The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Perioperative Events and Prognosis of GC Patients
2.1. Radical Gastrectomy
2.2. Anesthesia and/or Analgesia
2.3. POCs
2.4. Anemia, Intraoperative Blood Loss and Blood Transfusion
2.5. Malnutrition and Nutritional Support
3. Mechanisms Underlying the Increased Recurrence Rate Resulting from Perioperative Events
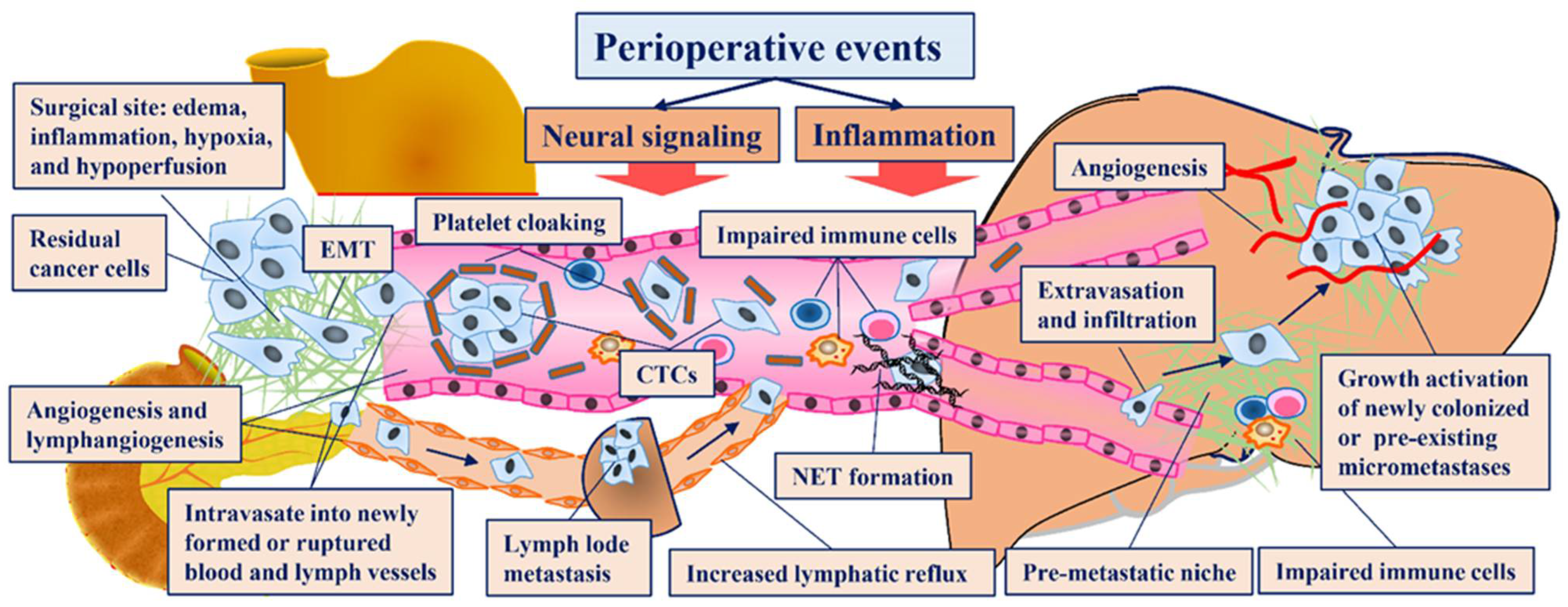
3.1. Perioperative Events Involved in Each Step of the Invasion-Metastasis Cascade
3.2. Physiological Responses to Perioperative Events
3.2.1. Activation of Neural Signaling
3.2.2. Activation of Inflammatory Responses
3.2.3. Suppression of Anticancer Immunity
3.3. Individual Event Aspects Affecting Recurrence
3.3.1. Radical Gastrectomy
3.3.2. Anesthetic and Analgesic Drugs
3.3.3. POCs
3.3.4. Anemia, Hemorrhage and Blood Transfusion
3.3.5. Malnutrition and Nutritional Support
4. Perspectives on Translating Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S. Tumor excision as a metastatic Russian Roulette: Perioperative interventions to improve long-term survival of cancer patients. Trends Cancer 2020, 6, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Hu, D.M.; Gong, T.P.; Xu, R.; Gao, J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: A systematic review and meta-analysis of 64 follow-up studies. Asian J. Surg. 2020, 43, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Lirosi, M.C.; Panunzi, S.; Santocchi, P.; Persiani, R.; D’Ugo, D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur. J. Surg. Oncol. 2018, 44, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Yao, H.S.; Hu, Z.Q. Allogeneic blood transfusion and the prognosis of gastric cancer patients: Systematic review and meta-analysis. Int. J. Surg. 2015, 13, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, D.; Chen, X.; Huang, Y.; Ouyang, M.; Zhang, W. Perioperative allogenenic blood transfusion is associated with worse clinical outcome for patients undergoing gastric carcinoma surgery: A meta-analysis. Medicine 2015, 94, e1574. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, A.; Weeden, S.; Fielding, J.; Bancewicz, J.; Craven, J.; Joypaul, V.; Sydes, M.; Fayers, P. Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br. J. Cancer 1999, 79, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Sasako, M.; Sano, T.; Yoshikawa, T.; Iwasaki, Y.; Nashimoto, A.; Ito, S.; Kurita, A.; Mizusawa, J.; Nakamura, K. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br. J. Surg. 2015, 102, 341–348. [Google Scholar]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Kurokawa, Y.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N. Engl. J. Med. 2008, 359, 453–462. [Google Scholar] [CrossRef]
- Sano, T.; Sasako, M.; Mizusawa, J.; Yamamoto, S.; Katai, H.; Yoshikawa, T.; Nashimoto, A.; Ito, S.; Kaji, M.; Imamura, H.; et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann. Surg. 2017, 265, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Doki, Y.; Mizusawa, J.; Terashima, M.; Katai, H.; Yoshikawa, T.; Kimura, Y.; Takiguchi, S.; Nishida, Y.; Fukushima, N.; et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): A phase 3, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 460–468. [Google Scholar] [CrossRef]
- Yu, W.; Choi, G.S.; Chung, H.Y. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br. J. Surg. 2006, 93, 559–563. [Google Scholar] [CrossRef]
- Katai, H.; Mizusawa, J.; Katayama, H.; Morita, S.; Yamada, T.; Bando, E.; Ito, S.; Takagi, M.; Takagane, A.; Teshima, S.; et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): A multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol. Hepatol. 2020, 5, 142–151. [Google Scholar] [CrossRef]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: The KLASS-01 randomized clinical trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef]
- Yu, J.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Wang, K.; Suo, J.; Tao, K.; He, X.; et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 Randomized Clinical trial. JAMA 2019, 321, 1983–1992. [Google Scholar] [CrossRef] [Green Version]
- Hyung, W.J.; Yang, H.K.; Park, Y.K.; Lee, H.J.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; Hur, H.; et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: The KLASS-02-RCT randomized clinical trial. J. Clin. Oncol. 2020, 38, 3304–3313. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef] [Green Version]
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br. J. Surg. 2014, 101, 653–660. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.Y.; Li, W.; Feng, Y.; Hou, J.; Ji, Y.; Sun, Y.H.; Shen, K.T.; Shen, Z.B.; Qin, X.Y.; et al. A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother. Pharmacol. 2014, 73, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Toriumi, T.; Terashima, M. Disadvantages of complete No. 10 lymph node dissection in gastric cancer and the possibility of spleen-preserving dissection: Review. J. Gastric Cancer 2020, 20, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raa, S.T.; Oosterling, S.J.; van der Kaaij, N.P.; van den Tol, M.P.; Beelen, R.H.; Meijer, S.; van Eijck, C.H.; van der Sijp, J.R.; van Egmond, M.; Jeekel, J. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. J. Surg. Oncol. 2005, 92, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Hamai, Y.; Takiyama, W.; Hirai, T.; Ikeda, T. Enhancing effect of thoracotomy on tumor growth in rats with special reference to the duration and timing of the operation. GANN Jpn. J. Cancer Res. 1980, 71, 280–284. [Google Scholar]
- Montejano, J.; Jevtovic-Todorovic, V. Anesthesia and cancer, friend or foe? A narrative review. Front. Oncol. 2021, 11, 803266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, H.; Xu, Y.; Zheng, X.; Wang, G. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: A retrospective study. Oncotarget 2017, 8, 62658–62665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiller, J.G.; Hacking, M.B.; Link, E.K.; Wessels, K.L.; Riedel, B.J. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol. Scand. 2014, 58, 281–290. [Google Scholar] [CrossRef]
- Cummings, K.C., 3rd; Patel, M.; Htoo, P.T.; Bakaki, P.M.; Cummings, L.C.; Koroukian, S. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: A population-based study. Reg. Anesth. Pain Med. 2014, 39, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, W.; Wu, Q.; Zhang, R.; Fang, J. Impact of combination epidural and general anesthesia on the long-term survival of gastric cancer patients: A retrospective study. Med. Sci. Monit. 2016, 22, 2379–2385. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Kim, H.I.; Kim, N.Y.; Lee, K.Y.; Kim, D.W.; Yoo, Y.C. Effect of postoperative analgesia technique on the prognosis of gastric cancer: A retrospective analysis. Oncotarget 2017, 8, 104594–104604. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.P.; Zhang, C.D.; Liang, Y.; Zhang, C.; Wu, K.Z.; Zhao, Z.M.; Dai, D.Q. Effects of epidural combined with general anesthesia versus general anesthesia alone in gastric cancer surgery: A propensity score matching analysis. Ann. Transl. Med. 2020, 8, 473. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Dong, L.; Zhao, S.; Wang, L.; Chen, H.; Xu, Y.; Wang, G. Effects of propofol-based total intravenous anesthesia on gastric cancer: A retrospective study. Onco. Targets. Ther. 2018, 11, 1141–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, N.C.; Lee, M.S.; Lai, H.C.; Lin, H.T.; Huang, Y.H.; Lu, C.H.; Hsu, C.H.; Wu, Z.F. Propofol-based total intravenous anesthesia improves survival compared to desflurane anesthesia in gastric cancer surgery: A retrospective analysis. Medicine 2020, 99, e20714. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, H.H.; Jeon, Y.T. Retrospective analysis of 1-year mortality after gastric cancer surgery: Total intravenous anesthesia versus volatile anesthesia. Acta Anaesthesiol. Scand. 2019, 63, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhou, N.B.; Li, H.; Wang, B.S.; Wang, X.Q.; Wang, X.W.; Wang, K.G.; Xue, F.S. Morphine and ketamine inhibit immune function of gastric cancer patients by increasing percentage of CD4(+)CD25(+)Foxp3(+) regulatory T cells in vitro. J. Surg. Res. 2016, 203, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Matzner, P.; Sandbank, E.; Neeman, E.; Zmora, O.; Gottumukkala, V.; Ben-Eliyahu, S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 2020, 17, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, L.; Wang, Q.; Li, J.; Bai, B.; Li, Z.; Wu, X.; Yu, P.; Li, X.; Yin, J. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Eto, K.; Hiki, N.; Kumagai, K.; Shoji, Y.; Tsuda, Y.; Kano, Y.; Yasufuku, I.; Okumura, Y.; Tsujiura, M.; Ida, S.; et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 2018, 21, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Z.; Yang, Y.C.; Chen, Y.; Wu, C.C.; Lin, R.F.; Wang, Z.N.; Zhang, X. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: A meta-analysis. Dis. Markers 2019, 2019, 7606128. [Google Scholar] [CrossRef]
- Xu, R.; Chen, X.D.; Ding, Z. Perioperative nutrition management for gastric cancer. Nutrition 2022, 93, 111492. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Hu, D.M.; Zhang, Y.; Gong, T.P.; Wu, X.L. Prognostic nutritional index predicts outcomes of patients after gastrectomy for cancer: A Systematic review and meta-analysis of nonrandomized studies. Nutr. Cancer 2019, 71, 557–568. [Google Scholar] [CrossRef]
- Huang, D.D.; Yu, D.Y.; Song, H.N.; Wang, W.B.; Luo, X.; Wu, G.F.; Yu, Z.; Liu, N.X.; Dong, Q.T.; Chen, X.L.; et al. The relationship between the GLIM-defined malnutrition, body composition and functional parameters, and clinical outcomes in elderly patients undergoing radical gastrectomy for gastric cancer. Eur. J. Surg. Oncol. 2021, 47, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Domagala, P.; Polak, W.G.; Buettner, S.; Wijnhoven, B.; Ijzermans, J. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. BMC Surg. 2019, 19, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijs, N.; van Bokhorst-de van der Schueren, M.A.; Langius, J.A.; Leemans, C.R.; Kuik, D.J.; Vermeulen, M.A.; van Leeuwen, P.A. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am. J. Clin. Nutr. 2010, 92, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, L.; O’Reilly, M.S.; Folkman, J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef]
- Garg, M. Emerging roles of epithelial-mesenchymal plasticity in invasion-metastasis cascade and therapy resistance. Cancer Metastasis Rev. 2022, 41, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The platelet lifeline to cancer: Challenges and opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Karolak, M.R.; Ma, Y.; Perrien, D.S.; Masood-Campbell, S.K.; Penner, N.L.; Munoz, S.A.; Zijlstra, A.; Yang, X.; Sterling, J.A.; et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012, 10, e1001363. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Goto, T. Links between inflammation and postoperative cancer recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B.M. Can stopping nerves, stop cancer. Trends Neurosci. 2016, 39, 880–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, S.; Yang, Q.; Li, J.; Yu, F.; Zhao, E. Norepinephrine enhances aerobic glycolysis and may act as a predictive factor for immunotherapy in gastric cancer. J. Immunol. Res. 2021, 2021, 5580672. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Li, B.; Li, Z.; Zhang, J.; Yu, J.; Zhang, L.; Xu, Z. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int. J. Oncol. 2019, 54, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Koh, M.; Takahashi, T.; Kurokawa, Y.; Kobayashi, T.; Saito, T.; Ishida, T.; Serada, S.; Fujimoto, M.; Naka, T.; Wada, N.; et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer 2021, 24, 1037–1049. [Google Scholar] [CrossRef]
- Lu, Y.J.; Geng, Z.J.; Sun, X.Y.; Li, Y.H.; Fu, X.B.; Zhao, X.Y.; Wei, B. Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol. Cell. Biochem. 2015, 408, 1–13. [Google Scholar] [CrossRef]
- Shan, T.; Cui, X.; Li, W.; Lin, W.; Li, Y.; Chen, X.; Wu, T. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014, 105, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, Y.; Zhao, H.; Li, Q.; Liu, Y.; Zuo, Y.; Xu, Q.; Zuo, H.; Li, Y.; Li, Y. Chronic stress model simulated by salbutamol promotes tumorigenesis of gastric cancer cells through β2-AR/ERK/EMT pathway. J. Cancer 2022, 13, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Han, C.; Wei, B.; Qian, L.; Chen, C.; Guo, L.; Hu, M.; Yu, M.; et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol. Cancer 2010, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.Y.; Nuyten, D.S.; Sneddon, J.B.; Hastie, T.; Tibshirani, R.; Sørlie, T.; Dai, H.; He, Y.D.; van’t Veer, L.J.; Bartelink, H.; et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl. Acad. Sci. USA 2005, 102, 3738–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Chen, Y.; Zhu, Y.; Wu, Q.; Yao, C.; Xia, H.; Li, C. Postoperative Systemic Immune-Inflammation Index (SII): A superior prognostic factor of endometrial cancer. Front. Surg. 2021, 8, 704235. [Google Scholar] [CrossRef]
- Yasui, K.; Shida, D.; Nakamura, Y.; Ahiko, Y.; Tsukamoto, S.; Kanemitsu, Y. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br. J. Cancer 2021, 124, 933–941. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, J.; Zhi, M.; Shen, D.; Shao, M.; Zhang, C.; Wang, G.; Jiang, Z. Stress and autonomic nerve dysfunction monitoring in perioperative gastric cancer patients using a smart device. Ann. Noninvasive Electrocardiol. 2022, 27, e12903. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Carpinteri, S.; Sampurno, S.; Bernardi, M.P.; Germann, M.; Malaterre, J.; Heriot, A.; Chambers, B.A.; Mutsaers, S.E.; Lynch, A.C.; Ramsay, R.G. Peritoneal tumorigenesis and inflammation are ameliorated by humidified-warm carbon dioxide insufflation in the mouse. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1540–S1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Tang, B.; Yu, P.W.; Peng, Z.H.; Qian, F.; Sun, G. Systemic and peritoneal inflammatory response after laparoscopic-assisted gastrectomy and the effect of inflammatory cytokines on adhesion of gastric cancer cells to peritoneal mesothelial cells. Surg. Endosc. 2010, 24, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Ricon, I.; Hanalis-Miller, T.; Haldar, R.; Jacoby, R.; Ben-Eliyahu, S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling. Cancer 2019, 125, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echizen, K.; Hirose, O.; Maeda, Y.; Oshima, M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016, 107, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.C.; Kang, W.; Xu, J.; Qian, Y.; Luk, S.; Chen, H.; Li, W.; Zhao, L.; Zhang, X.; Chiu, P.W.; et al. Prostaglandin E(2) induces DNA hypermethylation in gastric cancer in vitro and in vivo. Theranostics 2019, 9, 6256–6268. [Google Scholar] [CrossRef]
- Ruan, D.; So, S.P. Prostaglandin E2 produced by inducible COX-2 and mPGES-1 promoting cancer cell proliferation in vitro and in vivo. Life Sci. 2014, 116, 43–50. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Q.; Jiang, Y.; Yu, J.; Hu, Y.; Mou, T.; Chen, G.; Li, G. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2016, 5, e1069936. [Google Scholar] [CrossRef] [Green Version]
- Takaya, S.; Saito, H.; Ikeguchi, M. Upregulation of immune checkpoint molecules, PD-1 and LAG-3, on CD4+ and CD8+ T cells after gastric cancer surgery. Yonago Acta. Med. 2015, 58, 39–44. [Google Scholar]
- Zhang, Q.; Shan, F.; Li, Z.; Gao, J.; Li, Y.; Shen, L.; Ji, J.; Lu, M. A prospective study on the changes and clinical significance of pre-operative and post-operative circulating tumor cells in resectable gastric cancer. J. Transl. Med. 2018, 16, 171. [Google Scholar] [CrossRef]
- Hayashi, K.; Jiang, P.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007, 67, 8223–8228. [Google Scholar] [CrossRef] [Green Version]
- Tvedskov, T.F.; Jensen, M.B.; Kroman, N.; Balslev, E. Iatrogenic displacement of tumor cells to the sentinel node after surgical excision in primary breast cancer. Breast Cancer Res. Treat. 2012, 131, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.V.; Lara, P.F.; Oliveira-Filho, R.M.; Greco, R.V.; Sudo-Hayashi, L.S. Lymphatic regeneration across an incisional wound: Inhibition by dexamethasone and aspirin, and acceleration by a micronized purified flavonoid fraction. Eur. J. Pharmacol. 2006, 551, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sano, T.; Yamamoto, S.; Sairenji, M.; Arai, K.; Kinoshita, T.; Nashimoto, A.; Hiratsuka, M. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol. 2006, 7, 644–651. [Google Scholar] [CrossRef]
- Soltanizadeh, S.; Degett, T.H.; Gögenur, I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: A systematic review. J. Clin. Anesth. 2017, 42, 19–25. [Google Scholar] [CrossRef]
- Tavare, A.N.; Perry, N.J.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2012, 130, 1237–1250. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, D.; Gu, J.; Qu, M.; Guo, K.; Chen, W.; Miao, C. Targeting the mu-Opioid Receptor for Cancer Treatment. Curr. Oncol. Rep. 2021, 23, 111. [Google Scholar] [CrossRef]
- Carli, M.; Donnini, S.; Pellegrini, C.; Coppi, E.; Bocci, G. Opioid receptors beyond pain control: The role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol. Res. 2020, 159, 104938. [Google Scholar] [CrossRef]
- Shimizu, S.; Saito, H.; Kono, Y.; Murakami, Y.; Shishido, Y.; Miyatani, K.; Matsunaga, T.; Fukumoto, Y.; Fujiwara, Y. The prognostic significance of the comprehensive complication index in patients with gastric cancer. Surg. Today 2019, 49, 913–920. [Google Scholar] [CrossRef]
- Vicente, D.; Ikoma, N.; Chiang, Y.J.; Fournier, K.; Tzeng, C.D.; Song, S.; Mansfield, P.; Ajani, J.; Badgwell, B.D. Preoperative therapy for gastric adenocarcinoma is protective for poor oncologic outcomes in patients with complications after gastrectomy. Ann. Surg. Oncol. 2018, 25, 2720–2730. [Google Scholar] [CrossRef]
- Jin, L.X.; Sanford, D.E.; Squires, M.H., 3rd; Moses, L.E.; Yan, Y.; Poultsides, G.A.; Votanopoulos, K.I.; Weber, S.M.; Bloomston, M.; Pawlik, T.M.; et al. Interaction of postoperative morbidity and receipt of adjuvant therapy on long-term survival after resection for gastric adenocarcinoma: Results from the U.S. Gastric Cancer Collaborative. Ann. Surg. Oncol. 2016, 23, 2398–2408. [Google Scholar] [CrossRef]
- Li, S.S.; Udelsman, B.V.; Parikh, A.; Klempner, S.J.; Clark, J.W.; Roeland, E.J.; Wo, J.Y.; Hong, T.S.; Mullen, J.T. Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J. Am. Coll. Surg. 2020, 230, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kodera, Y.; Nakanishi, H.; Yokoyama, H.; Ohashi, N.; Ito, Y.; Nakayama, G.; Koike, M.; Fujiwara, M.; Nakao, A. The effect of chemotherapy against micrometastases and isolated tumor cells in lymph nodes: An in vivo study. In Vivo 2008, 22, 707–712. [Google Scholar]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misawa, K.; Kurokawa, Y.; Mizusawa, J.; Takiguchi, S.; Doki, Y.; Makino, S.; Choda, Y.; Takeno, A.; Tokunaga, M.; Sano, T.; et al. Negative impact of intraoperative blood loss on long-term outcome after curative gastrectomy for advanced gastric cancer: Exploratory analysis of the JCOG1001 phase III trial. Gastric Cancer 2021, 25, 459–467. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kanda, M.; Kodera, Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J. Gastroenterol. 2019, 25, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.; Sheridan, D.; Radosevic, J.; Burnouf, T.; Seghatchian, J. Transfusion-related immunomodulation and cancer. Transfus. Apher. Sci. 2017, 56, 336–340. [Google Scholar] [CrossRef]
- Huhmann, M.B.; August, D.A. Perioperative nutrition support in cancer patients. Nutr. Clin. Pract. 2012, 27, 586–592. [Google Scholar] [CrossRef]
- Hakkenbrak, N.; Jansma, E.P.; van der Wielen, N.; van der Peet, D.L.; Straatman, J. Laparoscopic versus open distal gastrectomy for gastric cancer: A systematic review and meta-analysis. Surgery 2022, 171, 1552–1561. [Google Scholar] [CrossRef]
- Kim, Y.M.; Hyung, W.J. Current status of robotic gastrectomy for gastric cancer: Comparison with laparoscopic gastrectomy. Updates Surg. 2021, 73, 853–863. [Google Scholar] [CrossRef]
- Claassen, Y.; van Amelsfoort, R.M.; Hartgrink, H.H.; Dikken, J.L.; de Steur, W.O.; van Sandick, J.W.; van Grieken, N.; Cats, A.; Boot, H.; Trip, A.K.; et al. Effect of hospital volume with respect to performing gastric cancer resection on recurrence and survival: Results from the CRITICS trial. Ann. Surg. 2019, 270, 1096–1102. [Google Scholar] [CrossRef]
- Liao, P.; Song, K.; Zhu, Z.; Liu, Z.; Zhang, W.; Li, W.; Hu, J.; Hu, Q.; Chen, C.; Chen, B.; et al. Propranolol suppresses the growth of colorectal cancer through simultaneously activating autologous CD8(+) T cells and inhibiting tumor AKT/MAPK pathway. Clin. Pharmacol. Ther. 2020, 108, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Haldar, R.; Ricon-Becker, I.; Radin, A.; Gutman, M.; Cole, S.W.; Zmora, O.; Ben-Eliyahu, S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 2020, 126, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missair, A.; Cata, J.P.; Votta-Velis, G.; Johnson, M.; Borgeat, A.; Tiouririne, M.; Gottumukkala, V.; Buggy, D.; Vallejo, R.; Marrero, E.B.; et al. Impact of perioperative pain management on cancer recurrence: An ASRA/ESRA special article. Reg. Anesth. Pain. Med. 2019, 44, 13–28. [Google Scholar] [CrossRef]
- Osorio, J.; Jericó, C.; Miranda, C.; Santamaría, M.; Artigau, E.; Galofré, G.; Garsot, E.; Luna, A.; Puértolas, N.; Aldeano, A.; et al. Improved postoperative outcomes and reduced transfusion rates after implementation of a Patient Blood Management program in gastric cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 1449–1457. [Google Scholar] [CrossRef]
- Keding, V.; Zacharowski, K.; Bechstein, W.O.; Meybohm, P.; Schnitzbauer, A.A. Patient Blood Management improves outcome in oncologic surgery. World J. Surg. Oncol. 2018, 16, 159. [Google Scholar] [CrossRef]
- Fidler, I.J.; Kripke, M.L. The challenge of targeting metastasis. Cancer Metastasis Rev. 2015, 34, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Cuschieri, A.; Fayers, P.; Fielding, J.; Craven, J.; Bancewicz, J.; Joypaul, V.; Cook, P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: Preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996, 347, 995–999. [Google Scholar] [CrossRef]
- Bonenkamp, J.J.; Hermans, J.; Sasako, M.; van de Velde, C.J.; Welvaart, K.; Songun, I.; Meyer, S.; Plukker, J.T.; Van Elk, P.; Obertop, H.; et al. Extended lymph-node dissection for gastric cancer. N. Engl. J. Med. 1999, 340, 908–914. [Google Scholar] [CrossRef]
- Sano, T.; Sasako, M.; Yamamoto, S.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; Yamamura, Y.; et al. Gastric cancer surgery: Morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J. Clin. Oncol. 2004, 22, 2767–2773. [Google Scholar] [CrossRef]
- Kim, W.; Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: Short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann. Surg. 2016, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Xue, Y.; Suo, J.; Tao, K.; He, X.; et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: A randomized controlled trial. J. Clin. Oncol. 2016, 34, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ouyang, J.; Liu, S.; Chen, J.; Zhang, H.; Wang, C.; Wu, W.; Zhang, C.; He, Y. The prognostic impact of pretreatment anemia in patients with gastric cancer and nonhypoalbuminemia undergoing curative resection: A retrospective study. Ann. Transl. Med. 2021, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, H.; Huang, Y.; Xu, D.; Li, W.; Li, Y.; Chen, Y.; Zhou, Z.; Sun, X. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: A single-institution retrospective analysis of 2163 Chinese patients. Cancer Med. 2018, 7, 360–369. [Google Scholar] [CrossRef]
- Wang, S.L.; Ma, L.L.; Chen, X.Y.; Zhou, D.L.; Li, B.; Huang, D.D.; Yu, Z.; Shen, X.; Zhuang, C.L. Impact of visceral fat on surgical complications and long-term survival of patients with gastric cancer after radical gastrectomy. Eur. J. Clin. Nutr. 2018, 72, 436–445. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Y.; Zhu, L.; Cong, X.; Li, S.; Song, S.; Song, H.; Xue, Y. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag. Res. 2017, 9, 849–867. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Lin, F.; Yin, M.; Feng, N.; Zhang, X.; Cui, Y.G.; Yi, Y.P.; Kong, X.Y.; Chen, X.; Liu, W.Z. Preoperative albumin/globulin ratio is a potential prognosis predicting biomarker in patients with resectable gastric cancer. Turk. J. Gastroenterol. 2017, 28, 439–445. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, D.Y.; Duan, Y.H.; Zhang, Y.Q.; Cui, X.N.; Luo, Z. Correlations of hemoglobin level and perioperative blood transfusion with the prognosis of gastric cancer: A retrospective study. Med. Sci. Monit. 2017, 23, 2470–2478. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, M.S.; Chung, I.K.; Son, M.W.; Cho, Y.S.; Lee, S.M. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J. Gastroenterol. 2017, 23, 2385–2395. [Google Scholar] [CrossRef]
- Eo, W.K.; Jeong, D.W.; Chang, H.J.; Won, K.Y.; Choi, S.I.; Kim, S.H.; Chun, S.W.; Oh, Y.L.; Lee, T.H.; Kim, Y.O.; et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J. Gastroenterol. 2015, 21, 2668–2676. [Google Scholar] [CrossRef]
- Rausei, S.; Ruspi, L.; Galli, F.; Tirotta, F.; Inversini, D.; Frattini, F.; Chiappa, C.; Rovera, F.; Boni, L.; Dionigi, G.; et al. Peri-operative blood transfusion in gastric cancer surgery: Prognostic or confounding factor. Int. J. Surg. 2013, 11 (Suppl. 1), S100–S103. [Google Scholar] [CrossRef] [Green Version]
- Mohri, Y.; Tanaka, K.; Ohi, M.; Yokoe, T.; Miki, C.; Kusunoki, M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J. Surg. 2010, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.G.; Cheong, J.H.; Hyung, W.J.; Kim, J.; Choi, S.H.; Noh, S.H. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J. Surg. Oncol. 2005, 91, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yoshikawa, T.; Yura, M.; Otsuki, S.; Yamagata, Y.; Morita, S.; Katai, H.; Nishida, T. Intraoperative blood loss as an independent prognostic factor for curative resection after neoadjuvant chemotherapy for gastric cancer: A single-center retrospective cohort study. Surg. Today 2021, 51, 293–302. [Google Scholar] [CrossRef]
- Tamagawa, H.; Aoyama, T.; Kano, K.; Numata, M.; Atsumi, Y.; Hara, K.; Kazama, K.; Koumori, K.; Murakawa, M.; Hashimoto, I.; et al. The impact of intraoperative blood loss on the long-term prognosis after curative resection for borrmann type IV gastric cancer: A retrospective multicenter study. Anticancer Res. 2020, 40, 405–412. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, X.; Lu, H.; Zhang, J.; Luo, R.; Xu, H.; Huang, B. Intraoperative blood loss does not independently affect the survival outcome of gastric cancer patients who underwent curative resection. Clin. Transl. Oncol. 2019, 21, 1197–1206. [Google Scholar] [CrossRef]
- Ito, Y.; Kanda, M.; Ito, S.; Mochizuki, Y.; Teramoto, H.; Ishigure, K.; Murai, T.; Asada, T.; Ishiyama, A.; Matsushita, H.; et al. Intraoperative blood loss is associated with shortened postoperative survival of patients with stage II/III gastric cancer: Analysis of a multi-institutional dataset. World J. Surg. 2019, 43, 870–877. [Google Scholar] [CrossRef]
- Nizuno, A.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Iwata, N.; Yamada, S.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Koike, M.; et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig. Surg. 2016, 33, 121–128. [Google Scholar]
- Ishino, Y.; Saigusa, S.; Ohi, M.; Yasuda, H.; Tanaka, K.; Toiyama, Y.; Mohri, Y.; Kusunoki, M. Preoperative C-reactive protein and operative blood loss predict poor prognosis in patients with gastric cancer after laparoscopy-assisted gastrectomy. Asian J. Endosc. Surg. 2014, 7, 287–294. [Google Scholar] [CrossRef]
- Liang, Y.X.; Guo, H.H.; Deng, J.Y.; Wang, B.G.; Ding, X.W.; Wang, X.N.; Zhang, L.; Liang, H. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J. Gastroenterol. 2013, 19, 5542–5550. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, X.; Kuang, X.; Li, J. The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review. Cancers 2022, 14, 3496. https://doi.org/10.3390/cancers14143496
Zhi X, Kuang X, Li J. The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review. Cancers. 2022; 14(14):3496. https://doi.org/10.3390/cancers14143496
Chicago/Turabian StyleZhi, Xing, Xiaohong Kuang, and Jian Li. 2022. "The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review" Cancers 14, no. 14: 3496. https://doi.org/10.3390/cancers14143496
APA StyleZhi, X., Kuang, X., & Li, J. (2022). The Impact of Perioperative Events on Cancer Recurrence and Metastasis in Patients after Radical Gastrectomy: A Review. Cancers, 14(14), 3496. https://doi.org/10.3390/cancers14143496





