A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Literature overview
2.1. Breast Size and Chest Wall Separation
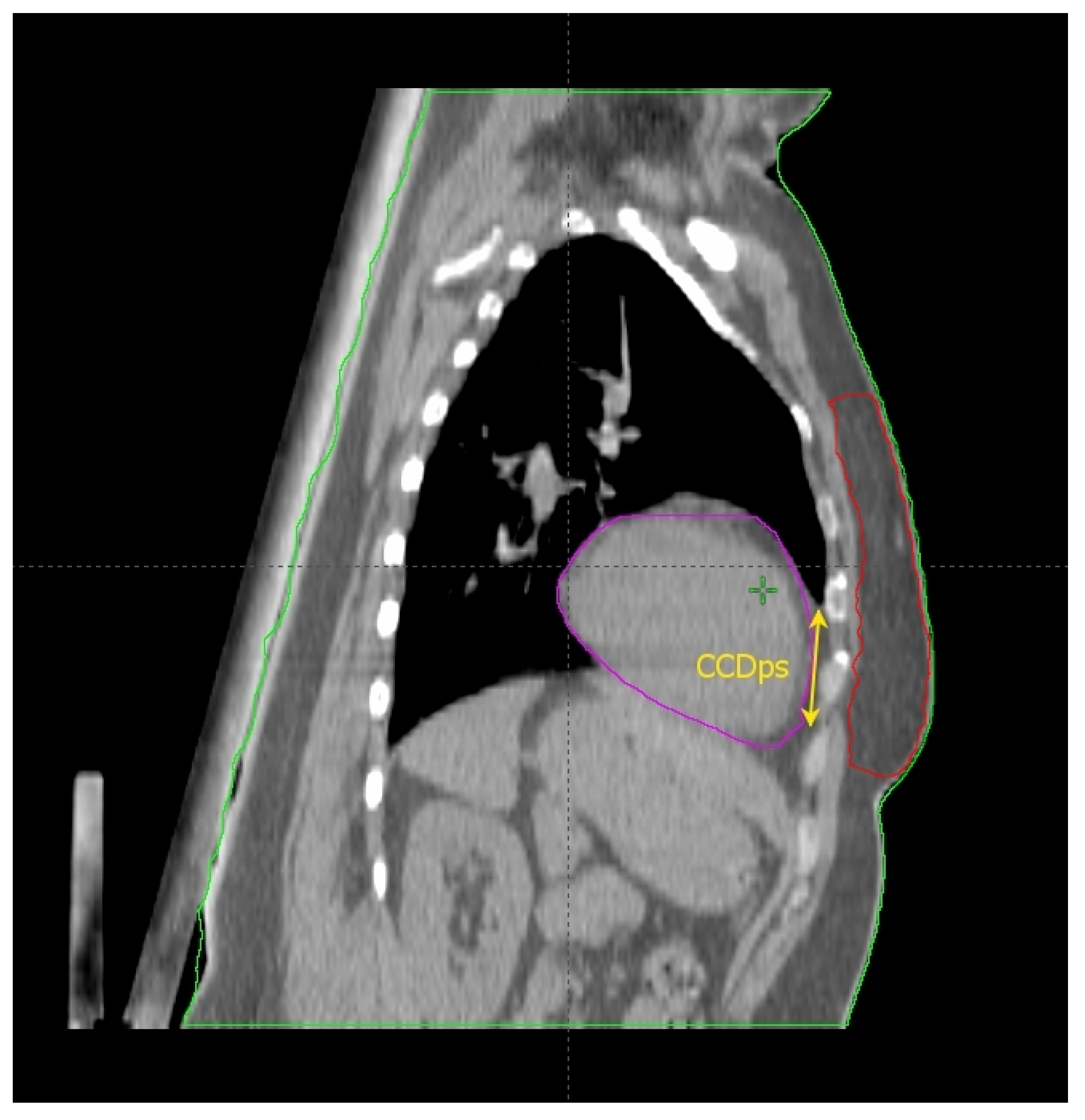
2.2. The Cardiac Contact Distance in the Axial (CCDax) and Parasagittal (CCDps) Planes and the Lateral Heart-to-Chest Distance (HCD)
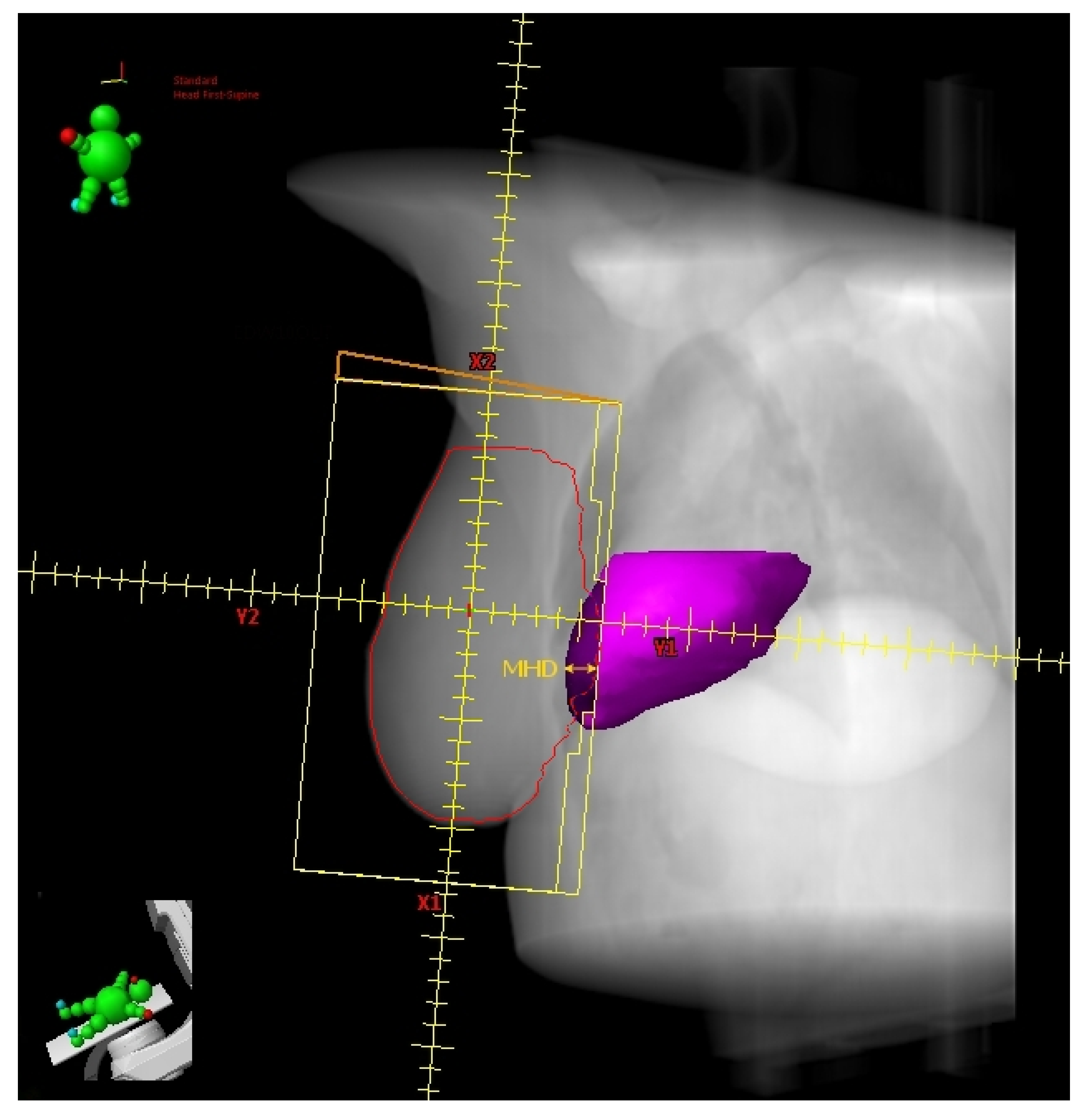
2.3. Heart Volume in Field
2.4. BMI, the Seasonal Effect, and Lung Predictors
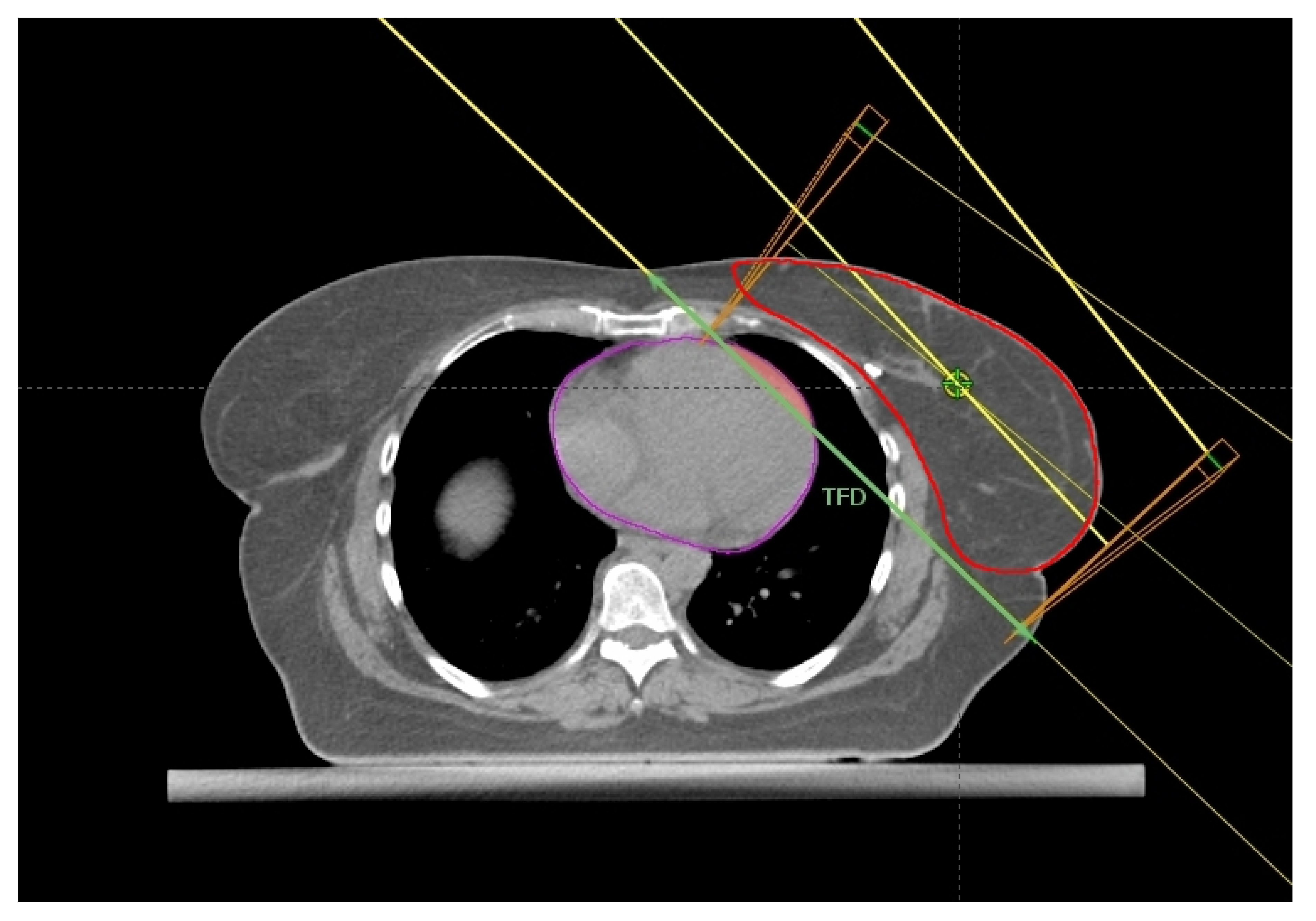
2.5. Maximum Heart Depth and Tumor Bed Site
3. Further Considerations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 25 June 2022).
- Sindoni, A.; Severo, C.; Vadala’, R.E.; Ferini, G.; Mazzei, M.M.; Vaccaro, M.; Iatì, G.; Pontoriero, A.; Pergolizzi, S. Levetiracetam-induced radiation recall dermatitis in a patient undergoing stereotactic radiotherapy. J. Dermatol. 2016, 43, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Prestifilippo, A.; Cantale, O.; Ferini, G.; Fisichella, G.; Fontana, P.; Sciacca, D.; Giuffrida, D. Role of the Combination of Cyclin-Dependent Kinase Inhibitors (CDKI) and Radiotherapy (RT) in the Treatment of Metastatic Breast Cancer (MBC): Advantages and Risks in Clinical Practice. Front. Oncol. 2021, 11, 643155. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin. Cancer Biol. 2020, in press. [CrossRef]
- Pontoriero, A.; Iatì, G.; Cacciola, A.; Conti, A.; Brogna, A.; Siragusa, C.; Ferini, G.; Davì, V.; Tamburella, C.; Molino, L.; et al. Stereotactic Body Radiation Therapy with Simultaneous Integrated Boost in Patients with Spinal Metastases. Technol. Cancer Res. Treat. 2020, 19, 1533033820904447. [Google Scholar] [CrossRef]
- Ferini, G.; Castorina, P.; Valenti, V.; Illari, S.I.; Sachpazidis, I.; Castorina, L.; Marrale, M.; Pergolizzi, S. A Novel Radiotherapeutic Approach to Treat Bulky Metastases Even From Cutaneous Squamous Cell Carcinoma: Its Rationale and a Look at the Reliability of the Linear-Quadratic Model to Explain Its Radiobiological Effects. Front. Oncol. 2022, 12, 809279. [Google Scholar] [CrossRef]
- Vadalà, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.I.; et al. Stereotactic body radiotherapy in non-operable lung cancer patients. Clin. Transl. Oncol. 2016, 18, 1158–1159. [Google Scholar] [CrossRef]
- Ferini, G.; Tripoli, A.; Molino, L.; Cacciola, A.; Lillo, S.; Parisi, S.; Umina, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; et al. How Much Daily Image-guided Volumetric Modulated Arc Therapy Is Useful for Proctitis Prevention with Respect to Static Intensity Modulated Radiotherapy Supported by Topical Medications Among Localized Prostate Cancer Patients? Anticancer Res. 2021, 41, 2101–2110. [Google Scholar] [CrossRef]
- Shah, C.; Badiyan, S.; Berry, S.; Khan, A.; Goyal, S.; Schulte, K.; Nanavati, A.; Lynch, M.; Vicini, F.A. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother. Oncol. 2014, 112, 9–16. [Google Scholar] [CrossRef]
- Corradini, S.; Ballhausen, H.; Weingandt, H.; Freislederer, P.; Schönecker, S.R.; Niyazi, M.; Simonetto, C.; Eidemüller, M.; Ganswindt, U.; Belka, C. Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease. Strahlenther. Onkol. 2017, 194, 196–205. [Google Scholar] [CrossRef]
- Taylor, C.W.; Kirby, A.M. Cardiac Side-effects From Breast Cancer Radiotherapy. Clin. Oncol. 2015, 27, 621–629. [Google Scholar] [CrossRef]
- Oonsiri, P.; Wisetrinthong, M.; Chitnok, M.; Saksornchai, K.; Suriyapee, S. An effective patient training for deep inspiration breath hold technique of left-sided breast on computed tomography simulation procedure at King Chulalongkorn Memorial Hospital. Radiat. Oncol. J. 2019, 37, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Li, J.; Zhao, Y.; Wang, P.; Tang, B.; Yao, X.; Liao, X.; Ma, J.; Orlandini, L.C. Retrospective Study on Left-Sided Breast Radiotherapy: Dosimetric Results and Correlation with Physical Factors for Free Breathing and Breath Hold Irradiation Techniques. Technol. Cancer Res. Treat. 2021, 20, 15330338211062429. [Google Scholar] [CrossRef]
- Ferini, G.; Molino, L.; Tripoli, A.; Valenti, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; Borzi, G.R. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy Among Women with Left Breast Cancer. Anticancer Res. 2021, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Kalet, A.M.; Young, L.A.; Fang, L.C.; Kim, J.N.; Mayr, N.A.; Meyer, J. Predictors of cardiac and lung dose sparing in DIBH for left breast treatment. Phys. Med. 2019, 67, 27–33. [Google Scholar] [CrossRef]
- Wennstig, A.-K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintelä, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of Coronary Artery Stenosis After Radiation for Breast Cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef]
- Rochet, N.; Drake, J.I.; Harrington, K.; Wolfgang, J.A.; Napolitano, B.; Sadek, B.T.; Shenouda, M.N.; Keruakous, A.; Niemierko, A.; Taghian, A.G. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: Evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract. Radiat. Oncol. 2015, 5, e127–e134. [Google Scholar] [CrossRef]
- Register, S.; Takita, C.; Reis, I.; Zhao, W.; Amestoy, W.; Wright, J. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med. Dosim. 2015, 40, 89–95. [Google Scholar] [CrossRef]
- Ferdinand, S.; Mondal, M.; Mallik, S.; Goswami, J.; Das, S.; Manir, K.S.; Sen, A.; Palit, S.; Sarkar, P.; Mondal, S.; et al. Dosimetric analysis of Deep Inspiratory Breath-hold technique (DIBH) in left-sided breast cancer radiotherapy and evaluation of pre-treatment predictors of cardiac doses for guiding patient selection for DIBH. Tech. Innov. Patient Support Radiat. Oncol. 2021, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Reardon, K.; Trifiletti, D.M.; Geesey, C.; Sukovich, K.; Crandley, E.; Read, P.W.; Wijesooriya, K. How dose sparing of cardiac structures correlates with in-field heart volume and sternal displacement. J. Appl. Clin. Med. Phys. 2016, 17, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanguturi, S.K.; Lyatskaya, Y.; Chen, Y.; Catalano, P.J.; Chen, M.H.; Yeo, W.-P.; Marques, A.; Truong, L.; Yeh, M.; Orlina, L.; et al. Prospective assessment of deep inspiration breath-hold using 3-dimensional surface tracking for irradiation of left-sided breast cancer. Pract. Radiat. Oncol. 2015, 5, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Mkanna, A.; Mohamad, O.; Ramia, P.; Thebian, R.; Makki, M.; Tamim, H.; Jalbout, W.; Youssef, B.; Eid, T.; Geara, F.; et al. Predictors of Cardiac Sparing in Deep Inspiration Breath-Hold for Patients with Left Sided Breast Cancer. Front. Oncol. 2018, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Shimizu, H.; Aoyama, T.; Kitagawa, T.; Miyauchi, R.; Watanabe, Y.; Tachibana, H.; Kodaira, T. Preoperative spirometry and BMI in deep inspiration breath-hold radiotherapy: The early detection of cardiac and lung dose predictors without radiation exposure. Radiat. Oncol. 2022, 17, 35. [Google Scholar] [CrossRef]
- Dell’Oro, M.; Giles, E.; Sharkey, A.; Borg, M.; Connell, C.; Bezak, E. A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique. Cancers 2019, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Tanna, N.; McLauchlan, R.; Karis, S.; Welgemoed, C.; Gujral, D.; Cleator, S. Assessment of Upfront Selection Criteria to Prioritise Patients for Breath-hold Left-sided Breast Radiotherapy. Clin. Oncol. 2017, 29, 356–361. [Google Scholar] [CrossRef]
- Gaasch, A.; Schönecker, S.; Simonetto, C.; Eidemüller, M.; Pazos, M.; Reitz, D.; Rottler, M.; Freislederer, P.; Braun, M.; Würstlein, R.; et al. Heart sparing radiotherapy in breast cancer: The importance of baseline cardiac risks. Radiat. Oncol. 2020, 15, 117. [Google Scholar] [CrossRef]
- Wang, X.; Fargier-Bochaton, O.; Dipasquale, G.; Laouiti, M.; Kountouri, M.; Gorobets, O.; Nguyen, N.P.; Miralbell, R.; Vinh-Hung, V. Is prone free breathing better than supine deep inspiration breath-hold for left whole-breast radiotherapy? A dosimetric analysis. Strahlenther. Onkol. 2021, 197, 317–331. [Google Scholar] [CrossRef]
- Johansen, S.; Vikstrom, J.; Hjelstuen, M.H.B.; Mjaaland, I.; Dybvik, K.I.; Olsen, D.R. Dose evaluation and risk estimation for secondary cancer in contralateral breast and a study of correlation between thorax shape and dose to organs at risk following tangentially breast irradiation during deep inspiration breath-hold and free breathing. Acta Oncol. 2011, 50, 563–568. [Google Scholar] [CrossRef]
- Lin, H.; Liu, T.; Shi, C.; Petillion, S.; Kindts, I.; Weltens, C.; Depuydt, T.; Song, Y.; Saleh, Z.; Xu, X.G.; et al. Feasibility study of individualized optimal positioning selection for left-sided whole breast radiotherapy: DIBH or prone. J. Appl. Clin. Med. Phys. 2018, 19, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.M.; Douglass, M.; Nguyen, G.T.; Cunningham, L.; Le, H.; Hu, Y.; Penfold, S.N. Individualised selection of left-sided breast cancer patients for proton therapy based on cost-effectiveness. J. Med. Radiat. Sci. 2020, 68, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; West, K.; Latty, D.; Beldham-Collins, R.; Jia, D.; Wang, W.; Cross, S.; Gebski, V.; Ahern, V.; Stuart, K. Patient-Specific Heart Constraint: A Tool for Optimization and Evaluation of Mean Heart Dose in Breast Cancer Patients. Pract. Radiat. Oncol. 2020, 11, e154–e162. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.; Fennell, L.; Folliard, T.; Kelly, C. Using a neural network to predict deviations in mean heart dose during the treatment of left-sided deep inspiration breath hold patients. Phys. Med. 2019, 65, 137–142. [Google Scholar] [CrossRef]
- Wang, X.; Pan, T.; Pinnix, C.; Zhang, S.X.; Salehpour, M.; Sun, T.L.; Gladish, G.; Strom, E.A.; Perkins, G.H.; Tereffe, W.; et al. Cardiac Motion During Deep-Inspiration Breath-Hold: Implications for Breast Cancer Radiotherapy. Int. J. Radiat. Oncol. 2011, 82, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Laaksomaa, M.; Sarudis, S.; Rossi, M.; Lehtonen, T.; Pehkonen, J.; Remes, J.; Luukkanen, H.; Skyttä, T.; Kapanen, M. AlignRT ® and Catalyst ™ in whole-breast radiotherapy with DIBH: Is IGRT still needed? J. Appl. Clin. Med. Phys. 2019, 20, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Otton, J.; Holloway, L.; Delaney, G.P.; Liney, G.; George, A.; Jameson, M.; Tran, D.; Batumalai, V.; Thomas, L.; et al. Quantification of cardiac subvolume dosimetry using a 17 segment model of the left ventricle in breast cancer patients receiving tangential beam radiotherapy. Radiother. Oncol. 2019, 132, 257–265. [Google Scholar] [CrossRef]
- Duma, M.N.; Herr, A.-C.; Borm, K.J.; Trott, K.R.; Molls, M.; Oechsner, M.; Combs, S.E. Tangential Field Radiotherapy for Breast Cancer—The Dose to the Heart and Heart Subvolumes: What Structures Must Be Contoured in Future Clinical Trials? Front. Oncol. 2017, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.-O.; Laurier, D.; et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: A dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat. Oncol. 2019, 14, 29. [Google Scholar] [CrossRef]
- Rice, A.; Zoller, I.; Kocos, K.; Weller, D.; DiCostanzo, D.; Hunzeker, A.; Lenards, N. The implementation of RapidPlan in predicting deep inspiration breath-hold candidates with left-sided breast cancer. Med. Dosim. 2018, 44, 210–218. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Zhao, F.; Hu, W.; Yao, G.; Lu, Z.; Yan, S. The benefits evaluation of abdominal deep inspiration breath hold based on knowledge-based radiotherapy treatment planning for left-sided breast cancer. J. Appl. Clin. Med. Phys. 2020, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Shimizu, H.; Wakabayashi, K.; Kitagawa, T.; Aoyama, T.; Miyauchi, R.; Tachibana, H.; Kodaira, T. Synthetic breath-hold CT generation from free-breathing CT: A novel deep learning approach to predict cardiac dose reduction in deep-inspiration breath-hold radiotherapy. J. Radiat. Res. 2021, 62, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Zellars, R.; Bravo, P.E.; Tryggestad, E.; Hopfer, K.; Myers, L.; Tahari, A.; Asrari, F.; Ziessman, H.; Garrett-Mayer, E. SPECT Analysis of Cardiac Perfusion Changes After Whole-Breast/Chest Wall Radiation Therapy with or without Active Breathing Coordinator: Results of a Randomized Phase 3 Trial. Int. J. Radiat. Oncol. 2014, 88, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Bergler-Klein, J.; Rainer, P.P.; Wallner, M.; Zaruba, M.-M.; Dörler, J.; Böhmer, A.; Buchacher, T.; Frey, M.; Adlbrecht, C.; Bartsch, R.; et al. Cardio-oncology in Austria: Cardiotoxicity and surveillance of anti-cancer therapies. Wien. Klin. Wochenschr. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]

| Authors | N° of LBC Patients | Irradiation Technique | Highlights |
|---|---|---|---|
| Xin et al. [15] | 155 (82 DIBH; 73 FB) | 3Dimensional-Conformal Radiation Therapy (3D-CRT, tangential fields) |
|
| Ferini et al. [16] | 116 | 3D-CRT (tangential fields) |
|
| Cao et al. [17] | 67 | 3D-CRT (tangential fields) |
|
| Rochet et al. [20] | 35 | 3D-CRT (tangential fields) |
|
| Register et al. [21] | 64 | 3D-CRT (tangential fields) |
|
| Ferdinand et al. [22] | 31 | 3D-CRT (tangential fields) |
|
| Kim et al. [23] | 97 | 3D-CRT (tangential fields) |
|
| Tanguturi et al. [24] | 148 (110 DIBH; 38 FB) | 3D-CRT (tangential fields) |
|
| Mkanna et al. [25] | 103 | 3D-CRT (tangential fields) |
|
| Koide et al. [26] | 100 | 3D-CRT (tangential fields) |
|
| Dell’Oro et al. [27] | 20 | 3D-CRT (tangential fields) |
|
| Tanna et al. [28] | 134 | Forward planned Intensity Modulated Radiation Therapy (IMRT) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferini, G.; Valenti, V.; Viola, A.; Umana, G.E.; Martorana, E. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers 2022, 14, 3477. https://doi.org/10.3390/cancers14143477
Ferini G, Valenti V, Viola A, Umana GE, Martorana E. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers. 2022; 14(14):3477. https://doi.org/10.3390/cancers14143477
Chicago/Turabian StyleFerini, Gianluca, Vito Valenti, Anna Viola, Giuseppe Emmanuele Umana, and Emanuele Martorana. 2022. "A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients" Cancers 14, no. 14: 3477. https://doi.org/10.3390/cancers14143477
APA StyleFerini, G., Valenti, V., Viola, A., Umana, G. E., & Martorana, E. (2022). A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers, 14(14), 3477. https://doi.org/10.3390/cancers14143477








