Visceral Obesity in Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data of the NSCLC Cohort
2.2. Assessment of CT Images for Visceral Obesity
2.3. Targeted Gene Expression Profiling of NSCLC Tumors
2.4. Statistical Analyses
3. Results
3.1. Measurement of Visceral Obesity with Fat Tissue Areas of Abdominal CT Images
3.2. Visceral Obesity at Different Body Levels
3.3. Change in Visceral Obesity over Time
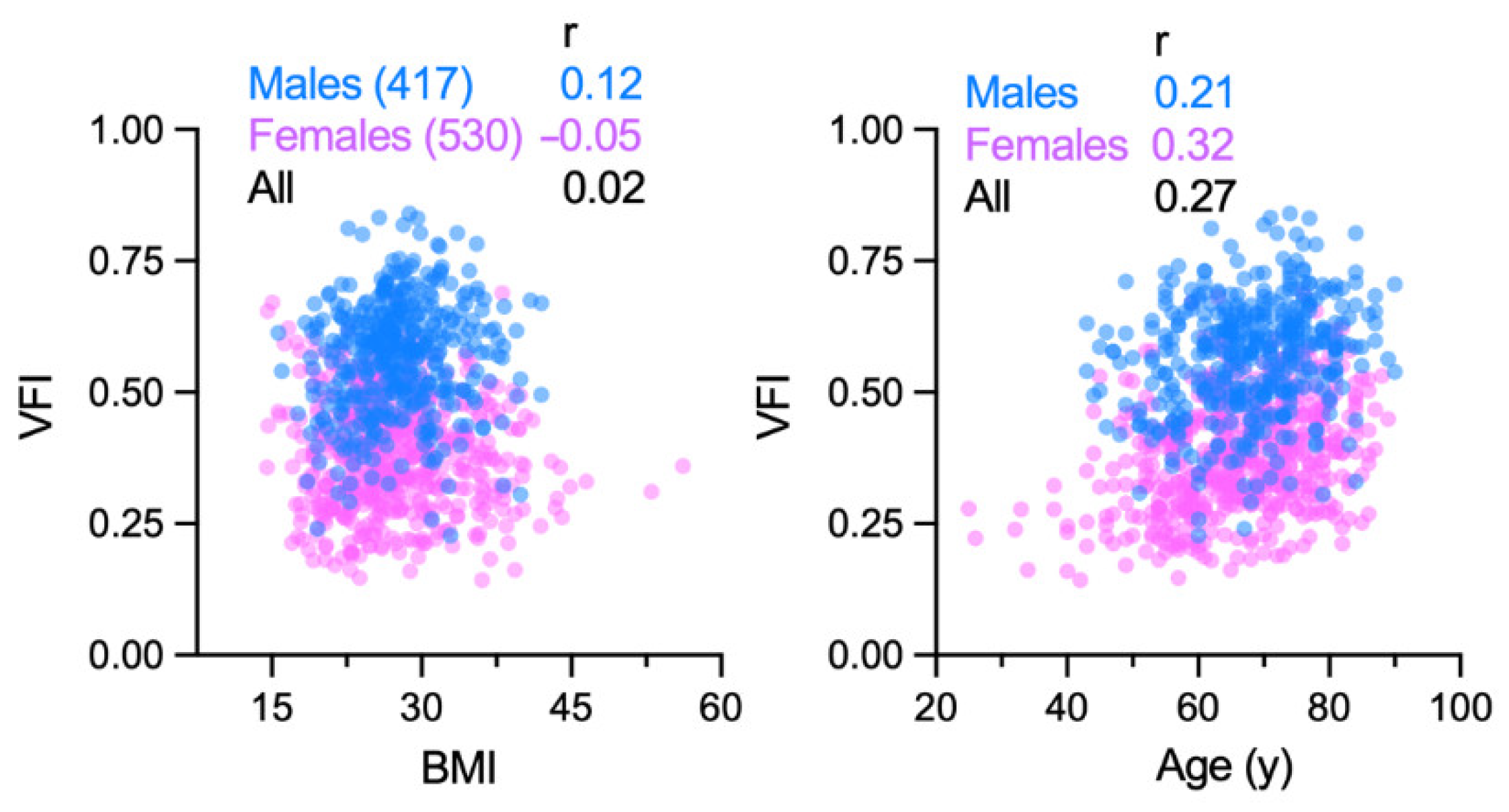
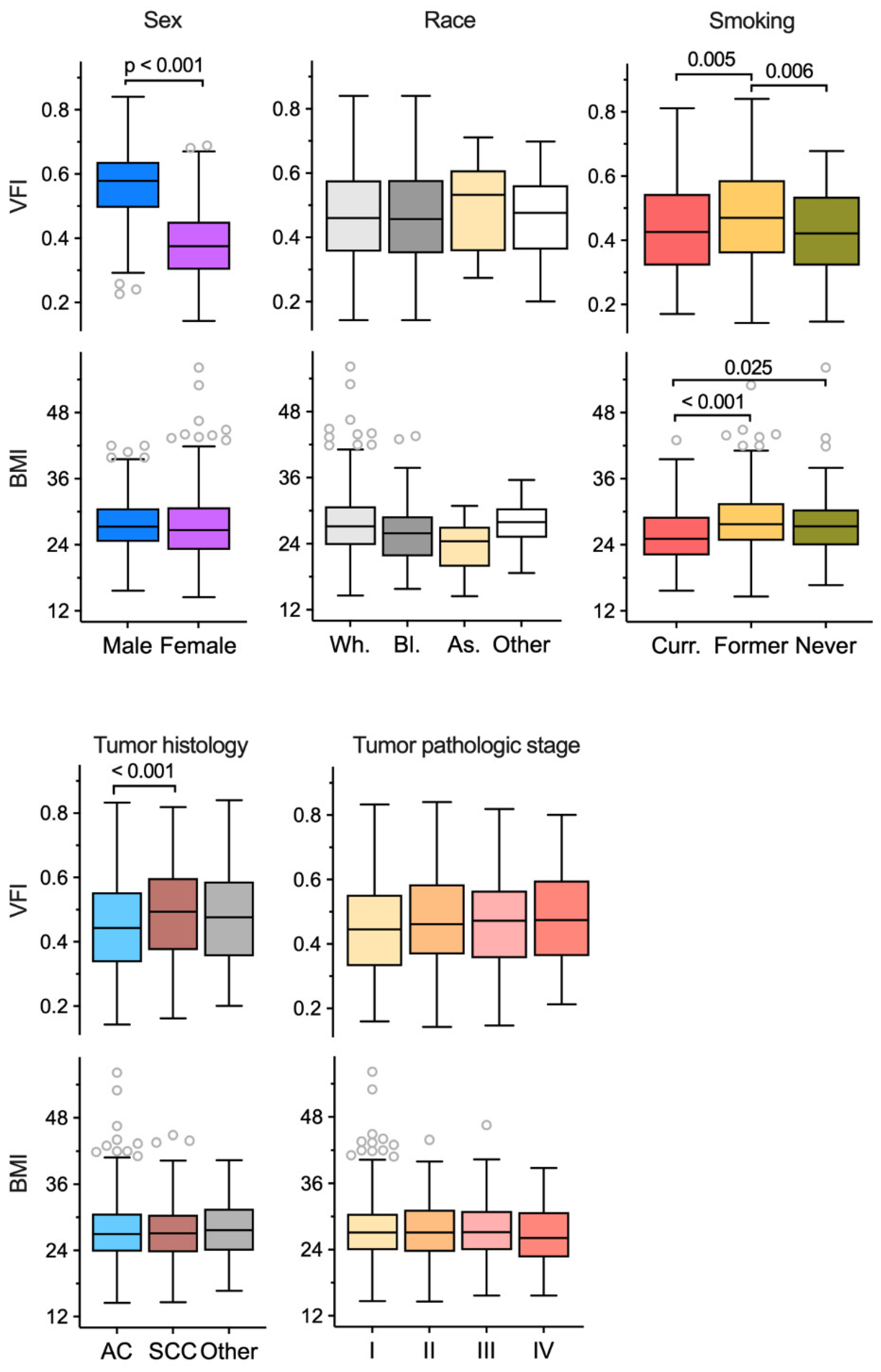
3.4. Associations of Visceral Obesity with Demographic and Clinical Features
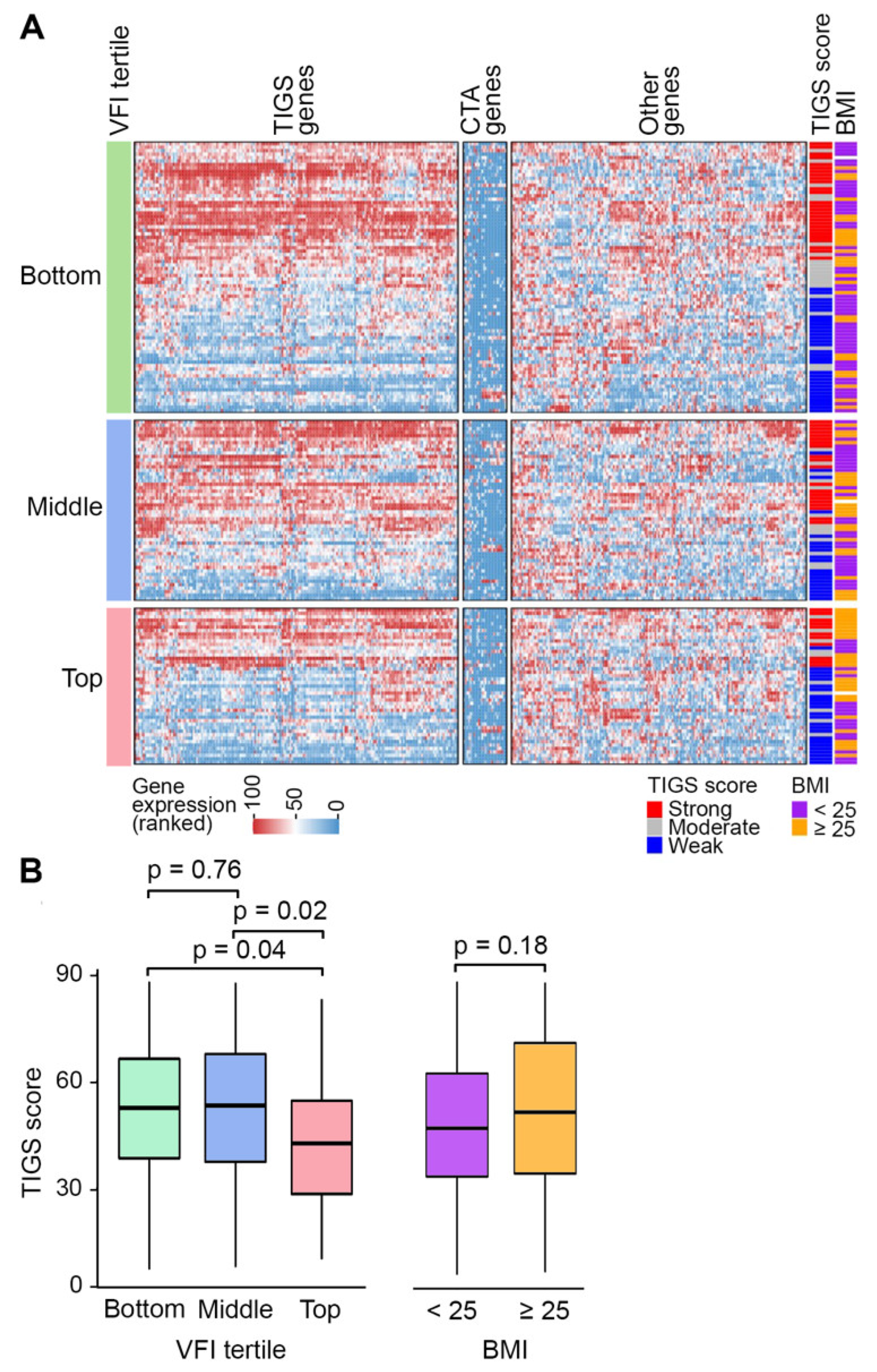
3.5. Variation of Tumor Inflammatory Gene Expression with VFI and BMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donini, L.M.; Pinto, A.; Giusti, A.M.; Lenzi, A.; Poggiogalle, E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zheng, W.; Johansson, M.; Lan, Q.; Park, Y.; White, E.; Matthews, C.E.; Sawada, N.; Gao, Y.T.; Robien, K.; et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. JNCI J. Natl. Cancer Inst. 2018, 110, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Huang, J.; Fan, J.; Du, H.; Liu, L.; Che, G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: Does the ‘obesity paradox’ really exist? Eur. J. Cardio-Thorac. Surg. 2017, 51, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Majumder, K.; Arora, N.; Mayo, H.G.; Singh, P.P.; Beg, M.S.; Hughes, R.; Singh, S.; Johnson, D.H. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer 2016, 102, 49–59. [Google Scholar] [CrossRef]
- Dahlberg, S.E.; Schiller, J.H.; Bonomi, P.B.; Sandler, A.B.; Brahmer, J.R.; Ramalingam, S.S.; Johnson, D.H. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J. Thorac. Oncol. 2013, 8, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef]
- Collet, L.; Delrieu, L.; Bouhamama, A.; Crochet, H.; Swalduz, A.; Nerot, A.; Marchal, T.; Chabaud, S.; Heudel, P.E. Association between Body Mass Index and Survival Outcome in Metastatic Cancer Patients Treated by Immunotherapy: Analysis of a French Retrospective Cohort. Cancers 2021, 13, 2200. [Google Scholar] [CrossRef]
- Patnaik, S.K.; Petrucci, C.; Barbi, J.; Seager, R.J.; Pabla, S.; Yendamuri, S. Obesity-Specific Association of Statin Use and Reduced Risk of Recurrence of Early Stage NSCLC. JTO Clin. Res. Rep. 2021, 2, 100254. [Google Scholar] [CrossRef]
- Nitsche, L.J.; Mukherjee, S.; Cheruvu, K.; Krabak, C.; Rachala, R.; Ratnakaram, K.; Sharma, P.; Singh, M.; Yendamuri, S. Exploring the Impact of the Obesity Paradox on Lung Cancer and Other Malignancies. Cancers 2022, 14, 1440. [Google Scholar] [CrossRef]
- Walker, G.E.; Marzullo, P.; Ricotti, R.; Bona, G.; Prodam, F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm. Mol. Biol. Clin. Investig. 2014, 19, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Vijay, J.; Gauthier, M.F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2020, 2, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Moore, S.C.; Koster, A.; Harris, T.B.; Park, Y.; Hollenbeck, A.; Schatzkin, A. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS ONE 2011, 6, e18582. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, K.; Du, X.; Chen, G.; Shi, M.; Shi, B. Abdominal Obesity and Lung Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2016, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Ardesch, F.H.; Ruiter, R.; Mulder, M.; Lahousse, L.; Stricker, B.H.C.; Jong, J.C.K.-D. The Obesity Paradox in Lung Cancer: Associations with Body Size Versus Body Shape. Front. Oncol. 2020, 10, 591110. [Google Scholar] [CrossRef]
- Christakoudi, S.; Tsilidis, K.K.; Evangelou, E.; Riboli, E. A Body Shape Index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. Cancer Med. 2021, 10, 5614–5628. [Google Scholar] [CrossRef]
- Bertin, E.; Marcus, C.; Ruiz, J.C.; Eschard, J.P.; Leutenegger, M. Measurement of visceral adipose tissue by DXA combined with anthropometry in obese humans. Int. J. Obes. 2000, 24, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Camhi, S.M.; Bray, G.A.; Bouchard, C.; Greenway, F.L.; Johnson, W.D.; Newton, R.L.; Ravussin, E.; Ryan, D.H.; Smith, S.R.; Katzmarzyk, P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity 2011, 19, 402–408. [Google Scholar] [CrossRef]
- Staunstrup, L.M.; Nielsen, H.B.; Pedersen, B.K.; Karsdal, M.; Blair, J.P.M.; Christensen, J.F.; Bager, C.L. Cancer risk in relation to body fat distribution, evaluated by DXA-scans, in postmenopausal women—The Prospective Epidemiological Risk Factor (PERF) study. Sci. Rep. 2019, 9, 5379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vugt, J.L.; Levolger, S.; Gharbharan, A.; Koek, M.; Niessen, W.J.; Burger, J.W.; Willemsen, S.P.; de Bruin, R.W.; Ijzermans, J.N. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J. Cachex-Sarcopenia Muscle 2017, 8, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Nattenmüller, J.; Wochner, R.; Muley, T.; Steins, M.; Hummler, S.; Teucher, B.; Wiskemann, J.; Kauczor, H.-U.; Wielpütz, M.O.; Heussel, C.P. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS ONE 2017, 12, e0169136. [Google Scholar] [CrossRef] [PubMed]
- Barbi, J.; Patnaik, S.K.; Pabla, S.; Zollo, R.; Smith, R.J., Jr.; Sass, S.N.; Srinivasan, A.; Petrucci, C.; Seager, R.; Conroy, J.; et al. Visceral Obesity Promotes Lung Cancer Progression—Toward Resolution of the Obesity Paradox in Lung Cancer. J. Thorac. Oncol. 2021, 16, 1333–1348. [Google Scholar] [CrossRef]
- Conroy, J.M.; Pabla, S.; Glenn, S.T.; Burgher, B.; Nesline, M.; Papanicolau-Sengos, A.; Andreas, J.; Giamo, V.; Lenzo, F.L.; Hyland, F.C.L.; et al. Analytical Validation of a Next-Generation Sequencing Assay to Monitor Immune Responses in Solid Tumors. J. Mol. Diagn. 2018, 20, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Pabla, S.; Seager, R.J.; Van Roey, E.; Gao, S.; Hoefer, C.; Nesline, M.K.; DePietro, P.; Burgher, B.; Andreas, J.; Giamo, V.; et al. Integration of tumor inflammation, cell proliferation, and traditional biomarkers improves prediction of immunotherapy resistance and response. Biomark. Res. 2021, 9, 56. [Google Scholar] [CrossRef]
- Barbalho, E.R.; Rocha, I.; Medeiros, G.O.C.; Friedman, R.; Fayh, A.P.T. Agreement between software programmes of body composition analyses on abdominal computed tomography scans of obese adults. Arch. Endocrinol. Metab. 2020, 64, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Heo, M.; Faith, M.S.; Pietrobelli, A. Resistance to change of adulthood body mass index. Int. J. Obes. 2002, 26, 1404–1405. [Google Scholar] [CrossRef] [Green Version]
- Leitner, B.P.; Givechian, K.B.; Ospanova, S.; Beisenbayeva, A.; Politi, K.; Perry, R.J. Multimodal analysis suggests differential immuno-metabolic crosstalk in lung squamous cell carcinoma and adenocarcinoma. NPJ Precis. Oncol. 2022, 6, 8. [Google Scholar] [CrossRef]
- He, S.; Yang, J.; Li, X.; Gu, H.; Su, Q.; Qin, L. Visceral adiposity index is associated with lung function impairment: A population-based study. Respir. Res. 2021, 22, 2. [Google Scholar] [CrossRef]
- Rizzo, S.; Petrella, F.; Bardoni, C.; Bramati, L.; Cara, A.; Mohamed, S.; Radice, D.; Raia, G.; Del Grande, F.; Spaggiari, L. CT-Derived Body Composition Values and Complications after Pneumonectomy in Lung Cancer Patients: Time for a Sex-Related Analysis? Front. Oncol. 2022, 12, 826058. [Google Scholar] [CrossRef] [PubMed]
- Katsui, K.; Ogata, T.; Sugiyama, S.; Yoshio, K.; Kuroda, M.; Yamane, M.; Hiraki, T.; Kiura, K.; Toyooka, S.; Kanazawa, S. Visceral Adipose Mass and Radiation Pneumonitis after Concurrent Chemoradiotherapy in Patients With Non-small-cell Lung Cancer. Cancer Diagn. Progn. 2021, 1, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Wang, S.C.; Su, G.L. Healthy US population reference values for CT visceral fat measurements and the impact of IV contrast, HU range, and spinal levels. Sci. Rep. 2022, 12, 2374. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.N.; Haddad, F.H. Suitability of Visceral Adiposity Index as a Marker for Cardiometabolic Risks in Jordanian Adults. Nutr. Hosp. 2015, 32, 2701–2709. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1009. [Google Scholar] [CrossRef] [Green Version]
- Machann, J.; Stefan, N.; Wagner, R.; Fritsche, A.; Bell, J.D.; Whitcher, B.; Häring, H.-U.; Birkenfeld, A.L.; Nikolaou, K.; Schick, F.; et al. Normalized Indices Derived from Visceral Adipose Mass Assessed by Magnetic Resonance Imaging and Their Correlation with Markers for Insulin Resistance and Prediabetes. Nutrients 2020, 12, 2064. [Google Scholar] [CrossRef]
- Demerath, E.W.; Shen, W.; Lee, M.; Choh, A.C.; Czerwinski, S.A.; Siervogel, R.M.; Towne, B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am. J. Clin. Nutr. 2007, 85, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Pu, L.; Ashraf, S.F.; Gezer, N.S.; Ocak, I.; Dresser, D.E.; Leader, J.K.; Dhupar, R. Estimating 3-D whole-body composition from a chest CT scan. Med. Phys. 2022. [Google Scholar] [CrossRef]
- Mohan, A.; Poulose, R.; Ansari, A.; Madan, K.; Hadda, V.; Khilnani, G.C.; Guleria, R. Alterations in body composition in Indian patients with non-small cell lung cancer. Lung India Off. Organ Indian Chest Soc. 2019, 36, 295–298. [Google Scholar] [CrossRef]
- Paris, M.T. Body Composition Analysis of Computed Tomography Scans in Clinical Populations: The Role of Deep Learning. Lifestyle Genom. 2020, 13, 28–31. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | Median (IQR)/n (%) |
|---|---|
| Age at time of analyzed CT (years) | 66 (59, 74) |
| Sex | |
| Female | 530 (56.0%) |
| Male | 417 (44.0%) |
| Race | |
| White | 849 (90.4%) |
| Black | 67 (7.1%) |
| Asian | 11 (1.2%) |
| Other | 12 (1.3%) |
| Smoking history at time of analyzed CT | |
| Current | 205 (26.0%) |
| Former | 510 (64.8%) |
| cNever | 72 (9.2%) |
| FEV1 (percent-predicted; 276 unknown) | 82 (66, 95) |
| DLCO (percent-predicted; 337 unknown) | 75 (62, 89) |
| Histology of NSCLC tumor | |
| Adenocarcinoma | 585 (61.8%) |
| Squamous cell carcinoma | 251 (26.5%) |
| Other | 111 (11.7%) |
| Pathological stage of NSCLC tumor | |
| I | 464 (56.3%) |
| II | 200 (24.3%) |
| III | 91 (11.0%) |
| IV | 69 (8.4%) |
| Body mass index | 27.1 (23.7, 30.7) |
| Body fat area in analyzed CT at L3 level | |
| Subcutaneous (mm2) | 19,395 (13,654, 27,628) |
| Visceral (mm2) | 17,601 (10,265, 26,112) |
| Total (mm2) | 39,679 (27,070, 53,227) |
| Visceral fat index | 0.46 (0.35, 0.58) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsche, L.; Vedire, Y.; Kannisto, E.; Wang, X.; Seager, R.J.; Pabla, S.; Patnaik, S.K.; Yendamuri, S. Visceral Obesity in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3450. https://doi.org/10.3390/cancers14143450
Nitsche L, Vedire Y, Kannisto E, Wang X, Seager RJ, Pabla S, Patnaik SK, Yendamuri S. Visceral Obesity in Non-Small Cell Lung Cancer. Cancers. 2022; 14(14):3450. https://doi.org/10.3390/cancers14143450
Chicago/Turabian StyleNitsche, Lindsay, Yeshwanth Vedire, Eric Kannisto, Xiaolong Wang, Robert J. Seager, Sarabjot Pabla, Santosh K. Patnaik, and Sai Yendamuri. 2022. "Visceral Obesity in Non-Small Cell Lung Cancer" Cancers 14, no. 14: 3450. https://doi.org/10.3390/cancers14143450
APA StyleNitsche, L., Vedire, Y., Kannisto, E., Wang, X., Seager, R. J., Pabla, S., Patnaik, S. K., & Yendamuri, S. (2022). Visceral Obesity in Non-Small Cell Lung Cancer. Cancers, 14(14), 3450. https://doi.org/10.3390/cancers14143450






