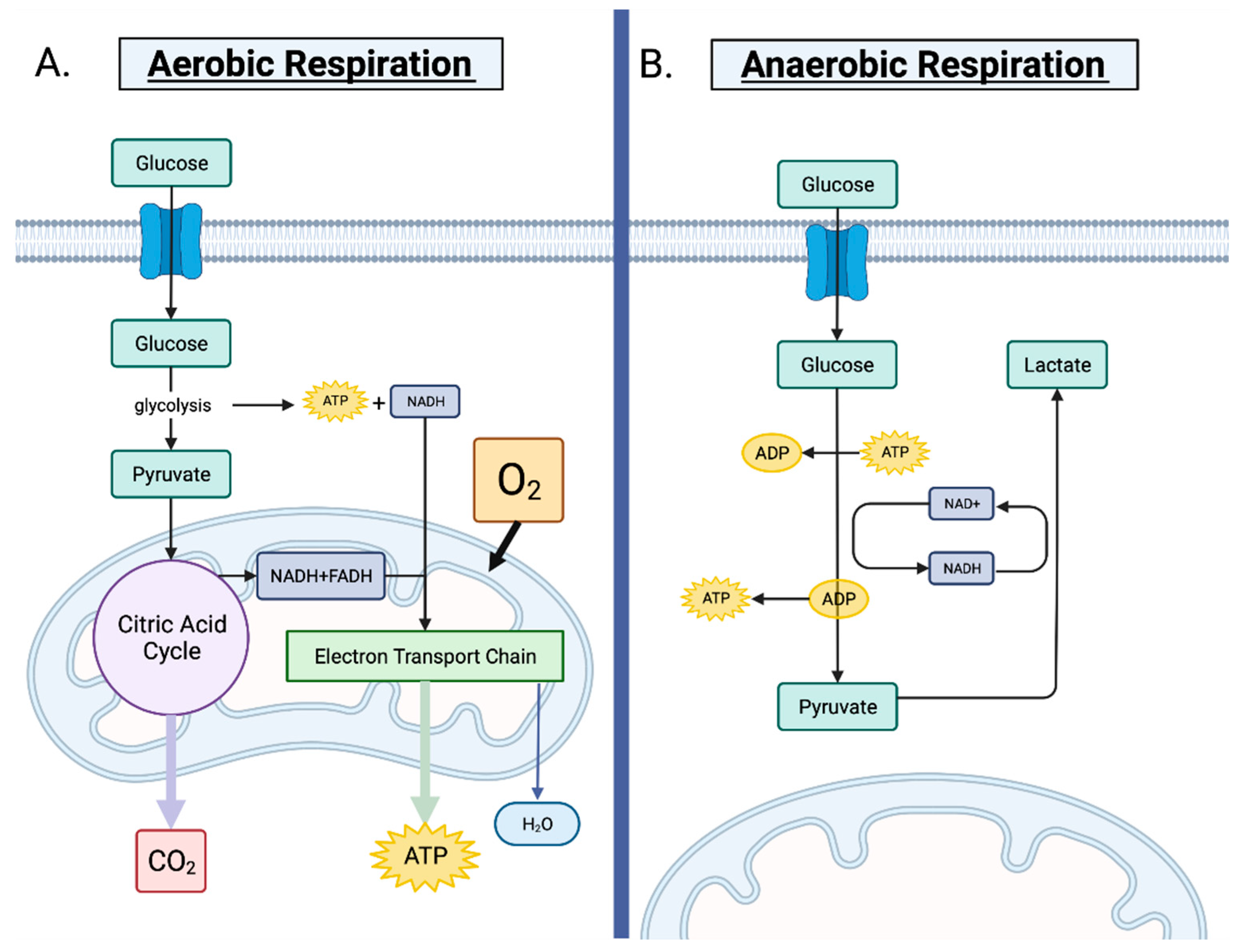
1.1. Cellular Metabolism in Normal Non-Cancerous Cells
1.2. The Role of Metabolism in Tumorigenesis
2. The Mevalonate Pathway
2.1. Regulation of Metabolic Reprograming by Tumor Suppressor and Proto-Oncogenic Genes
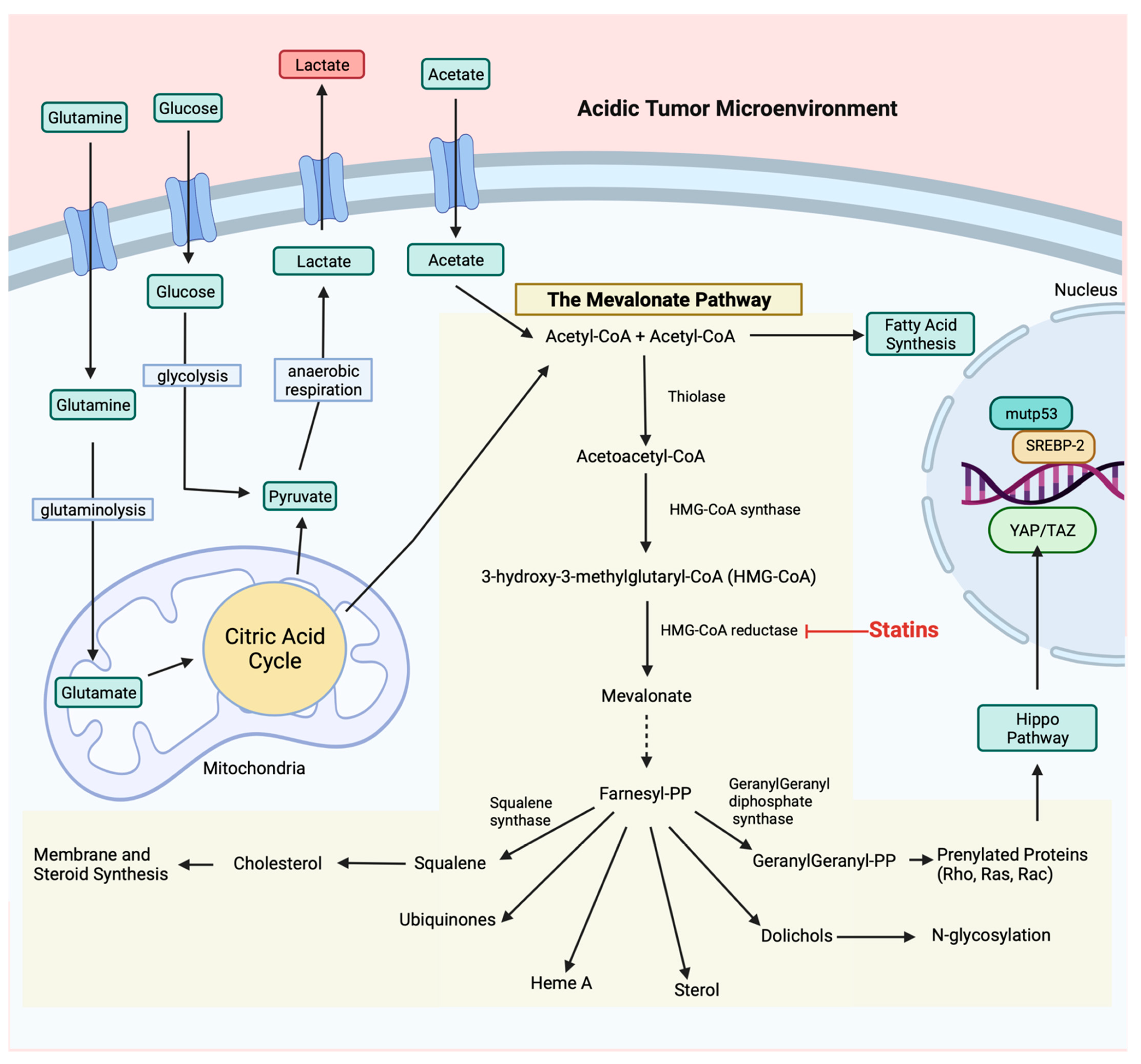
2.2. Mevalonate Pathway
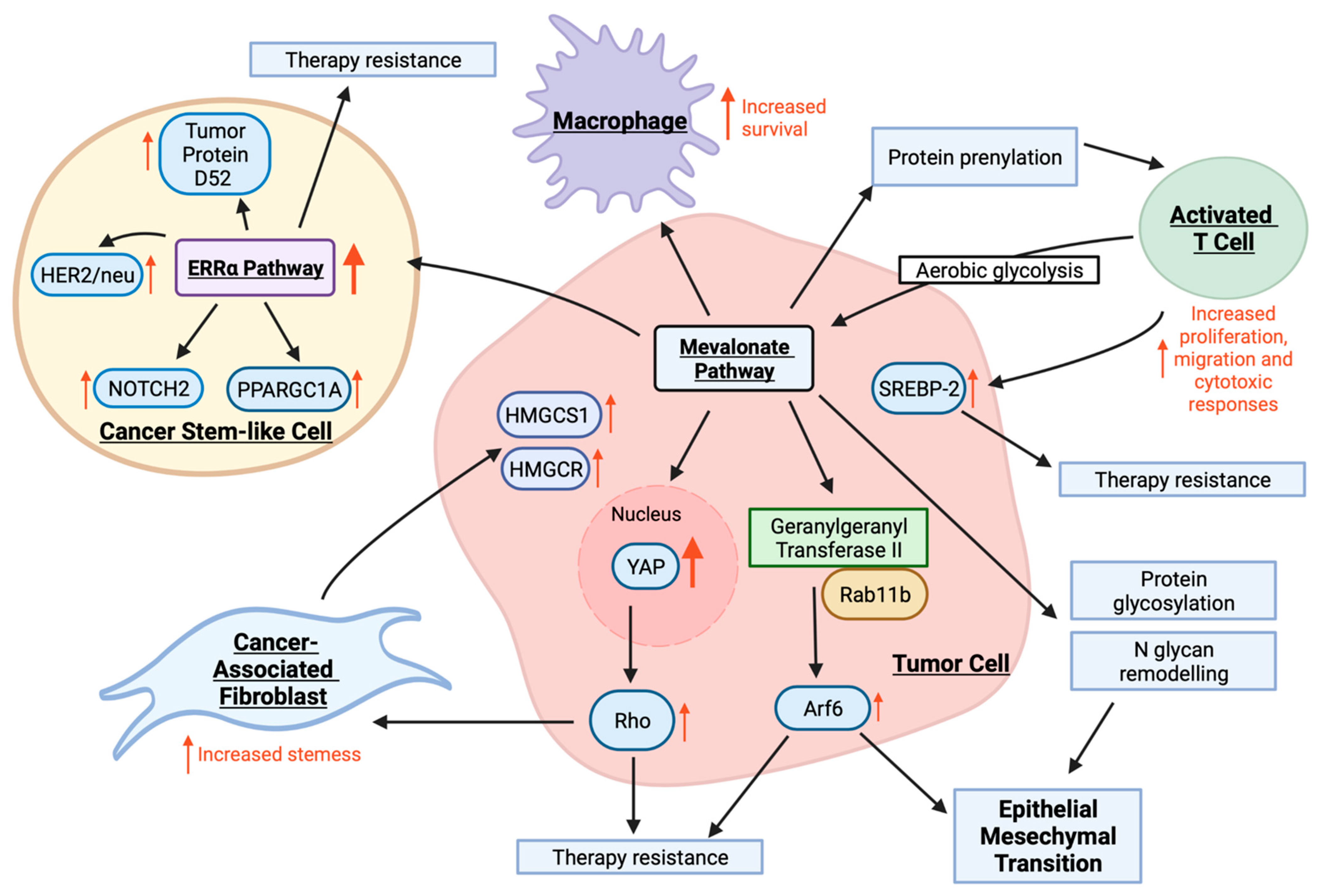
2.3. Mevalonate Pathway in the Tumor Microenvironment
2.4. The Mevalonate Pathway and Therapy Resistance
3. Targeting the Mevalonate Pathway
3.1. Statin Drugs
3.2. Pre-Clinical In Vitro and In Vivo Studies
3.3. Statins and Cancer Incidence and Mortality
3.4. Statins and Current Clinical Trials
4. Considerations When Targeting Cancer Metabolism with Statins
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reiter, R.J.; Sharma, R.; Ma, Q. Switching Diseased Cells from Cytosolic Aerobic Glycolysis to Mitochondrial Oxidative Phosphorylation: A Metabolic Rhythm Regulated by Melatonin? J. Pineal Res. 2021, 70, e12677. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Varacallo, M. Biochemistry, Glycolysis; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, Anaerobic Glycolysis; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2020, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- DE LA Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agnihotri, N.; Kumar, S. Targeting fuel pocket of cancer cell metabolism: A focus on glutaminolysis. Biochem. Pharmacol. 2022, 198, 114943. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Semba, H.; Takeda, N.; Isagawa, T.; Sugiura, Y.; Honda, K.; Wake, M.; Miyazawa, H.; Yamaguchi, Y.; Miura, M.; Jenkins, D.M.R.; et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016, 7, 11635. [Google Scholar] [CrossRef]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet. Med. Int. 2020, 2020, 5346483. [Google Scholar] [CrossRef]
- Lucantoni, F.; Salvucci, M.; Düssmann, H.; Lindner, A.U.; Lambrechts, D.; Prehn, J.H.M. BCL(X)L and BCL2 increase the metabolic fitness of breast cancer cells: A single-cell imaging study. Cell Death Differ. 2020, 28, 1512–1531. [Google Scholar] [CrossRef]
- Pereira, M.; Matuszewska, K.; Jamieson, C.; Petrik, J. Characterizing Endocrine Status, Tumor Hypoxia and Immunogenicity for Therapy Success in Epithelial Ovarian Cancer. Front. Endocrinol. 2021, 12, 772349. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Guo, F.; Li, Q.; Luo, H.; Shu, Y.; Shen, Y.; Gan, J.; Xu, L.; Yang, H. Mitochondrial UQCC3 Modulates Hypoxia Adaptation by Orchestrating OXPHOS and Glycolysis in Hepatocellular Carcinoma. Cell Rep. 2020, 33, 108340. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Marbaniang, C.; Kma, L. Dysregulation of Glucose Metabolism by Oncogenes and Tumor Suppressors in Cancer Cells. Asian Pac. J. Cancer Prev. 2018, 19, 2377–2390. [Google Scholar]
- Wang, W.; Bai, L.; Li, W.; Cui, J. The Lipid Metabolic Landscape of Cancers and New Therapeutic Perspectives. Front. Oncol. 2020, 10, 605154. [Google Scholar] [CrossRef] [PubMed]
- Sellers, W.R.; Novitch, B.G.; Miyake, S.; Heith, A.; Otterson, G.A.; Kaye, F.J.; Lassar, A.B.; Kaelin, W.G. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998, 12, 95–106. [Google Scholar] [CrossRef]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a Putative Protein Tyrosine Phosphatase Gene Mutated in Human Brain, Breast, and Prostate Cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef]
- Smith, S.; Easton, D.; Evans, D.; Ponder, B. Allele losses in the region 17q12–21 in familial breast and ovarian cancer involve the wild–type chromosome. Nat. Genet. 1992, 2, 128–131. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, N.; Murugan, A.K.; Grieco, M. Kirsten Ras* oncogene: Significance of its discovery in human cancer research. Oncotarget 2016, 7, 46717–46733. [Google Scholar] [CrossRef] [PubMed]
- Malynn, B.A.; de Alboran, I.M.; O’Hagan, R.C.; Bronson, R.; Davidson, L.; DePinho, R.A.; Alt, F.W. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000, 14, 1390–1399. [Google Scholar] [CrossRef]
- Gray, A.; Dull, T.J.; Ullrich, A.; Gray, T.J.D.A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature 1983, 303, 722–725. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Raj, S.; DePamphilis, M.L. Developmental Acquisition of p53 Functions. Genes 2021, 12, 1675. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2018, 26, 199–212. [Google Scholar] [CrossRef]
- Song, W.; Liu, Z.; Wang, K.; Tan, K.; Zhao, A.; Li, X.; Yuan, Y.; Yang, Z. Pyroptosis-related genes regulate proliferation and invasion of pancreatic cancer and serve as the prognostic signature for modeling patient survival. Discov. Oncol. 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Parrales, A.; Ranjan, A.; Iyer, S.; Padhye, S.; Weir, S.J.; Roy, A.; Iwakuma, T. DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat. Cell Biol. 2016, 18, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Soriano, O.; Alcón-Pérez, M.; Vicente-Manzanares, M.; Castellano, E. The Crossroads between RAS and RHO Signaling Pathways in Cellular Transformation, Motility and Contraction. Genes 2021, 12, 819. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Shao, J.; Zou, Z.; Qiu, X.; Wang, E.; Wu, G. Glucose Transporter 1 Promotes the Malignant Phenotype of Non-Small Cell Lung Cancer through Integrin β1/Src/FAK Signaling. J. Cancer 2019, 10, 4989–4997. [Google Scholar] [CrossRef]
- Houde, V.P.; Donzelli, S.; Sacconi, A.; Galic, S.; Hammill, J.A.; Bramson, J.L.; Foster, R.A.; Tsakiridis, T.; Kemp, B.E.; Grasso, G.; et al. AMPK β1 reduces tumor progression and improves survival in p53 null mice. Mol. Oncol. 2017, 11, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, J.B.; Virtanen, C.; Osz, K.; Revay, T.; Hardy, D.; Shepherd, T.; DiMattia, G.; Petrik, J. Ovarian tumour growth is characterized by mevalonate pathway gene signature in an orthotopic, syngeneic model of epithelial ovarian cancer. Oncotarget 2016, 7, 47343–47365. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front. Endocrinol. 2019, 9, 807. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Liu, S.; Cao, Q. Cholesterol promotes the migration and invasion of renal carcinoma cells by regulating the KLF5/miR-27a/FBXW7 pathway. Biochem. Biophys. Res. Commun. 2018, 502, 69–75. [Google Scholar] [CrossRef]
- Jeong, A.; Suazo, K.F.; Wood, W.G.; Distefano, M.D.; Li, L. Isoprenoids and protein prenylation: Implications in the pathogenesis and therapeutic intervention of Alzheimer’s disease. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 279–310. [Google Scholar] [CrossRef] [PubMed]
- Toma-Fukai, S.; Shimizu, T. Structural Insights into the Regulation Mechanism of Small GTPases by GEFs. Molecules 2019, 24, 3308. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K.-L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Di Agostino, S.; Sorrentino, G.; Ingallina, E.; Valenti, F.; Ferraiuolo, M.; Bicciato, S.; Piazza, S.; Strano, S.; Del Sal, G.; Blandino, G. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2015, 17, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Peng, K.; Zhu, J.; Wang, L.; Han, T.; Sun, A.; Shao, G.; Liu, H.; Yang, W.; Lin, Q. Geranylgeranylation promotes proliferation, migration and invasion of gastric cancer cells through the YAP signaling pathway. Am. J. Transl. Res. 2020, 12, 5296–5307. [Google Scholar]
- Xiao, Y.; Liu, Q.; Peng, N.; Li, Y.; Qiu, D.; Yang, T.; Kang, R.; Usmani, A.; Amadasu, E.; Borlongan, C.V.; et al. Lovastatin Inhibits RhoA to Suppress Canonical Wnt/β-Catenin Signaling and Alternative Wnt-YAP/TAZ Signaling in Colon Cancer. Cell Transplant. 2022, 31, 09636897221075749. [Google Scholar] [CrossRef]
- Ngai, D.; Mohabeer, A.L.; Mao, A.; Lino, M.; Bendeck, M.P. Stiffness-responsive feedback autoregulation of DDR1 expression is mediated by a DDR1-YAP/TAZ axis. Matrix Biol. 2022, 110, 129–140. [Google Scholar] [CrossRef]
- Zindel, D.; Mensat, P.; Vol, C.; Homayed, Z.; Charrier-Savournin, F.; Trinquet, E.; Banères, J.; Pin, J.; Pannequin, J.; Roux, T.; et al. G protein-coupled receptors can control the Hippo/YAP pathway through Gq signaling. FASEB J. 2021, 35, e21668. [Google Scholar] [CrossRef]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef]
- Cárdenas-León, C.G.; Klaas, M.; Mäemets-Allas, K.; Arak, T.; Eller, M.; Jaks, V. Olfactomedin 4 regulates migration and proliferation of immortalized non-transformed keratinocytes through modulation of the cell cycle machinery and actin cytoskeleton remodelling. Exp. Cell Res. 2022, 415, 113111. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin Use and Reduced Cancer-Related Mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.H.; Wang, W.; Yeung, W.; Deng, Y.; Yuan, P.; Mak, K.K.-L. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 2014, 33, 4857–4866. [Google Scholar] [CrossRef] [PubMed]
- Na Kim, Y.; Choe, S.R.; Cho, K.H.; Cho, D.Y.; Kang, J.; Park, C.G.; Lee, H.Y. Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Exp. Mol. Med. 2017, 49, e296. [Google Scholar] [CrossRef]
- Du, X.; Ou, Y.; Zhang, M.; Li, K.; Huang, W.; Jiang, D. The mevalonate pathway promotes the metastasis of osteosarcoma by regulating YAP1 activity via RhoA. Genes Dis. 2022, 9, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Yu, R.; Longo, J.; van Leeuwen, J.E.; Zhang, C.; Branchard, E.; Elbaz, M.; Cescon, D.W.; Drake, R.R.; Dennis, J.W.; Penn, L.Z. Mevalonate Pathway Inhibition Slows Breast Cancer Metastasis via Reduced N-glycosylation Abundance and Branching. Cancer Res. 2021, 81, 2625–2635. [Google Scholar] [CrossRef]
- Brindisi, M.; Fiorillo, M.; Frattaruolo, L.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Cholesterol and Mevalonate: Two Metabolites Involved in Breast Cancer Progression and Drug Resistance through the ERRα Pathway. Cells 2020, 9, 1819. [Google Scholar] [CrossRef]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Pariti, R.K.R.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, T.-S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Wang, T.; Guo, Q.; Xi, T.; Zheng, L. Emerging agents that target signaling pathways in cancer stem cells. J. Hematol. Oncol. 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, M.; Zheng, Y. Role of Rho GTPases in stem cell regulation. Biochem. Soc. Trans. 2021, 49, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Z.; Wu, Q.; Prager, B.C.; Mack, S.C.; Yang, K.; Kim, L.J.; Gimple, R.C.; Shi, Y.; Lai, S.; et al. MYC-Regulated Mevalonate Metabolism Maintains Brain Tumor–Initiating Cells. Cancer Res. 2017, 77, 4947–4960. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, Y.S.; Han, Y. Simvastatin Suppresses Self-Renewal of Mouse Embryonic Stem Cells by Inhibiting RhoA Geranylgeranylation. Stem Cells 2007, 25, 1654–1663. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ashok, V.; Mohanty, S.; Das, A.; Das, S.; Kumar, S.; Sen, S.; Purwar, R. Blockade of Rho-associated protein kinase (ROCK) inhibits the contractility and invasion potential of cancer stem like cells. Oncotarget 2017, 8, 21418–21428. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Monville, F.; Wicinski, J.; Cabaud, O.; Cervera, N.; Josselin, E.; Finetti, P.; Guille, A.; Larderet, G.; Viens, P.; et al. Mevalonate Metabolism Regulates Basal Breast Cancer Stem Cells and Is a Potential Therapeutic Target. Stem Cells 2012, 30, 1327–1337. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wang, H.; Zhang, Y.; Mei, L.; Fang, X.; Zhang, X.; Zhang, F.; Chen, H.; Liu, Y.; et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl. Acad. Sci. USA 2013, 111, E89–E98. [Google Scholar] [CrossRef]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sørensen, H.T.; Lash, T.L. Statin Prescriptions and Breast Cancer Recurrence Risk: A Danish Nationwide Prospective Cohort Study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532. [Google Scholar] [CrossRef]
- Yu, T.; Yang, G.; Hou, Y.; Tang, X.; Wu, C.; Wu, X.-A.; Guo, L.; Zhu, Q.; Luo, H.; Du, Y.-E.; et al. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene 2016, 36, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Neuwirt, H.; Bouchal, J.; Kharaishvili, G.; Ploner, C.; Jöhrer, K.; Pitterl, F.; Weber, A.; Klocker, H.; Eder, I.E. Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun. Signal. 2020, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Ashida, S.; Kawada, C.; Inoue, K. Stromal regulation of prostate cancer cell growth by mevalonate pathway enzymes HMGCS1 and HMGCR. Oncol. Lett. 2017, 14, 6533–6542. [Google Scholar] [CrossRef] [PubMed]
- Thurnher, M.; Gruenbacher, G. T lymphocyte regulation by mevalonate metabolism. Sci. Signal. 2015, 8, re4. [Google Scholar] [CrossRef] [PubMed]
- Gruenbacher, G.; Thurnher, M. Mevalonate metabolism governs cancer immune surveillance. OncoImmunology 2017, 6, e1342917. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR Signaling Couples Sterol Metabolism to Proliferation in the Acquired Immune Response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Murthy, S.; Ryan, A.J.; Carter, A.B. Modulation of the Mevalonate Pathway by Akt Regulates Macrophage Survival and Development of Pulmonary Fibrosis. J. Biol. Chem. 2014, 289, 36204–36219. [Google Scholar] [CrossRef]
- Feingold, K.R.; Shigenaga, J.K.; Kazemi, M.R.; McDonald, C.M.; Patzek, S.M.; Cross, A.S.; Moser, A.; Grunfeld, C. Mechanisms of triglyceride accumulation in activated macrophages. J. Leukoc. Biol. 2012, 92, 829–839. [Google Scholar] [CrossRef]
- Na, Y.R.; Gu, G.J.; Jung, D.; Kim, Y.W.; Na, J.; Woo, J.S.; Cho, J.Y.; Youn, H.; Seok, S.H. GM-CSF Induces Inflammatory Macrophages by Regulating Glycolysis and Lipid Metabolism. J. Immunol. 2016, 197, 4101–4109. [Google Scholar] [CrossRef]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006, 6, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, L.; Wang, D.; Jiang, S.; Cao, D.; Zhao, Z.; Huang, M.; Jin, J. Lipidomics reveals that sustained SREBP-1-dependent lipogenesis is a key mediator of gefitinib-acquired resistance in EGFR-mutant lung cancer. Cell Death Discov. 2021, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Dehairs, J.; Rambow, F.; Rogiers, A.; Nittner, D.; Derua, R.; Vanderhoydonc, F.; Duarte, J.A.G.; Bosisio, F.; Van Den Eynde, K.; et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat. Commun. 2018, 9, 2500. [Google Scholar] [CrossRef]
- Hong, X.; Roh, W.; Sullivan, R.J.; Wong, K.H.K.; Wittner, B.S.; Guo, H.; Dubash, T.D.; Sade-Feldman, M.; Wesley, B.; Horwitz, E.; et al. The Lipogenic Regulator SREBP2 Induces Transferrin in Circulating Melanoma Cells and Suppresses Ferroptosis. Cancer Discov. 2021, 11, 678–695. [Google Scholar] [CrossRef]
- Sethunath, V.; Hu, H.; De Angelis, C.; Veeraraghavan, J.; Qin, L.; Wang, N.; Simon, L.M.; Wang, T.; Fu, X.; Nardone, A.; et al. Targeting the Mevalonate Pathway to Overcome Acquired Anti-HER2 Treatment Resistance in Breast Cancer. Mol. Cancer Res. 2019, 17, 2318–2330. [Google Scholar] [CrossRef]
- Hashimoto, A.; Oikawa, T.; Hashimoto, S.; Sugino, H.; Yoshikawa, A.; Otsuka, Y.; Handa, H.; Onodera, Y.; Nam, J.-M.; Oneyama, C.; et al. P53-and mevalonate pathway–driven malignancies require Arf6 for metastasis and drug resistance. J. Cell Biol. 2016, 213, 81–95. [Google Scholar] [CrossRef]
- Sharma, S.; Santiskulvong, C.; Rao, J.; Gimzewski, J.K.; Dorigo, O. The role of Rho GTPase in cell stiffness and cisplatin resistance in ovarian cancer cells. Integr. Biol. 2014, 6, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Calvayrac, O.; Mazières, J.; Figarol, S.; Marty-Detraves, C.; Raymond-Letron, I.; Bousquet, E.; Farella, M.; Clermont-Taranchon, E.; Milia, J.; Rouquette, I.; et al. The RAS-related GTP ase RHOB confers resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer via an AKT-dependent mechanism. EMBO Mol. Med. 2016, 9, 238–250. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Zhou, W.; Chen, T.; Wu, Q.; Chutturghoon, V.K.; Lin, B.; Geng, L.; Yang, Z.; Zhou, L.; et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019, 19, 179. [Google Scholar] [CrossRef]
- Li, W.; Cao, Y.; Xu, J.; Wang, Y.; Li, W.; Wang, Q.; Hu, Z.; Hao, Y.; Hu, L.; Sun, Y.; et al. YAP transcriptionally regulates COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor, overcomes drug resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2017, 36, 144. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kashima, H.; Rahmanto, Y.S.; Banno, K.; Yu, Y.; Matoba, Y.; Watanabe, K.; Iijima, M.; Takeda, T.; Kunitomi, H.; et al. Drug repositioning of mevalonate pathway inhibitors as antitumor agents for ovarian cancer. Oncotarget 2017, 8, 72147–72156. [Google Scholar] [CrossRef] [PubMed]
- Srivichit, B.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Impacts of bisphosphonates on the bone and its surrounding tissues: Mechanistic insights into medication-related osteonecrosis of the jaw. Arch. Toxicol. 2022, 96, 1227–1255. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.A.; Rogers, M.J. Cellular and Molecular Actions of Bisphosphonates. In Bone Cancer: Bone Sarcomas and Bone Metastases—From Bench to Bedside; Heymann, D., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 921–942. [Google Scholar]
- Conroy, M.; Cowzer, D.; Kolch, W.; Duffy, A.G. Emerging RAS-directed therapies for cancer. Cancer Drug Resist. 2021, 4, 543–558. [Google Scholar] [CrossRef]
- Karasic, T.B.; Chiorean, E.G.; Sebti, S.M.; O’Dwyer, P.J. A Phase I Study of GGTI-2418 (Geranylgeranyl Transferase I Inhibitor) in Patients with Advanced Solid Tumors. Target. Oncol. 2019, 14, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.; Tam, L.C.S.; Hale, A.B.; Cioroch, M.; Douglas, G.; Agkatsev, S.; Hibbitt, O.; Mason, J.; Holt-Martyn, J.; Bataille, C.J.R.; et al. A Genomic DNA Reporter Screen Identifies Squalene Synthase Inhibitors That Act Cooperatively with Statins to Upregulate the Low-Density Lipoprotein Receptor. J. Pharmacol. Exp. Ther. 2017, 361, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Figorilli, F.; Mannarino, M.R.; Bianconi, V.; Pirro, M. Cholesterol-Lowering Therapy in Patients at Low-to-Moderate Cardiovascular Risk. High Blood Press. Cardiovasc. Prev. 2022, 29, 327–336. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Li, H.; Tang, J.-J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.-K.; Shi, X.-J.; Cui, H.-W.; Tang, J.; et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.L.; Ruan, X.Z.; Liu, B.C. Dysregulation of the Low-Density Lipoprotein Receptor Pathway Is Involved in Lipid Disorder-Mediated Organ Injury. Int. J. Biol. Sci. 2016, 12, 569–579. [Google Scholar] [CrossRef]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef]
- Harrington, R.A. Statins—Almost 30 Years of Use in the United States and Still Not Quite There. JAMA Cardiol. 2017, 2, 66. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, I.R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arter. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Furberg, C.D.; Pitt, B. Withdrawal of cerivastatin from the world market. Curr. Control. Trials Cardiovasc. Med. 2001, 2, 205–207. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 491. [Google Scholar] [CrossRef]
- Murphy, C.; Deplazes, E.; Cranfield, C.G.; Garcia, A. The Role of Structure and Biophysical Properties in the Pleiotropic Effects of Statins. Int. J. Mol. Sci. 2020, 21, 8745. [Google Scholar] [CrossRef]
- Fong, C.W. Statins in therapy: Understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur. J. Med. Chem. 2014, 85, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Cassagnol, M. Antilipemic Agents, HMG-CoA Reductase Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Liu, C.; Liu, Q.; Xiao, X. Effectiveness and safety of combinational therapy compared with intensified statin monotherapy in patients with coronary heart disease. Exp. Ther. Med. 2018, 15, 4683–4688. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.M.; Fang, Z.; Sun, W.; Clark, L.H.; Stine, J.E.; Tran, A.; Sullivan, S.A.; Gilliam, T.P.; Zhou, C.; Bae-Jump, V.L. Atorvastatin exhibits anti-tumorigenic and anti-metastatic effects in ovarian cancer in vitro. Am. J. Cancer Res. 2017, 7, 2478–2490. [Google Scholar]
- Hu, M.-B.; Zhang, J.-W.; Gao, J.-B.; Qi, Y.-W.; Gao, Y.; Xu, L.; Ma, Y.; Wei, Z.-Z. Atorvastatin induces autophagy in MDA-MB-231 breast cancer cells. Ultrastruct. Pathol. 2018, 42, 409–415. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Ghasemi, F.; Flahatgar, F.; Rahmanian, N.; Ghasemi, A.; Asgarian-Omran, H. Atorvastatin sensitizes breast and lung cancer cells to ionizing radiation. Iran. J. Pharm. Res. 2020, 19, 80–88. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, X.-X.; Avila, G.E.; Huang, M.-T.; Liu, Y.; Patel, J.; Kong, A.N.T.; Paulino, R.; Shih, W.J.; Lin, Y.; et al. Atorvastatin and Celecoxib Inhibit Prostate PC-3 Tumors in Immunodeficient Mice. Clin. Cancer Res. 2007, 13, 5480–5487. [Google Scholar] [CrossRef]
- Taylor-Harding, B.; Orsulic, S.; Karlan, B.Y.; Li, A.J. Fluvastatin and cisplatin demonstrate synergistic cytotoxicity in epithelial ovarian cancer cells. Gynecol. Oncol. 2010, 119, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Isono, M.; Miyai, K.; Asano, T.; Sato, A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2019, 111, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Das, B.; Suresh, V.; Parida, D.; Minz, A.P.; Nayak, U.; Mohapatra, A.P.; Swain, R.K.; Senapati, S. Fluvastatin sensitizes pancreatic cancer cells toward radiation therapy and suppresses radiation- and/or TGF-β-induced tumor-associated fibrosis. Lab. Investig. 2021, 102, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, E.; Yie, T.-A.; Rom, W.N. In Vitro Mechanisms of Lovastatin on Lung Cancer Cell Lines as a Potential Chemopreventive Agent. Lung 2007, 186, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, A.; Kikuchi, N.; Osada, R.; Wang, C.; Hayashi, A.; Nikaido, T.; Konishi, I. Overexpression of RhoA enhances peritoneal dissemination: RhoA suppression with Lovastatin may be useful for ovarian cancer. Cancer Sci. 2008, 99, 2532–2539. [Google Scholar] [CrossRef]
- Alonso, D.F.; Farina, H.G.; Skilton, G.; Gabri, M.R.; De Lorenzo, M.S.; Gomez, D.E. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res. Treat. 1998, 50, 83–93. [Google Scholar] [CrossRef]
- Zhang, W.-J.; You, H.-Y.; Xie, X.-M.; Zheng, Z.-H.; Zhu, H.-L.; Jiang, F.-Z. Pitavastatin suppressed liver cancer cells in vitro and in vivo. OncoTargets Ther. 2016, 9, 5383–5388. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, Y.-C.; Lin, C.-C.; Hsieh, Y.-P.; Hsu, C.-S.; Hsieh, M.-C. Synergistic Anticancer Effects of Gemcitabine with Pitavastatin on Pancreatic Cancer Cell Line MIA PaCa-2 in vitro and in vivo. Cancer Manag. Res. 2020, 12, 4645–4665. [Google Scholar] [CrossRef]
- Hu, T.; Shen, H.; Huang, H.; Yang, Z.; Zhou, Y.; Zhao, G. Cholesterol-lowering drug pitavastatin targets lung cancer and angiogenesis via suppressing prenylation-dependent Ras/Raf/MEK and PI3K/Akt/mTOR signaling. Anti-Cancer Drugs 2020, 31, 377–384. [Google Scholar] [CrossRef]
- Hijona, E.; Banales, J.M.; Hijona, L.; Medina, J.F.; Arenas, J.; Herreros-Villanueva, M.; Aldazabal, P.; Bujanda, L. Pravastatin inhibits cell proliferation and increased MAT1A expression in hepatocarcinoma cells and in vivo models. Cancer Cell Int. 2012, 12, 5. [Google Scholar] [CrossRef]
- Taras, D.; Blanc, J.-F.; Rullier, A.; Dugot-Senant, N.; Laurendeau, I.; Vidaud, M.; Rosenbaum, J. Pravastatin reduces lung metastasis of rat hepatocellular carcinoma via a coordinated decrease of MMP expression and activity. J. Hepatol. 2007, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.; Cheng, C.-C.; Lin, H.-C.; Luo, T.-Y.; Chang, J.; Ho, A.-S. Pravastatin inhibits tumor growth through elevating the levels of apolipoprotein A1. Adv. Dig. Med. 2015, 3, 3–10. [Google Scholar] [CrossRef][Green Version]
- Deezagi, A.; Safari, N. Rosuvastatin inhibit spheroid formation and epithelial–mesenchymal transition (EMT) in prostate cancer PC-3 cell line. Mol. Biol. Rep. 2020, 47, 8727–8737. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, N.D.; Gulcelik, N.E.; Kaymaz, F.F.; Sarisozen, C.; Vural, I.; Bodur, E.; Canpinar, H.; Usman, A.; Asan, E. Rosuvastatin induces apoptosis in cultured human papillary thyroid cancer cells. J. Endocrinol. 2011, 210, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Yu, Z.; Gao, X.; Gong, J.; Fan, L.; Liu, F. Simvastatin induces breast cancer cell death through oxidative stress up-regulating miR-140-5p. Aging 2019, 11, 3198–3219. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Xu, J.; Ma, L. Simvastatin enhances chemotherapy in cervical cancer via inhibition of multiple prenylation-dependent GTP ases-regulated pathways. Fundam. Clin. Pharmacol. 2019, 34, 32–40. [Google Scholar] [CrossRef]
- Kim, J.S.; Turbov, J.; Rosales, R.; Thaete, L.G.; Rodriguez, G.C. Combination simvastatin and metformin synergistically inhibits endometrial cancer cell growth. Gynecol. Oncol. 2019, 154, 432–440. [Google Scholar] [CrossRef]
- Oechsle, C.M.; Showalter, L.E.; Novak, C.M.; Czerniecki, B.J.; Koski, G.K. Statin Drugs Plus Th1 Cytokines Potentiate Apoptosis and Ras Delocalization in Human Breast Cancer Lines and Combine with Dendritic Cell-Based Immunotherapy to Suppress Tumor Growth in a Mouse Model of HER-2pos Disease. Vaccines 2020, 8, 72. [Google Scholar] [CrossRef]
- Gbelcová, H.; Rimpelová, S.; Ruml, T.; Fenclová, M.; Kosek, V.; Hajšlová, J.; Strnad, H.; Kolář, M.; Vítek, L. Variability in statin-induced changes in gene expression profiles of pancreatic cancer. Sci. Rep. 2017, 7, 44219. [Google Scholar] [CrossRef]
- Beckwitt, C.; Clark, A.M.; Ma, B.; Whaley, D.; Oltvai, Z.N.; Wells, A. Statins attenuate outgrowth of breast cancer metastases. Br. J. Cancer 2018, 119, 1094–1105. [Google Scholar] [CrossRef]
- Ren, Q.-W.; Yu, S.-Y.; Teng, T.-H.K.; Li, X.; Cheung, K.-S.; Wu, M.-Z.; Li, H.-L.; Wong, P.-F.; Tse, H.-F.; Lam, C.S.P.; et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur. Hear. J. 2021, 42, 3049–3059. [Google Scholar] [CrossRef]
- Chen, P.-H.; Jhou, H.-J.; Chung, C.-H.; Lee, C.-H.; Wu, Y.-Y.; Chang, W.-C.; Chien, W.-C.; Chang, P.-Y. The Effect of Statins in Cancer Risk Reduction in Patients on Dialysis: A Population-Based Case-Control Study. J. Clin. Med. 2021, 10, 5602. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Morimoto, T.; Ogawa, H.; Soejima, H.; Matsumoto, C.; Sakuma, M.; Nakayama, M.; Doi, N.; Jinnouchi, H.; Waki, M.; et al. Association Between Statins and Cancer Incidence in Diabetes: A Cohort Study of Japanese Patients with Type 2 Diabetes. J. Gen. Intern. Med. 2020, 36, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, H.J.; Ahn, H.S. Statins and the risk of gastric, colorectal, and esophageal cancer incidence and mortality: A cohort study based on data from the Korean national health insurance claims database. J. Cancer Res. Clin. Oncol. 2022, 1–11. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, S.-M.; Shin, D.W.; Cho, M.; Cho, J.H.; Kim, J. The Associations of Aspirin, Statins, and Metformin With Lung Cancer Risk and Related Mortality: A Time-Dependent Analysis of Population-Based Nationally Representative Data. J. Thorac. Oncol. 2020, 16, 76–88. [Google Scholar] [CrossRef]
- Borgquist, S.; Tamimi, R.M.; Chen, W.Y.; Garber, J.E.; Eliassen, A.H.; Ahern, T.P. Statin Use and Breast Cancer Risk in the Nurses’ Health Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 201–206. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, H.S.; Kim, H.J. Statin use and incidence and mortality of breast and gynecology cancer: A cohort study using the National Health Insurance claims database. Int. J. Cancer 2021, 150, 1156–1165. [Google Scholar] [CrossRef]
- Saito, K.; Sato, Y.; Nakatani, E.; Kaneda, H.; Yamamoto, S.; Miyachi, Y.; Itoh, H. Statin Exposure and Pancreatic Cancer Incidence: A Japanese Regional Population-Based Cohort Study, the Shizuoka Study. Cancer Prev. Res. 2021, 14, 863–872. [Google Scholar] [CrossRef]
- Koo, H.Y.; Jeong, S.-M.; Cho, M.H.; Chun, S.; Shin, D.W.; Park, J. Population-wide impacts of aspirin, statins, and metformin use on prostate cancer incidence and mortality. Sci. Rep. 2021, 11, 16171. [Google Scholar] [CrossRef]
- Chou, C.-W.; Lin, C.-H.; Hsiao, T.-H.; Lo, C.-C.; Hsieh, C.-Y.; Huang, C.-C.; Sher, Y.-P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-S.; Chen, I.-C.; Lee, C.-P.; Huang, R.-J.; Chen, P.-C.; Tsai, Y.-H.; Yang, Y.-H. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS ONE 2017, 12, e0171137. [Google Scholar] [CrossRef]
- Borgquist, S.; Broberg, P.; Tojjar, J.; Olsson, H. Statin use and breast cancer survival—a Swedish nationwide study. BMC Cancer 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Ro, V.; Steel, L.; Carrigan, E.; Nguyen, J.; Williams, A.; So, A.; Tchou, J. Impact of long-term lipid-lowering therapy on clinical outcomes in breast cancer. Breast Cancer Res. Treat. 2019, 176, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Mc Menamin, C.; Murray, L.J.; Hughes, C.M.; Cardwell, C.R. Statin use and breast cancer survival: A nationwide cohort study in Scotland. BMC Cancer 2016, 16, 600. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Huang, D.-W.; Chen, P.-T.; Tsai, C.-F.; Lin, M.-C.; Lin, C.-C.; Wang, S.-H.; Pan, Y.-J. Association between statin use and second cancer risk in breast cancer patients: A nationwide population-based cohort study. Breast Cancer Res. Treat. 2020, 185, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Jian-Yu, E.; Graber, J.M.; Lu, S.-E.; Lin, Y.; Yao, G.L.; Tan, X.-L. Effect of Metformin and Statin Use on Survival in Pancreatic Cancer Patients: A Systematic Literature Review and Meta-analysis. Curr. Med. Chem. 2018, 25, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, S.H.; Lee, H.J.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Bang, S. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine 2016, 95, e3607. [Google Scholar] [CrossRef]
- Couttenier, A.; Lacroix, O.; Vaes, E.; Cardwell, C.; De Schutter, H.; Robert, A. Statin use is associated with improved survival in ovarian cancer: A retrospective population-based study. PLoS ONE 2017, 12, e0189233. [Google Scholar] [CrossRef]
- Harding, B.N.; Delaney, J.A.; Urban, R.R.; Weiss, N.S. Use of Statin Medications Following Diagnosis in Relation to Survival among Women with Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1127–1133. [Google Scholar] [CrossRef]
- Yulian, E.D.; Siregar, N.C.; Bajuadji. Combination of Simvastatin and FAC Improves Response to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Cancer Res. Treat. 2021, 53, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, M.A.M.; Malhotra, N.; Mukherjee, S.D.; Sanger, S.; Dhesy-Thind, S.K.; Ellis, P.; Leong, D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0209486. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kim, H.S.; Kim, J.H.; Lee, J. The Effect of Statin Added to Systemic Anticancer Therapy: A Meta-Analysis of Randomized, Controlled Trials. J. Clin. Med. 2018, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.-K.; Hu, Z.-G.; Tian, Y.-F.; Zeng, F.-J. Statin use and prognosis of lung cancer: A systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des. Dev. Ther. 2019, 13, 405–422. [Google Scholar] [CrossRef]
- Abdullah, M.I.; de Wolf, E.; Jawad, M.J.; Richardson, A. The poor design of clinical trials of statins in oncology may explain their failure—Lessons for drug repurposing. Cancer Treat. Rev. 2018, 69, 84–89. [Google Scholar] [CrossRef]
- Tutuska, K.; Parrilla-Monge, L.; Di Cesare, E.; Nemajerova, A.; Moll, U.M. Statin as anti-cancer therapy in autochthonous T-lymphomas expressing stabilized gain-of-function mutant p53 proteins. Cell Death Dis. 2020, 11, 274. [Google Scholar] [CrossRef]
- Tabuso, M.; Christian, M.; Kimani, P.K.; Gopalakrishnan, K.; Arasaradnam, R.P. KRAS Status is Associated with Metabolic Parameters in Metastatic Colorectal Cancer According to Primary Tumour Location. Pathol. Oncol. Res. 2020, 26, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.-H.; Kwon, M.; Jung, H.; Ko, E.; Kim, S.A.; Choi, Y.; Song, S.J.; Kim, S.; Lee, Y.; Kim, G.B.; et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J. Immunother. Cancer 2021, 9, e002474. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Mirzaei, A.; Rashedi, S.; Akbari, M.R.; Khatami, F.; Aghamir, S.M.K. Combined anticancer effects of simvastatin and arsenic trioxide on prostate cancer cell lines via downregulation of the VEGF and OPN isoforms genes. J. Cell. Mol. Med. 2022, 26, 2728–2740. [Google Scholar] [CrossRef]
- Valipour, E.; Ranjbar, F.E.; Mousavi, M.; Ai, J.; Malekshahi, Z.V.; Mokhberian, N.; Taghdiri-Nooshabadi, Z.; Khanmohammadi, M.; Nooshabadi, V.T. The anti-angiogenic effect of atorvastatin loaded exosomes on glioblastoma tumor cells: An in vitro 3D culture model. Microvasc. Res. 2022, 143, 104385. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-R.; Tsai, Y.-Y.; Chen, K.-J.; Yang, Y.-H.; Shih, W.-T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers 2020, 12, 2055. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2018, 35, 153–159. [Google Scholar] [CrossRef]
- Ricco, N.; Flor, A.; Wolfgeher, D.; Efimova, E.V.; Ramamurthy, A.; Appelbe, O.K.; Brinkman, J.; Truman, A.W.; Spiotto, M.T.; Kron, S.J. Mevalonate pathway activity as a determinant of radiation sensitivity in head and neck cancer. Mol. Oncol. 2019, 13, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; E, J.Y.; Lin, Y.; Rebbeck, T.R.; Lu, S.E.; Shang, M.; Kelly, W.K.; D’Amico, A.; Stein, M.N.; Zhang, L.; et al. Individual and joint effects of metformin and statins on mortality among patients with high-risk prostate cancer. Cancer Med. 2020, 9, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, C.; Lynch, L.M. An Update on Common Pharmaceuticals in the Prevention of Pancreatic Cancer. Cureus 2022, 14, e25496. [Google Scholar] [CrossRef]
- Li, S.; Saviano, A.; Erstad, D.J.; Hoshida, Y.; Fuchs, B.C.; Baumert, T.; Tanabe, K.K. Risk Factors, Pathogenesis, and Strategies for Hepatocellular Carcinoma Prevention: Emphasis on Secondary Prevention and Its Translational Challenges. J. Clin. Med. 2020, 9, 3817. [Google Scholar] [CrossRef]
- Temkin, S.M.; Bergstrom, J.; Samimi, G.; Minasian, L. Ovarian Cancer Prevention in High-risk Women. Clin. Obstet. Gynecol. 2017, 60, 738–757. [Google Scholar] [CrossRef]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast Cancer Growth Prevention by Statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef]
- Bowman, C.M.; Okochi, H.; Benet, L.Z. The Presence of a Transporter-Induced Protein Binding Shift: A New Explanation for Protein-Facilitated Uptake and Improvement for In Vitro-In Vivo Extrapolation. Drug Metab. Dispos. 2019, 47, 358–363. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, H.; Li, X.; Sun, Y. Pay attention to cardiac remodeling in cancer cachexia. Support. Care Cancer 2016, 24, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D. Cachexia as a Major Underestimated and Unmet Medical Need: Facts and Numbers. J. Cachexia. Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.R.; Becher, H.; Fassbender, K.; Chu, Q.; Baracos, V.E. Concurrent evolution of cancer cachexia and heart failure: Bilateral effects exist. J. Cachex-Sarcopenia Muscle 2014, 5, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachex-Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Emami, H.; Vucic, E.; Singh, P.; Vijayakumar, J.; Fifer, K.M.; Alon, A.; Shankar, S.S.; Farkouh, M.; Rudd, J.H.; et al. High-Dose Atorvastatin Reduces Periodontal Inflammation. J. Am. Coll. Cardiol. 2013, 62, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, S.R.; Lu, L.; Rai, S.K.; Seeger, J.D.; Zhang, Y.; Choi, H.K. Statin use and mortality in rheumatoid arthritis: A general population-based cohort study. Ann. Rheum. Dis. 2015, 75, 1315–1320. [Google Scholar] [CrossRef]
- Vidt, D.G.; Cressman, M.D.; Harris, S.; Pears, J.S.; Hutchinson, H.G. Rosuvastatin-Induced Arrest in Progression of Renal Disease. Cardiology 2004, 102, 52–60. [Google Scholar] [CrossRef]
- Glynn, R.J.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, J.A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. A Randomized Trial of Rosuvastatin in the Prevention of Venous Thromboembolism. N. Engl. J. Med. 2009, 360, 1851–1861. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Statins and Blood Coagulation. Arter. Thromb. Vasc. Biol. 2005, 25, 287–294. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Meenakshisundaram, S.; Manickam, M.; Sankaranarayanan, M. A medicinal chemistry perspective of drug repositioning: Recent advances and challenges in drug discovery. Eur. J. Med. Chem. 2020, 195, 112275. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Amare, G.G.; Meharie, B.G.; Belayneh, Y.M. A drug repositioning success: The repositioned therapeutic applications and mechanisms of action of thalidomide. J. Oncol. Pharm. Pract. 2020, 27, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Cibeira, M.T.; Rosiñol, L.; Ramiro, L.; Esteve, J.; Torrebadell, M.; Bladé, J. Long-term results of thalidomide in refractory and relapsed multiple myeloma with emphasis on response duration. Eur. J. Haematol. 2006, 77, 486–492. [Google Scholar] [CrossRef]
- Weber, D.; Rankin, K.; Gavino, M.; Delasalle, K.; Alexanian, R. Thalidomide Alone or With Dexamethasone for Previously Untreated Multiple Myeloma. J. Clin. Oncol. 2003, 21, 16–19. [Google Scholar] [CrossRef]
- Breitkreutz, I.; Anderson, K.C. Thalidomide in multiple myeloma—clinical trials and aspects of drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2008, 4, 973–985. [Google Scholar] [CrossRef]
- Zi, F.; Zi, H.; Li, Y.; He, J.; Shi, Q.; Cai, Z. Metformin and cancer: An existing drug for cancer prevention and therapy (Review). Oncol. Lett. 2017, 15, 683–690. [Google Scholar] [CrossRef]
- Tang, C.-J.; Xu, J.; Ye, H.-Y.; Wang, X.-B. Metformin prevents PFKFB3-related aerobic glycolysis from enhancing collagen synthesis in lung fibroblasts by regulating AMPK/mTOR pathway. Exp. Ther. Med. 2021, 21, 581. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
| Statin | Statin Type | Side Ring | Solubility | Transport | Half-Life (h) | Dose (mg/day) | Optimal Dosing Time |
|---|---|---|---|---|---|---|---|
| Atorvastatin | II | Pyrrole | Lipophilic | Passive | 14 | 10–80 | Any time of day |
| Fluvastatin | II | Indole | Lipophilic | Passive | 1.2 | 20–80 | Bedtime |
| Lovastatin | I | Naphthalene | Lipophilic | Passive | 3 | 10–80 | Morning and evening |
| Pitavastatin | II | Quinoline | Lipophilic | Passive | 12 | 1–4 | Any time of day |
| Pravastatin | I | Naphthalene | Hydrophilic | Active | 1.8 | 10–80 | Bedtime |
| Rosuvastatin | II | Pyrimidine | Hydrophilic | Active | 19 | 5–40 | Any time of day |
| Simvastatin | I | Naphthalene | Lipophilic | Passive | 2 | 5–40 | Evening |
| Statin | Cancer Type | Dose (mg/day) | Combination Agent | Trial Phase | Recruitment Status | Clinical Trial Number |
|---|---|---|---|---|---|---|
| Atorvastatin | Breast | 40 | Fulvestrant, Letrozole | Phase 2 | Recruiting | NCT02958852 |
| 80 | NT | Phase 3 | Recruiting | NCT04601116 | ||
| 80 | ACT/NCT | Phase 2; Phase 3 | Recruiting | NCT05103644 | ||
| 20–80 | NCT | Phase 2 | Recruiting | NCT03872388 | ||
| Colorectal | 20 | Aspirin | Phase 1 | Recruiting | NCT04379999 | |
| Not Specified | N/A | Phase 2 | Recruiting | NCT04767984 | ||
| Head and Neck | 20 | ACT/NCT, ART/NRT | Phase 3 | Not Recruiting | NCT04915183 | |
| Hepatocellular | 10 | N/A | Phase 4 | Recruiting | NCT03024684 | |
| Pancreatic | 80 | Ezetimibe/Ezetrol, Evolocumab/Repatha, ACT | Phase 1 | Recruiting | NCT04862260 | |
| Prostate | 80 | ADT | Phase 3 | Recruiting | NCT04026230 | |
| 80 | Acetylsalicylic acid | Phase 3 | Recruiting | NCT03819101 | ||
| Not Specified | Pentoxifylline, Vitamin E, ART/NRT | Phase 2 | Recruiting | NCT03830164 | ||
| Solid tumor, AML | 80 | N/A | Phase 1 | Recruiting | NCT03560882 | |
| Pitavastatin | AML, CLL | 1–4 | Venetoclax | Phase 1 | Recruiting | NCT04512105 |
| Breast | 2 | NCT | Phase 2; Phase 3 | Not Recruiting | NCT04705909 | |
| 40 | ART | Phase 3 | Recruiting | NCT04385433 | ||
| 40 | ART | Phase 2 | Recruiting | NCT04356209 | ||
| AML | 1280 | ACT/NCT | Phase 2 | Not Recruiting | NCT00840177 | |
| Rosuvastatin | Endometrial | 10 | Megestrol Acetate | Phase 2 | Recruiting | NCT04491643 |
| Ovarian | Not Specified | Enoxaparin, Thromboprophylaxis | Phase 2 | Not Recruiting | NCT03532139 | |
| Melanoma, Solid tumor | 10 | Bupropion, ACT/NCT | Phase 1 | Recruiting | NCT03864042 | |
| Solid tumor | Not Specified | Sitravatinib, Nivolumab | Phase 1 | Recruiting | NCT04887194 | |
| Simvastatin | Breast | 40 | ACT/NCT | Phase 2 | Not Recruiting | NCT02096588 |
| 20 | N/A | Phase 3 | Recruiting | NCT03971019 | ||
| 80 | HER2 Therapy | Phase 2 | Recruiting | NCT03324425 | ||
| Not Specified | N/A | Phase 2 | Not Recruiting | NCT03454529 | ||
| Liver | Not Specified | N/A | Phase 2 | Not Recruiting | NCT02968810 | |
| Lung | 40 | AZD9291 | Phase 1 | Not Recruiting | NCT02197234 | |
| Ovarian | 40 | ACT/NCT | Phase 1 | Recruiting | NCT04457089 | |
| Lung | 20 | ACT/NCT | Phase 2 | Recruiting | NCT04698941 | |
| 40 | ACT/NCT | Phase 2 | Not Recruiting | NCT04985201 | ||
| 40 | ACT/NCT | Phase 2 | Not Recruiting | NCT01441349 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Matuszewska, K.; Glogova, A.; Petrik, J. Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy. Cancers 2022, 14, 3500. https://doi.org/10.3390/cancers14143500
Pereira M, Matuszewska K, Glogova A, Petrik J. Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy. Cancers. 2022; 14(14):3500. https://doi.org/10.3390/cancers14143500
Chicago/Turabian StylePereira, Madison, Kathy Matuszewska, Alice Glogova, and Jim Petrik. 2022. "Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy" Cancers 14, no. 14: 3500. https://doi.org/10.3390/cancers14143500
APA StylePereira, M., Matuszewska, K., Glogova, A., & Petrik, J. (2022). Mutant p53, the Mevalonate Pathway and the Tumor Microenvironment Regulate Tumor Response to Statin Therapy. Cancers, 14(14), 3500. https://doi.org/10.3390/cancers14143500







