Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Volunteers Recruitment
2.2. Cell Culture
2.3. Reverse-Transcription qPCR
2.4. Vital Colorant Assay
2.5. Cell Sorting and Flow Cytometry
2.6. In Vitro Modulation of Monocyte Polarisation
2.7. Soluble Cytokines Quantification
2.8. Transwell Migration Assay
2.9. In Vitro Proliferation Assays
2.10. Soluble Immune-Checkpoint Measurement
2.11. Tissue Sample Immunohistochemistry
2.12. Statistical Analysis
2.13. Ethics Approval
3. Results
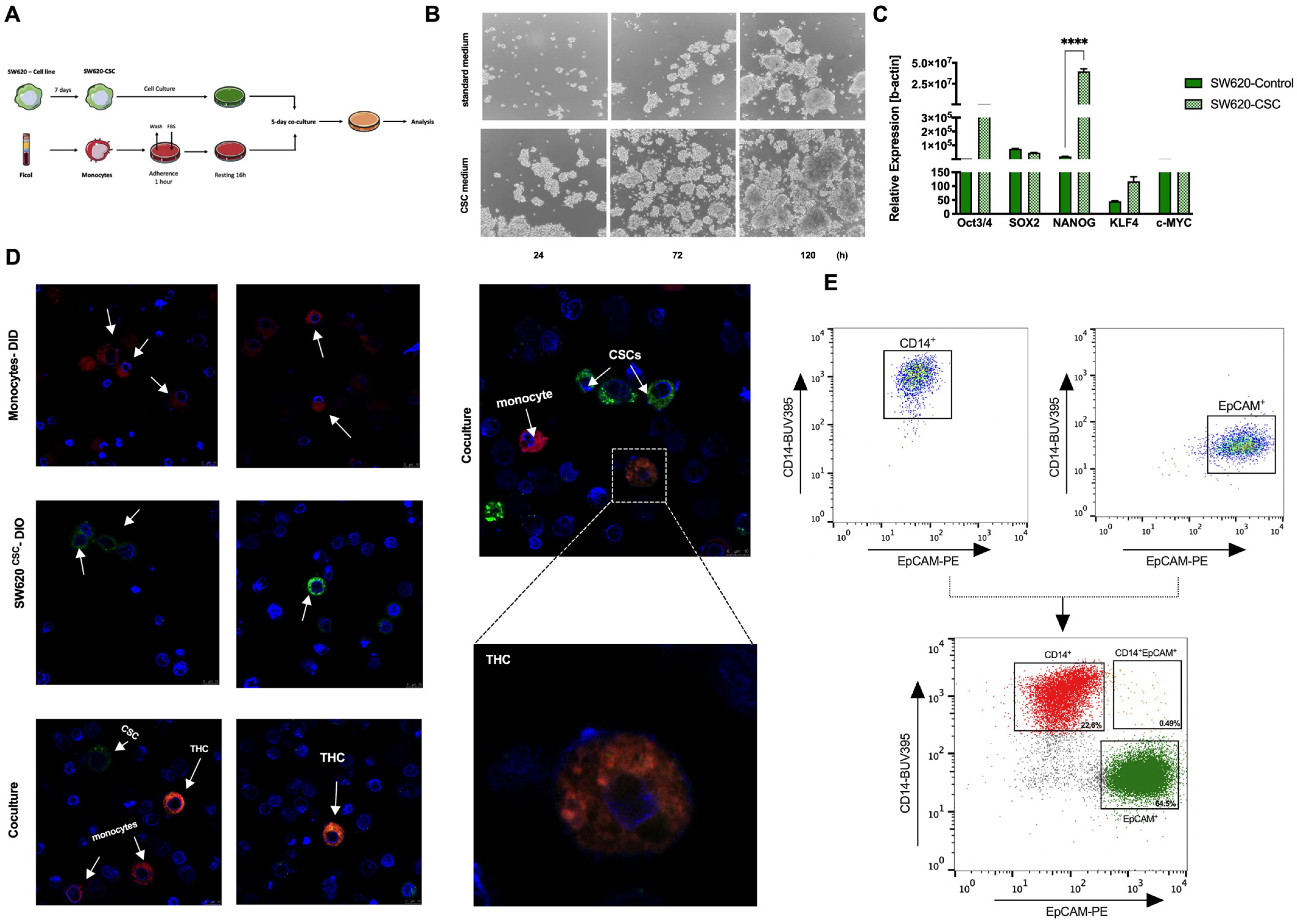
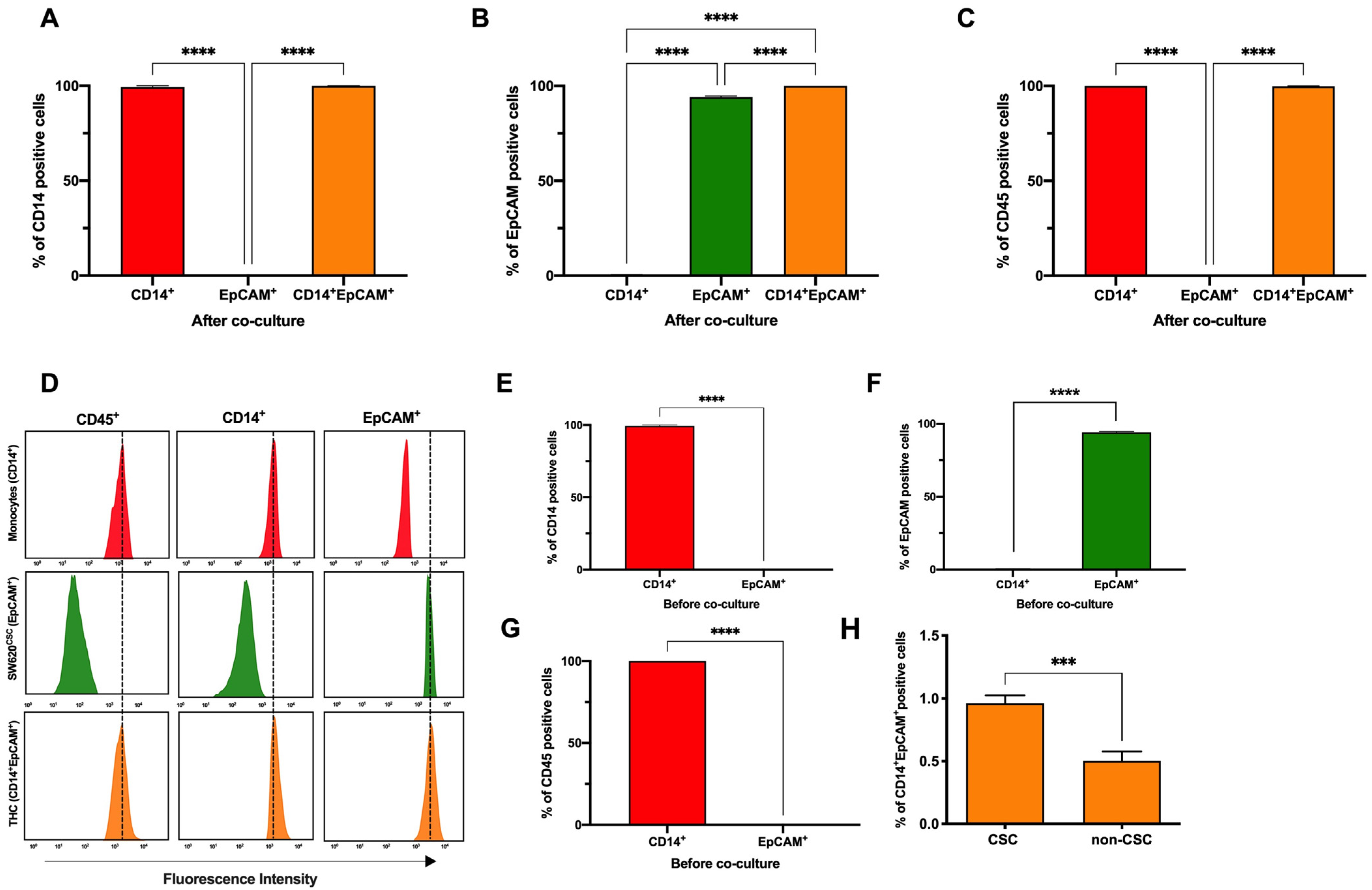
3.1. Co-Culture of Colorectal Cancer Stem Cells with Human Monocytes in Vitro Yields a New Hybrid Entity
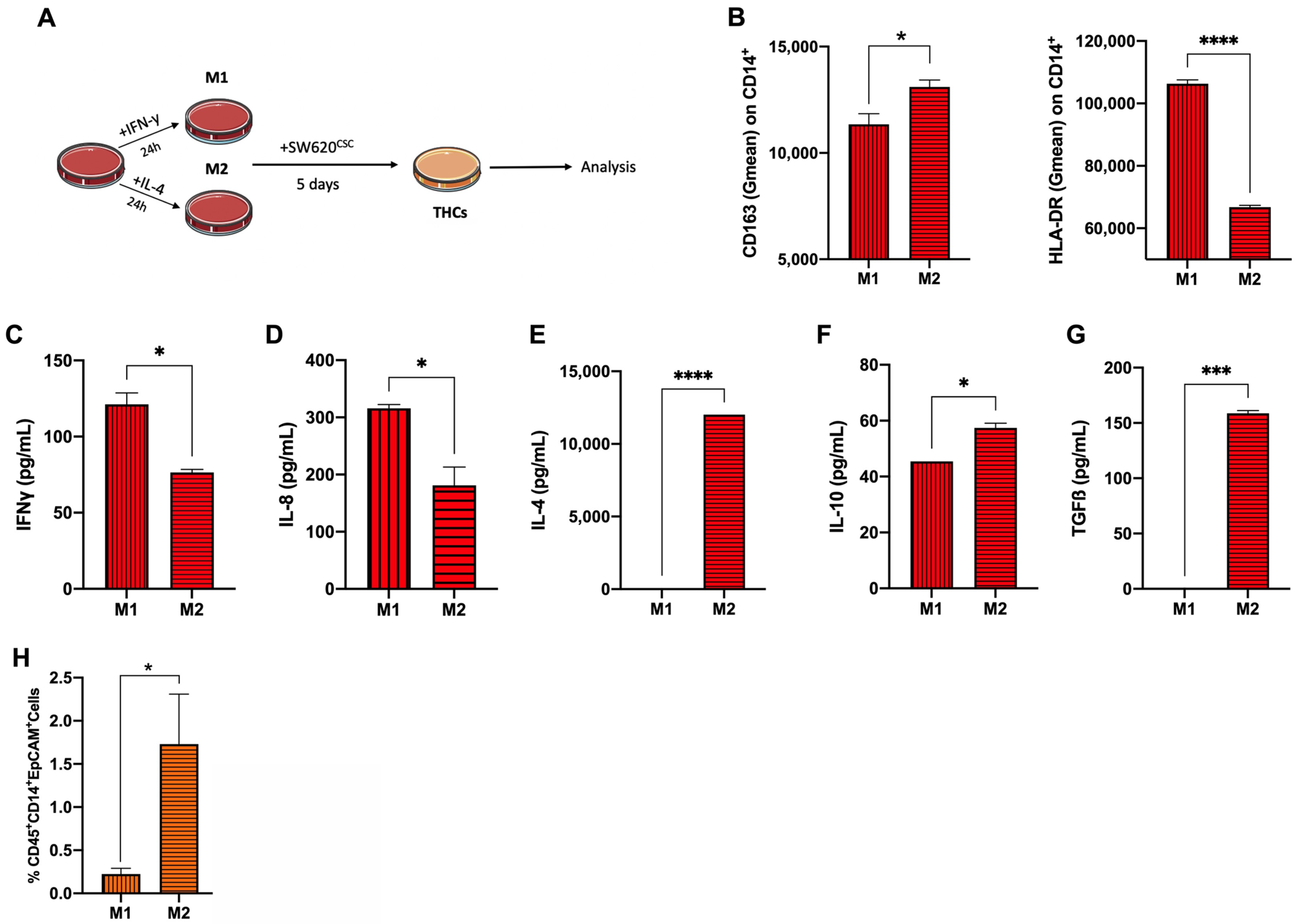
3.2. Anti-Inflammatory Phenotype Favours THC Generation
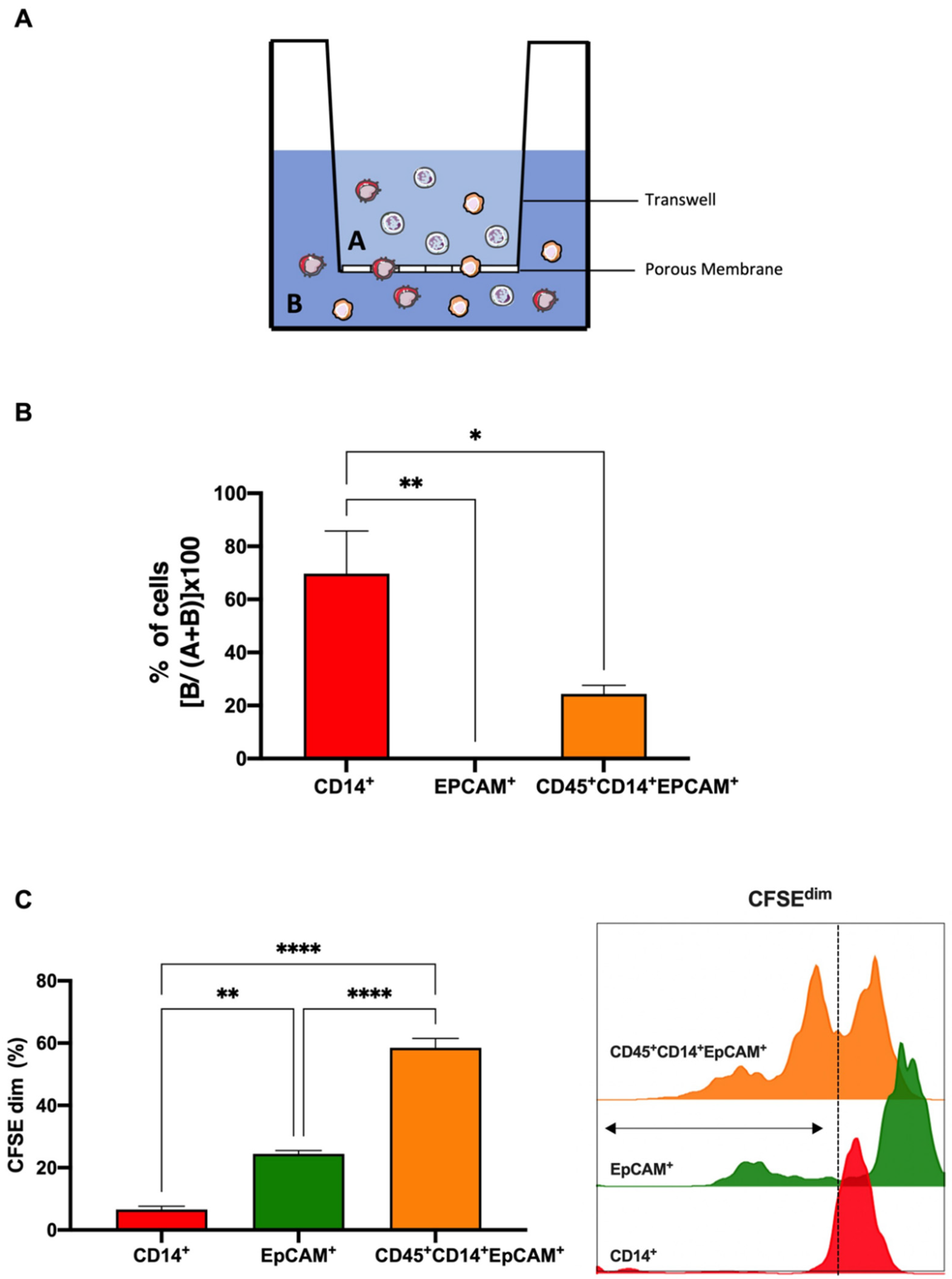
3.3. Colorectal THCs Exhibit a High Rate of Migration and Proliferation In Vitro
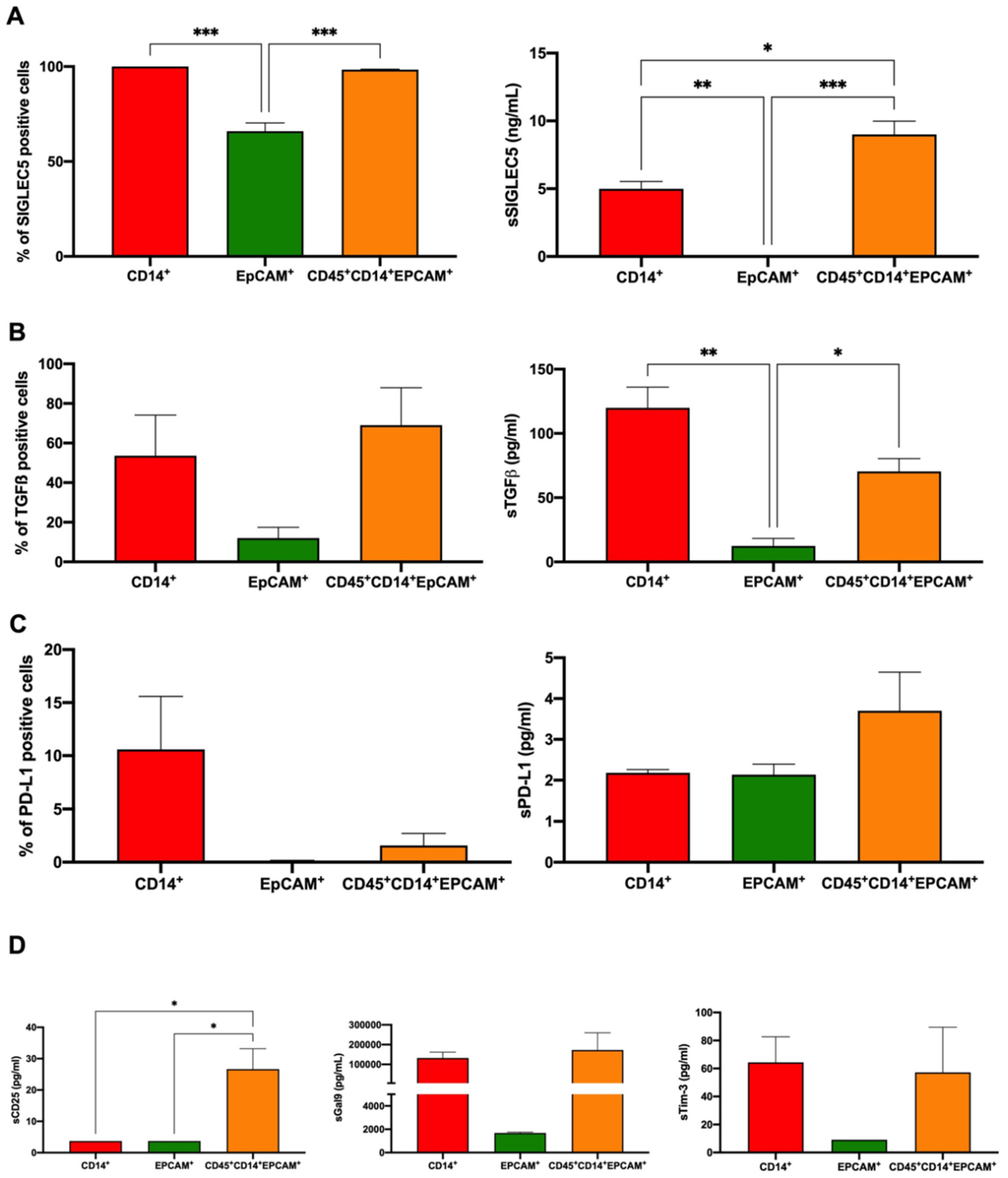
3.4. Colorectal THCs Inhibit Lymphocyte Proliferation through SIGLEC5 Immune Checkpoint
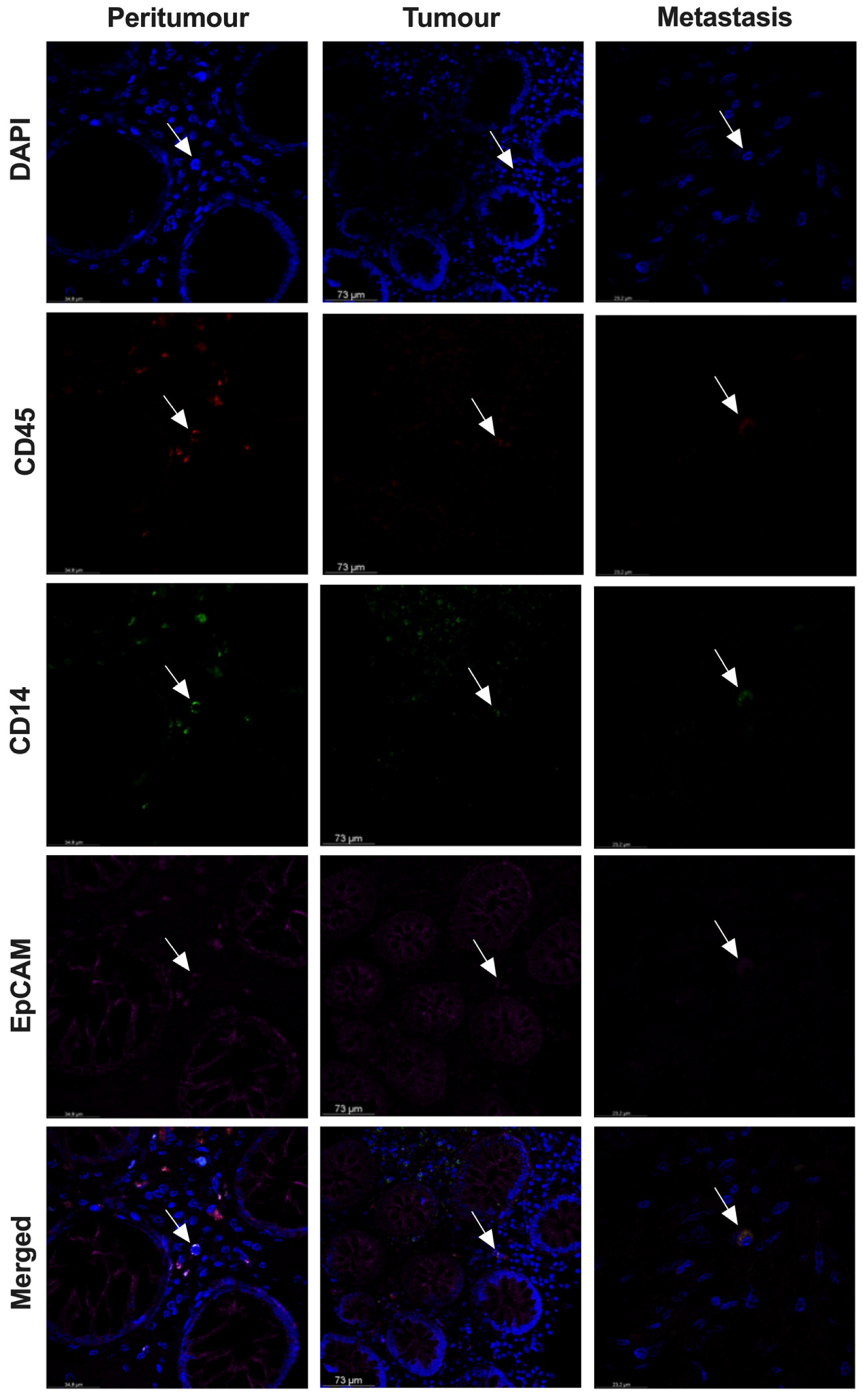
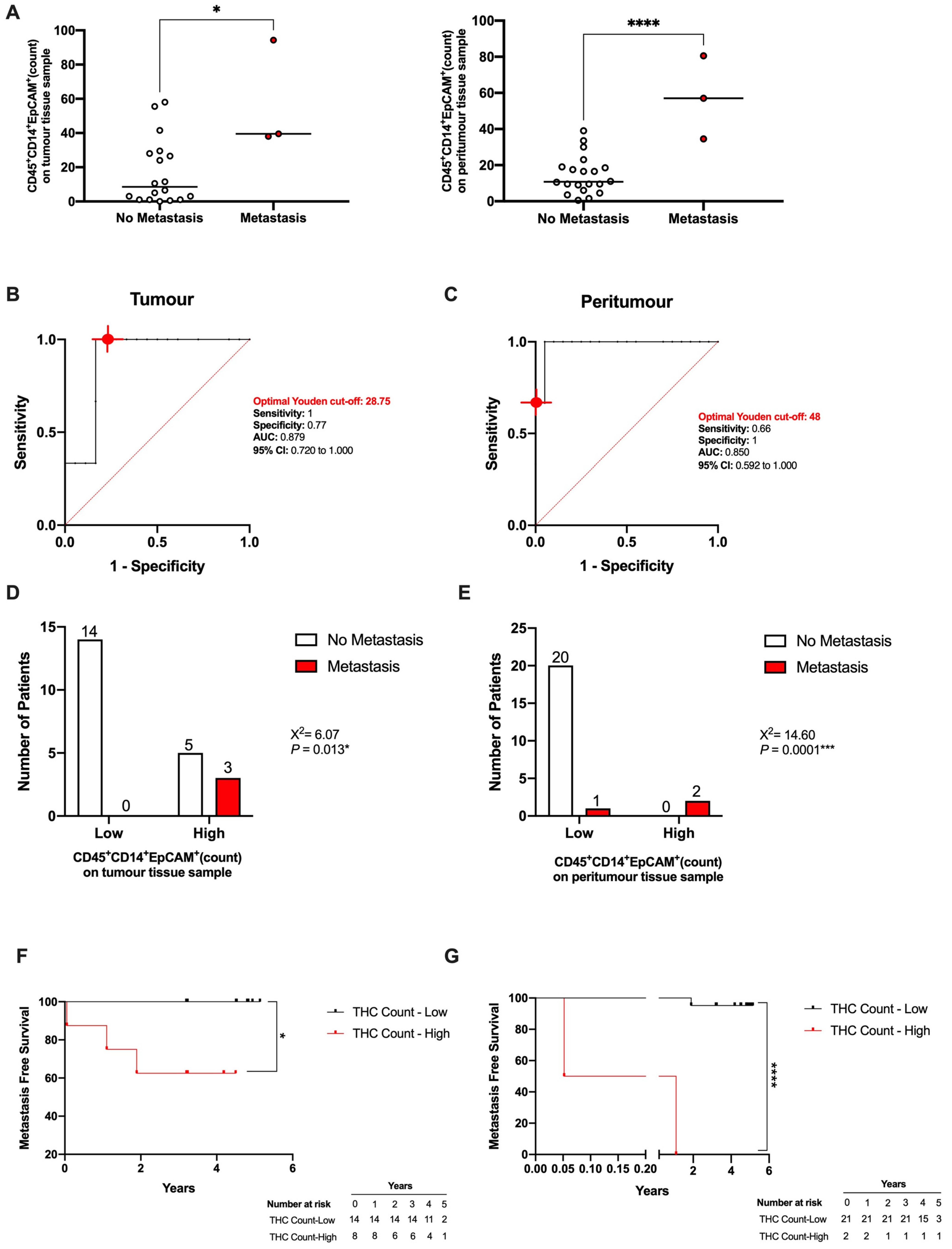
3.5. THC-Specific Signature Is Present in Primary Tissue and Metastatic Samples of Colorectal Cancer Patients
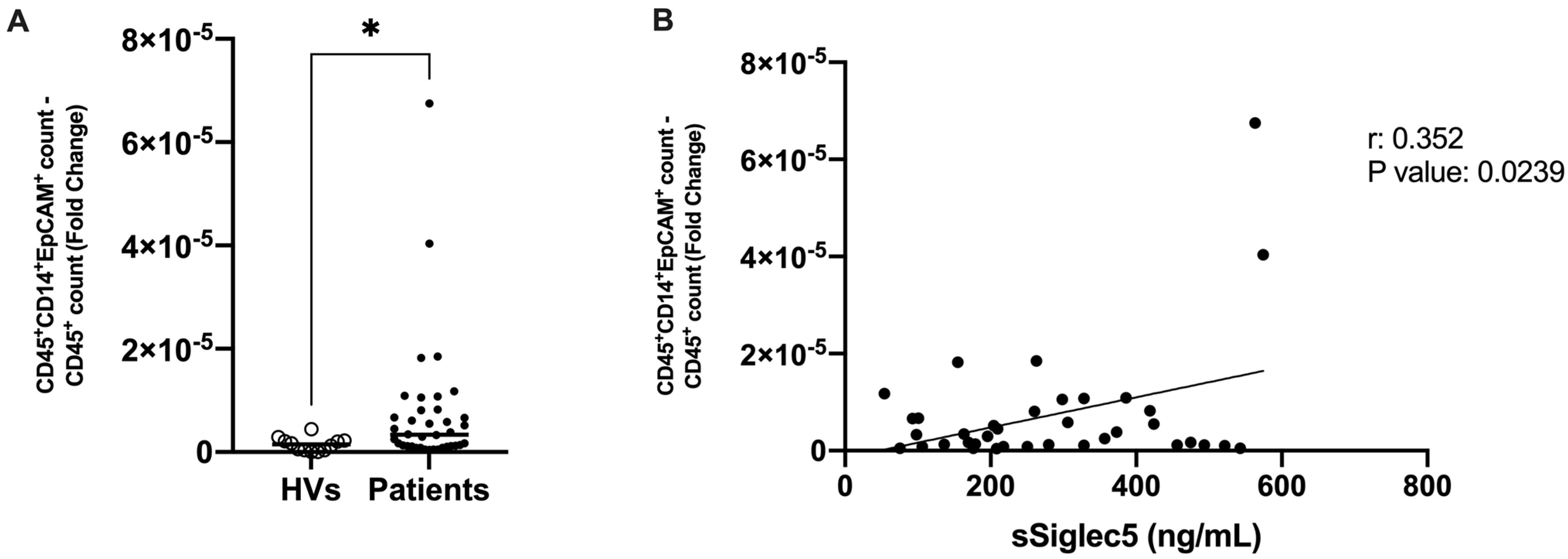
3.6. THC-Specific Signature Is Present in Circulation of Colorectal Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkin, D.; Whelan, S.; Ferlay, J.; Teppo, L.; Thomas, D. Cancer Incidence in Five Continents Volume VIII. IARC Sci. Publ. 2002, 155, 729990. [Google Scholar]
- Ferlay, J. Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. IARC Cancerbase 2001, 49, 1374–1403. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Speicher, M. The Biology of Circulating Tumor Cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef]
- Racila, E.; Euhus, D.; Weiss, A.J.; Rao, C.; McConnell, J.; Terstappen, L.W.; Uhr, J.W. Detection and Characterization of Carcinoma Cells in the Blood. Proc. Natl. Acad. Sci. USA 1998, 95, 4589–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaifi, J.T.; Li, G.; Clawson, G.; Kimchi, E.T.; Staveley-O’Carroll, K.F. Perioperative Circulating Tumor Cell Detection: Current Perspectives. Cancer Biol. Ther. 2016, 17, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Aichel, O. Über Zellverschmelzung Mit Qualitativ Abnormer Chromosomenverteilung Als Ursache Der Geschwulstbildung; W. Engelmann: Liepzig, Germany, 1911. [Google Scholar]
- Fernandes, C.; Prabhu, P.; Juvale, K.; Suares, D.; Mayur, Y. Cancer Cell Fusion: A Potential Target to Tackle Drug-Resistant and Metastatic Cancer Cells. Drug Discov. Today 2019, 24, 1836–1844. [Google Scholar] [CrossRef]
- Laberge, G.S.; Duvall, E.; Haedicke, K.; Pawelek, J. Leukocyte–Cancer Cell Fusion—Genesis of a Deadly Journey. Cells 2019, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Pawelek, J.M. Tumour Cell Hybridization and Metastasis Revisited. Melanoma Res. 2000, 10, 507–514. [Google Scholar] [CrossRef]
- Pawelek, J.M. Tumour-Cell Fusion as a Source of Myeloid Traits in Cancer. Lancet Oncol. 2005, 6, 988–993. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Chakraborty, A.K. The Cancer Cell--Leukocyte Fusion Theory of Metastasis. Adv. Cancer Res. 2008, 101, 397–444. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Kimchi, E.; Patrick, S.D.; Xin, P.; Harouaka, R.; Zheng, S.; Berg, A.; Schell, T.; Staveley-O’Carroll, K.F.; Neves, R.I. Circulating Tumor Cells in Melanoma Patients. PLoS ONE 2012, 7, e41052. [Google Scholar] [CrossRef] [Green Version]
- Shabo, I.; Midtbö, K.; Andersson, H.; Åkerlund, E.; Olsson, H.; Wegman, P.; Gunnarsson, C.; Lindström, A. Macrophage Traits in Cancer Cells Are Induced by Macrophage-Cancer Cell Fusion and Cannot Be Explained by Cellular Interaction. BMC Cancer 2015, 15, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, L.A.; Montalbán-Hernández, K.; Avendaño-Ortiz, J.; Marín, E.; Lozano, R.; Toledano, V.; Sánchez-Maroto, L.; Terrón, V.; Valentín, J.; Pulido, E.; et al. Tumor Stem Cells Fuse with Monocytes to Form Highly Invasive Tumor-Hybrid Cells. OncoImmunology 2020, 9, 1773204. [Google Scholar] [CrossRef]
- Rubio, C.; Avendaño-Ortiz, J.; Ruiz-Palomares, R.; Karaivanova, V.; Alberquilla, O.; Sánchez-Domínguez, R.; Casalvilla-Dueñas, J.C.; Montalbán-Hernández, K.; Lodewijk, I.; Rodríguez-Izquierdo, M.; et al. Toward Tumor Fight and Tumor Microenvironment Remodeling: PBA Induces Cell Cycle Arrest and Reduces Tumor Hybrid Cells’ Pluripotency in Bladder Cancer. Cancers 2022, 14, 287. [Google Scholar] [CrossRef]
- Pawelek, J.; Chakraborty, A.; Lazova, R.; Yilmaz, Y.; Cooper, D.; Brash, D.; Handerson, T. Co-Opting Macrophage Traits in Cancer Progression: A Consequence of Tumor Cell Fusion? Infect. Inflamm. Impacts Oncog. 2006, 13, 138–155. [Google Scholar]
- del Fresno, C.; García-Rio, F.; Gómez-Piña, V.; Soares-Schanoski, A.; Fernández-Ruíz, I.; Jurado, T.; Kajiji, T.; Shu, C.; Marín, E.; Gutierrez del Arroyo, A.; et al. Potent Phagocytic Activity with Impaired Antigen Presentation Identifying Lipopolysaccharide-Tolerant Human Monocytes: Demonstration in Isolated Monocytes from Cystic Fibrosis Patients. J. Immunol 2009, 182, 6494–6507. [Google Scholar] [CrossRef]
- Cave, D.D.; Hernando-Momblona, X.; Sevillano, M.; Minchiotti, G.; Lonardo, E. Nodal-Induced L1CAM/CXCR4 Subpopulation Sustains Tumor Growth and Metastasis in Colorectal Cancer Derived Organoids. Theranostics 2021, 11, 5686–5699. [Google Scholar] [CrossRef]
- Montalbán-Hernández, K.; Cantero-Cid, R.; Lozano-Rodríguez, R.; Pascual-Iglesias, A.; Avendaño-Ortiz, J.; Casalvilla-Dueñas, J.C.; Bonel Pérez, G.C.; Guevara, J.; Marcano, C.; Barragán, C.; et al. Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients. Cancers 2021, 13, 3896. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.E.; Anderson, E.C.; Davies, P.S.; Silk, A.D.; Pelz, C.; Impey, S.; Wong, M.H. Fusion between Intestinal Epithelial Cells and Macrophages in a Cancer Context Results in Nuclear Reprogramming. Cancer Res. 2011, 71, 1497–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, D.M.; Pavia, R.A.; Tsao, M.C. In Vivo Hybridisation of Human Tumour and Normal Hamster Cells. Nature 1974, 250, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Rachkovsky, M.; Sodi, S.; Chakraborty, A.; Avissar, Y.; Bolognia, J.; McNiff, J.M.; Platt, J.; Bermudes, D.; Pawelek, J. Melanoma x Macrophage Hybrids with Enhanced Metastatic Potential. Clin. Exp. Metastasis 1998, 16, 299–312. [Google Scholar] [CrossRef]
- Busund, L.-T.R.; Killie, M.K.; Bartnes, K.; Seljelid, R. Spontaneously Formed Tumorigenic Hybrids of Meth A Sarcoma and Macrophages Grow Faster and Are Better Vascularized than the Parental Tumor. Int. J. Cancer 2002, 100, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Mathur, S.R.; Mukhopadhyay, A. Fusion-Derived Epithelial Cancer Cells Express Hematopoietic Markers and Contribute to Stem Cell and Migratory Phenotype in Ovarian Carcinoma. Cancer Res. 2013, 73, 5360–5370. [Google Scholar] [CrossRef] [Green Version]
- Clawson, G.A.; Matters, G.L.; Xin, P.; McGovern, C.; Wafula, E.; DePamphilis, C.; Meckley, M.; Wong, J.; Stewart, L.; D’Jamoos, C. “Stealth Dissemination” of Macrophage-Tumor Cell Fusions Cultured from Blood of Patients with Pancreatic Ductal Adenocarcinoma. PLoS ONE 2017, 12, e0184451. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.-H.; Gao, X.; Luo, D.; Zhou, X.-D.; Xiong, W.; Liu, G.-X. EMT and Acquisition of Stem Cell-like Properties Are Involved in Spontaneous Formation of Tumorigenic Hybrids between Lung Cancer and Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e87893. [Google Scholar] [CrossRef]
- Clawson, G.A.; Matters, G.L.; Xin, P.; Imamura-Kawasawa, Y.; Du, Z.; Thiboutot, D.M.; Helm, K.F.; Neves, R.I.; Abraham, T. Macrophage-Tumor Cell Fusions from Peripheral Blood of Melanoma Patients. PLoS ONE 2015, 10, e0134320. [Google Scholar] [CrossRef] [Green Version]
- Vignery, A. Macrophage Fusion: Are Somatic and Cancer Cells Possible Partners? Trends Cell Biol. 2005, 15, 188–193. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell Fusion Potentiates Tumor Heterogeneity and Reveals Circulating Hybrid Cells That Correlate with Stage and Survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabo, I.; Olsson, H.; Stål, O.; Svanvik, J. Breast Cancer Expression of DAP12 Is Associated With Skeletal and Liver Metastases and Poor Survival. Clin. Breast Cancer 2013, 13, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Rodríguez, R.; Avendaño-Ortíz, J.; Montalbán-Hernández, K.; Ruiz-Rodríguez, J.C.; Ferrer, R.; Martín-Quirós, A.; Maroun-Eid, C.; González-López, J.J.; Fàbrega, A.; Terrón, V.; et al. SIGLEC5: An Immune Checkpoint Ligand in Sepsis. Infectious Diseases (except HIV/AIDS). MedRxiv 2020, in press. [Google Scholar] [CrossRef]
- Carter, A. Cell Fusion Theory: Can It Explain What Triggers Metastasis? JNCI J. Natl. Cancer Inst. 2008, 100, 1279–1281. [Google Scholar] [CrossRef] [Green Version]
- Cornish, A.L.; Freeman, S.; Forbes, G.; Ni, J.; Zhang, M.; Cepeda, M.; Gentz, R.; Augustus, M.; Carter, K.C.; Crocker, P.R. Characterization of Siglec-5, a Novel Glycoprotein Expressed on Myeloid Cells Related to CD33. Blood 1998, 92, 2123–2132. [Google Scholar] [CrossRef]
- Lin, H.-C.; Hsu, H.-C.; Hsieh, C.-H.; Wang, H.-M.; Huang, C.-Y.; Wu, M.-H.; Tseng, C.-P. A Negative Selection System PowerMag for Effective Leukocyte Depletion and Enhanced Detection of EpCAM Positive and Negative Circulating Tumor Cells. Clin. Chim. Acta 2013, 419, 77–84. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.J.; Alpaugh, R.K.; Gross, S.; O’Hara, S.M.; Smirnov, D.A.; Terstappen, L.W.M.M.; Allard, W.J.; Bilbee, M.; Cheng, J.D.; Hoffman, J.P.; et al. Isolation and Characterization of Circulating Tumor Cells in Patients with Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2006, 6, 125–132. [Google Scholar] [CrossRef]

| Characteristic | All Patients N = 23 | Stage I N = 4 | Stage II N = 11 | Stage III N = 8 | p-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 14 (61) | 2 (14) | 7 (50) | 5 (36) | 0.438 |
| Female | 9 (39) | 2 (22) | 4 (44) | 3 (34) | 0.986 |
| Age | |||||
| Median Range | 78 (63–92) | 78.5 (63–81) | 78 (68–87) | 73.5 (64–92) | 0.525 |
| Metastasis | |||||
| Synchronous | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Metachronous | 3 (13) | 0 (0) | 0 (0) | 3 (100) | 0.039 |
| Exitus | 2 (9) | 0 (0) | 1 (50) | 1 (50) | 0.767 |
| Tumour Site | |||||
| Ascending Colon | 7 (31) | 1 (14) | 4 (57) | 2 (29) | 0.839 |
| Transverse Colon | 4 (17) | 1 (25) | 3 (75) | 0 (0) | 0.273 |
| Descending Colon | 4 (17) | 1 (25) | 1 (25) | 2 (50) | 0.603 |
| Caecum | 8 (35) | 1 (12) | 3 (38) | 4 (50) | 0.532 |
| Comorbidities | |||||
| Smoker | 4 (17) | 0 (0) | 3 (75) | 1 (25) | 0.422 |
| Arterial Hypertension | 8 (35) | 3 (38) | 4 (50) | 1 (12) | 0.099 |
| Dyslipidaemia | 9 (39) | 2 (22) | 6 (67) | 1 (11) | 0.159 |
| Diabetes Mellitus | 3 (13) | 2 (66) | 1 (34) | 0 (0) | 0.045 * |
| BMI Median Range | 28.3 (23.2–35.5) | 28.4 (26.1–30.8) | 27.1 (23.2–35.4) | 29.7 (23.5–35.5) | 0.919 |
| Characteristic | Healthy Volunteers N = 12 | All Patients N = 38 | Stage I N = 10 | Stage II N = 10 | Stage III N = 16 | Stage IV N = 2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 8 (67) | 27(71) | 8 (30) | 8 (30) | 9 (33) | 2 (7) | 0.347 |
| Female | 4 (33) | 11 (29) | 2 (18) | 2 (18) | 7 (64) | 0 (0) | 0.500 |
| Age | |||||||
| Median Range | 44.5 (26–75) | 71.5 (50–92) | 64.5 (62–71) | 72 (50–85) | 75 (50–92) | 68 (55–81) | 0.298 |
| Metastasis | |||||||
| Synchronous | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | <0.0001 **** | |
| Metachronous | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Exitus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Tumour Site | |||||||
| Ascending Colon | 11 (30) | 2 (19) | 4 (36) | 4 (36) | 1 (9) | 0.609 | |
| Transverse Colon | 2 (5) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0.116 | |
| Descending Colon | 20 (54) | 4 (20) | 5 (25) | 11 (55) | 0 (0) | 0.207 | |
| Caecum | 4 (11) | 2 (50) | 1 (25) | 0 (0) | 1 (25) | 0.104 | |
| Comorbidities | |||||||
| Smoker | 15 (39) | 4 (27) | 4 (27) | 5 (33) | 2 (13) | 0.317 | |
| Arterial Hypertension | 18 (47) | 3 (17) | 5 (27) | 9 (50) | 1 (6) | 0.626 | |
| Dyslipidaemia | 11 (29) | 3 (27) | 4 (37) | 3 (27) | 1 (9) | 0.606 | |
| Diabetes Mellitus | 8 (21) | 3 (37) | 3 (37) | 1 (13) | 1 (13) | 0.252 | |
| BMI Median Range | 27.02 (19.7–42.2) | 27.86 (19.7 31.6) | 26.09 (23.8–33.9) | 26.65 (21.4–42.2) | 25.04 (20.3–29.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalbán-Hernández, K.; Cantero-Cid, R.; Casalvilla-Dueñas, J.C.; Avendaño-Ortiz, J.; Marín, E.; Lozano-Rodríguez, R.; Terrón-Arcos, V.; Vicario-Bravo, M.; Marcano, C.; Saavedra-Ambrosy, J.; et al. Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System. Cancers 2022, 14, 3445. https://doi.org/10.3390/cancers14143445
Montalbán-Hernández K, Cantero-Cid R, Casalvilla-Dueñas JC, Avendaño-Ortiz J, Marín E, Lozano-Rodríguez R, Terrón-Arcos V, Vicario-Bravo M, Marcano C, Saavedra-Ambrosy J, et al. Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System. Cancers. 2022; 14(14):3445. https://doi.org/10.3390/cancers14143445
Chicago/Turabian StyleMontalbán-Hernández, Karla, Ramón Cantero-Cid, José Carlos Casalvilla-Dueñas, José Avendaño-Ortiz, Elvira Marín, Roberto Lozano-Rodríguez, Verónica Terrón-Arcos, Marina Vicario-Bravo, Cristóbal Marcano, Jorge Saavedra-Ambrosy, and et al. 2022. "Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System" Cancers 14, no. 14: 3445. https://doi.org/10.3390/cancers14143445
APA StyleMontalbán-Hernández, K., Cantero-Cid, R., Casalvilla-Dueñas, J. C., Avendaño-Ortiz, J., Marín, E., Lozano-Rodríguez, R., Terrón-Arcos, V., Vicario-Bravo, M., Marcano, C., Saavedra-Ambrosy, J., Prado-Montero, J., Valentín, J., Pérez de Diego, R., Córdoba, L., Pulido, E., del Fresno, C., Dueñas, M., & López-Collazo, E. (2022). Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System. Cancers, 14(14), 3445. https://doi.org/10.3390/cancers14143445







