Type IV Collagen in Human Colorectal Liver Metastases—Cellular Origin and a Circulating Biomarker
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Sample Collection for Tissue Analysis
2.3. Patients and Sample Collection for Plasma Analysis
2.4. Expression of Type IV Collagen in Colorectal Liver Metastases and Cell Lines
2.4.1. Protein Expression of COL IV in Colorectal Liver Metastases
2.4.2. RNA Expression of COL IV in Colorectal Liver Metastases
2.4.3. Expression of CAF Markers in Colorectal Liver Metastases
2.4.4. Expression of COL IV in Cell Lines
Cell Lines and Cell Culturing
Analysis of COL IV Expression in Cell Lines
2.5. Expression of Type IV Collagen-Degrading Proteases in Colorectal Liver Metastases
2.6. Circulating Type IV Collagen in Patients with Colorectal Liver Metastases
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics of Tissue Cohort
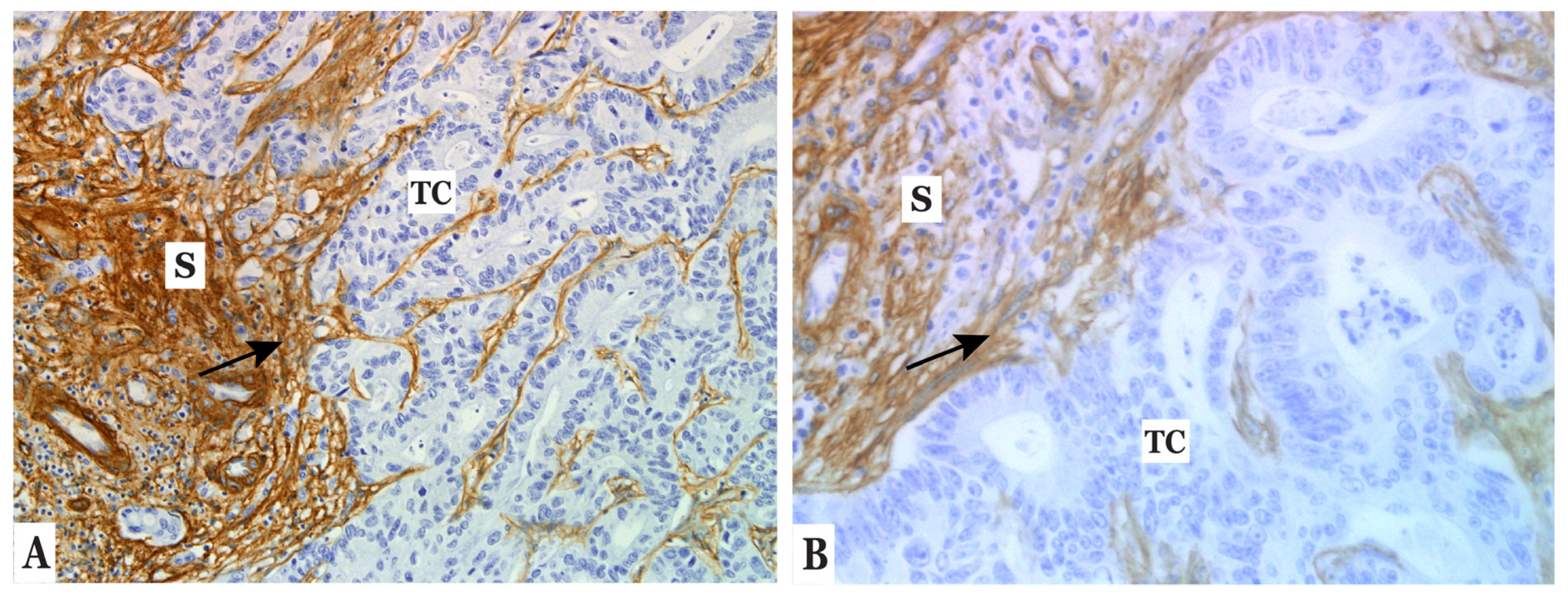
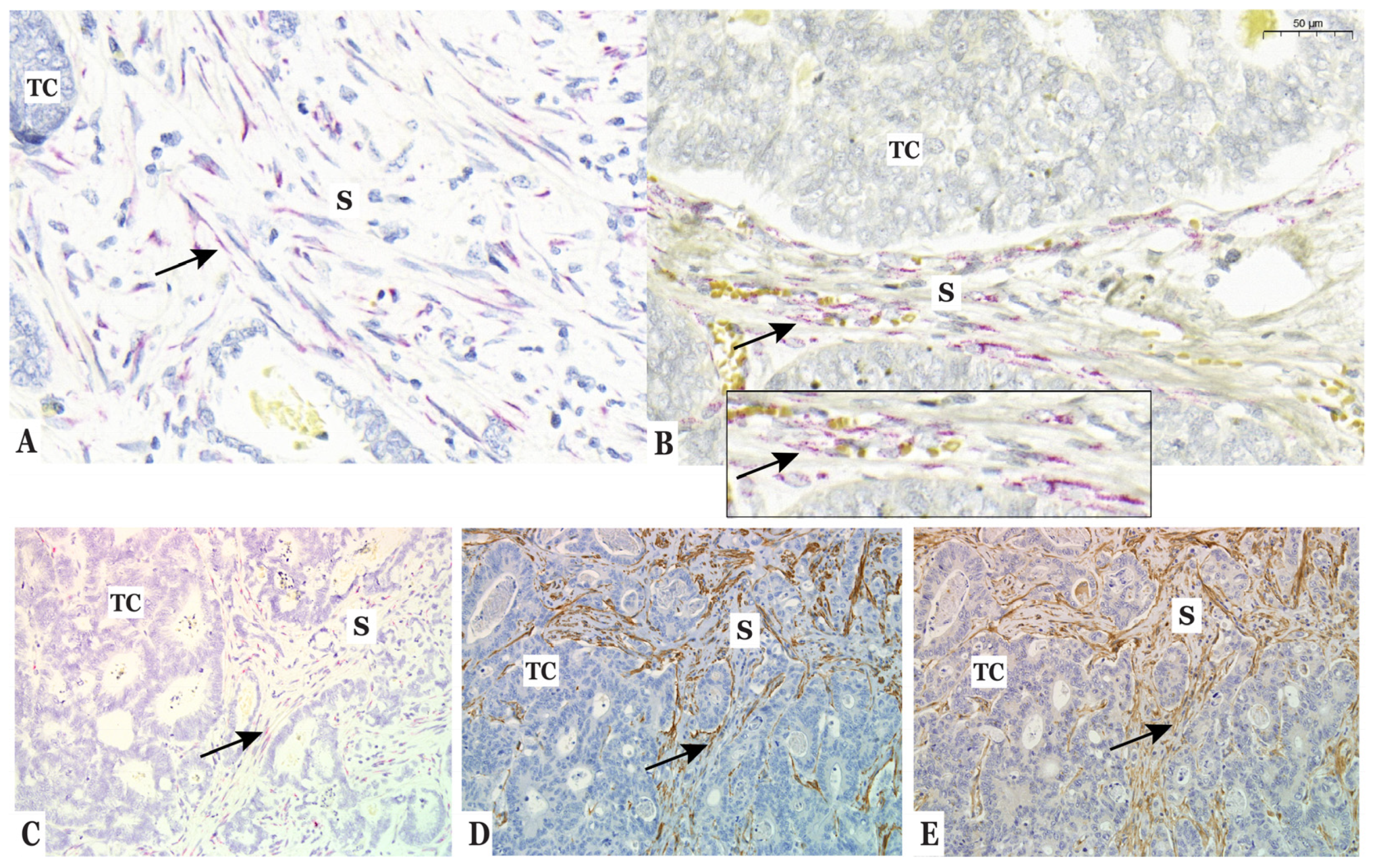
3.2. Type IV Collagen Is Expressed by CAFs in the Stroma of Colorectal Liver Metastases (CLM)
3.3. Type IV Collagen Is Produced by Fibroblasts In Vitro but Not by Colorectal Cancer Cells
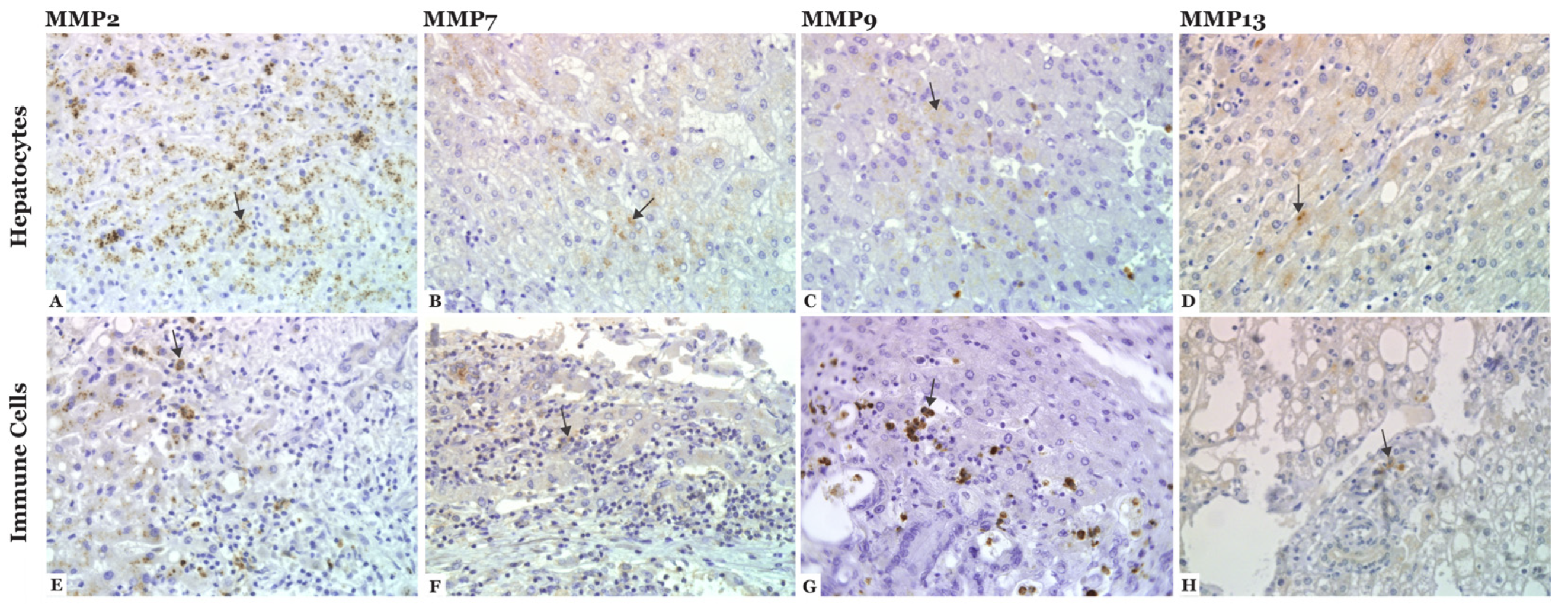
3.4. MMP-2, -7, -9, and -13 Are Expressed in CLM
3.5. Patient Characteristics of Plasma Cohort
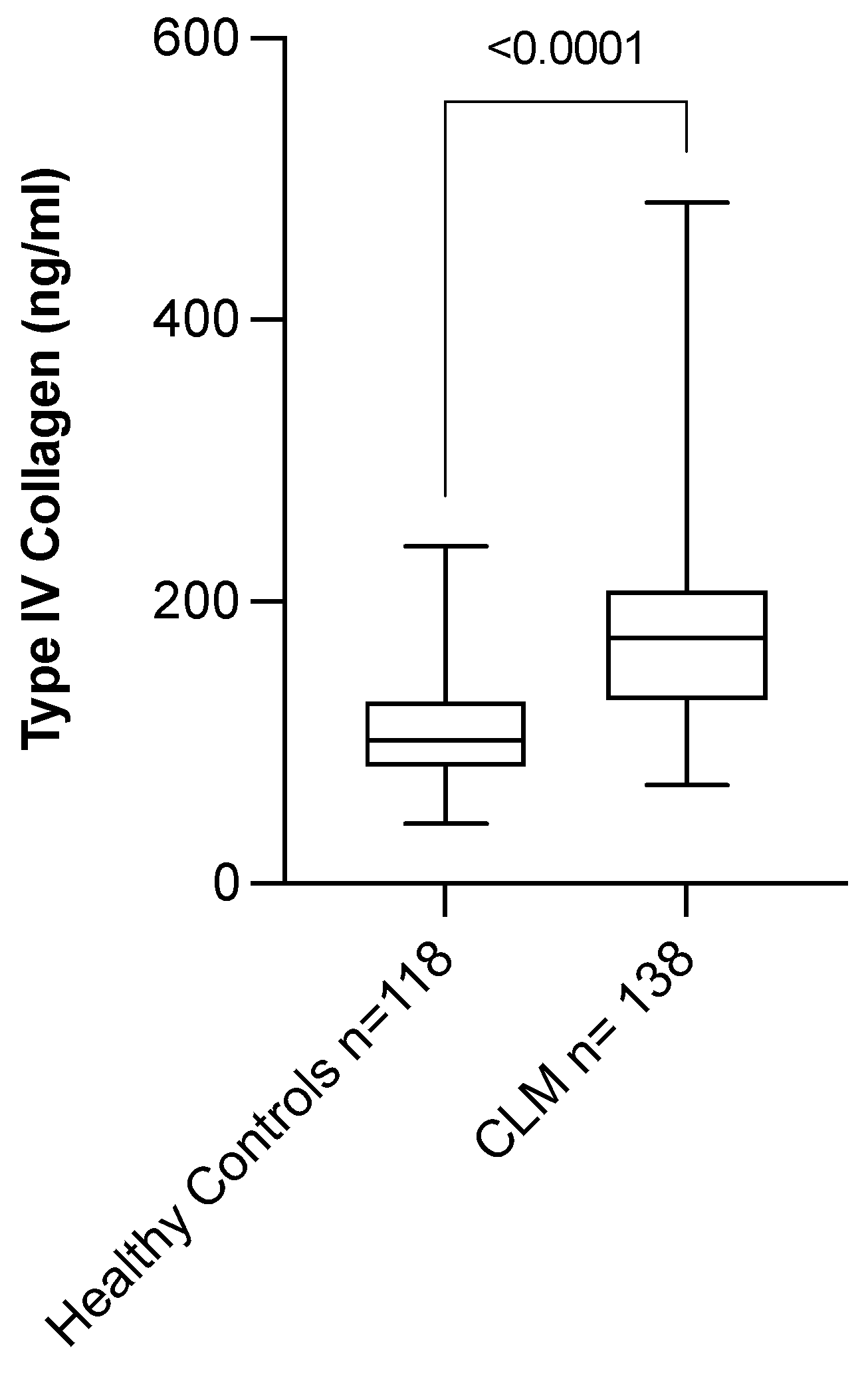
3.6. Circulating Level of COL IV Is Elevated in Patients with CLM
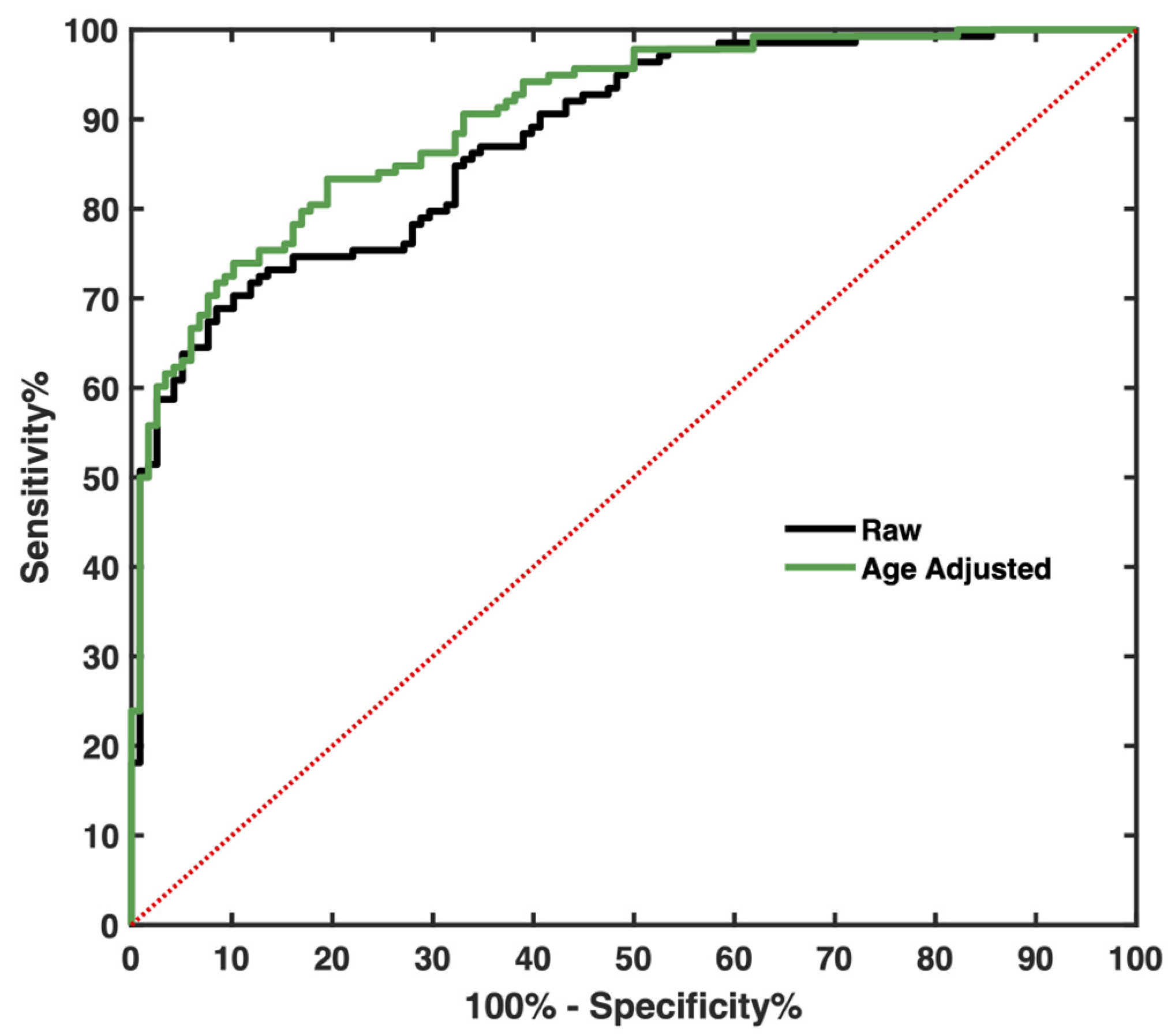
3.7. Circulating Level of COL IV Distinguishes CLM Patients from Controls
3.8. Levels of Circulating COL IV Do Not Correlate with Survival for CLM Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernard, W.; Stewart, C.P.W. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014; ISBN 978-92-832-0429-9. Available online: https://www.who.int/cancer/publications (accessed on 12 November 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. SEER Cancer Statistics Review, 1975–2016; Based on November 2018 SEER Data Submission, Posted to the SEER Website; National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 30 April 2019).
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Leporrier, J.; Maurel, J.; Chiche, L.; Bara, S.; Segol, P.; Launoy, G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br. J. Surg. 2006, 93, 465–474. [Google Scholar] [CrossRef]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef] [PubMed]
- RCC. Tjock-och ändtarmscancer. In Nationellt Vårdprogram; Version 3.0; RCC Samverkan: Stockholm, Sweden, 2021. [Google Scholar]
- Engstrand, J.; Kartalis, N.; Stromberg, C.; Broberg, M.; Stillstrom, A.; Lekberg, T.; Jonas, E.; Freedman, J.; Nilsson, H. The Impact of a Hepatobiliary Multidisciplinary Team Assessment in Patients with Colorectal Cancer Liver Metastases: A Population-Based Study. Oncologist 2017, 22, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Noren, A.; Sandstrom, P.; Gunnarsdottir, K.; Ardnor, B.; Isaksson, B.; Lindell, G.; Rizell, M. Identification of Inequalities in the Selection of Liver Surgery for Colorectal Liver Metastases in Sweden. Scand. J. Surg. 2018, 107, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef]
- House, M.G.; Ito, H.; Gonen, M.; Fong, Y.; Allen, P.J.; DeMatteo, R.P.; Brennan, M.F.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Survival after hepatic resection for metastatic colorectal cancer: Trends in outcomes for 1,600 patients during two decades at a single institution. J. Am. Coll. Surg. 2010, 210, 744–752. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Tangen, C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993, 270, 943–947. [Google Scholar] [CrossRef]
- McCall, J.L.; Black, R.B.; Rich, C.A.; Harvey, J.R.; Baker, R.A.; Watts, J.M.; Toouli, J. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis. Colon Rectum 1994, 37, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Su, B.B.; Shi, H.; Wan, J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J. Gastroenterol. 2012, 18, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Wanebo, H.J.; Rao, B.; Pinsky, C.M.; Hoffman, R.G.; Stearns, M.; Schwartz, M.K.; Oettgen, H.F. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N. Engl. J. Med. 1978, 299, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; De Paoli, M.; Basso, D.; Roveroni, G.; Giacomini, A.; Galeotti, F.; Corsini, A. Serum tumor markers in colorectal cancer staging, grading, and follow-up. J. Surg. Oncol. 1996, 62, 239–244. [Google Scholar] [CrossRef]
- Steele, G., Jr.; Ellenberg, S.; Ramming, K.; O’Connell, M.; Moertel, C.; Lessner, H.; Bruckner, H.; Horton, J.; Schein, P.; Zamcheck, N.; et al. CEA monitoring among patients in multi-institutional adjuvant G.I. therapy protocols. Ann. Surg. 1982, 196, 162–169. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Jelski, W.; Mroczko, B. Biochemical Markers of Colorectal Cancer—Present and Future. Cancer Manag. Res. 2020, 12, 4789–4797. [Google Scholar] [CrossRef]
- Fukuda, I.; Yamakado, M.; Kiyose, H. Influence of smoking on serum carcinoembryonic antigen levels in subjects who underwent multiphasic health testing and services. J. Med. Syst. 1998, 22, 89–93. [Google Scholar] [CrossRef]
- Huang, C.S.; Chen, C.Y.; Huang, L.K.; Wang, W.S.; Yang, S.H. Prognostic value of postoperative serum carcinoembryonic antigen levels in colorectal cancer patients who smoke. PLoS ONE 2020, 15, e0233687. [Google Scholar] [CrossRef]
- Tan, E.; Gouvas, N.; Nicholls, R.J.; Ziprin, P.; Xynos, E.; Tekkis, P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009, 18, 15–24. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C., Jr.; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, K.; Inoue, T.; Shimao, Y.; Sameshima, T. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol. Int. 2002, 52, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Peltonen, R.; Hagstrom, J.; Tervahartiala, T.; Sorsa, T.; Haglund, C.; Isoniemi, H. High Expression of MMP-9 in Primary Tumors and High Preoperative MPO in Serum Predict Improved Prognosis in Colorectal Cancer with Operable Liver Metastases. Oncology 2021, 99, 144–160. [Google Scholar] [CrossRef]
- Huang, M.Y.; Chang, H.J.; Chung, F.Y.; Yang, M.J.; Yang, Y.H.; Wang, J.Y.; Lin, S.R. MMP13 is a potential prognostic marker for colorectal cancer. Oncol. Rep. 2010, 24, 1241–1247. [Google Scholar]
- Collins, H.M.; Morris, T.M.; Watson, S.A. Spectrum of matrix metalloproteinase expression in primary and metastatic colon cancer: Relationship to the tissue inhibitors of metalloproteinases and membrane type-1-matrix metalloproteinase. Br. J. Cancer 2001, 84, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Chen, J.; Drachenberg, C.B.; Raufman, J.P.; Xie, G. Farnesoid X receptor represses matrix metalloproteinase 7 expression, revealing this regulatory axis as a promising therapeutic target in colon cancer. J. Biol. Chem. 2019, 294, 8529–8542. [Google Scholar] [CrossRef] [PubMed]
- Illemann, M.; Bird, N.; Majeed, A.; Sehested, M.; Laerum, O.D.; Lund, L.R.; Dano, K.; Nielsen, B.S. MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol. Cancer Res. 2006, 4, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.S.; Shu, W.P.; Cohen, A.M.; Guillem, J.G. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: Evidence for involvement of MMP-7 activation in human cancer metastases. Clin. Cancer Res. 2002, 8, 144–148. [Google Scholar]
- Conti, J.; Thomas, G. The role of tumour stroma in colorectal cancer invasion and metastasis. Cancers 2011, 3, 2160–2168. [Google Scholar] [CrossRef] [Green Version]
- Van Huizen, N.A.; Coebergh van den Braak, R.R.J.; Doukas, M.; Dekker, L.J.M.; IJzermans, J.N.M.; Luider, T.M. Up-regulation of collagen proteins in colorectal liver metastasis compared with normal liver tissue. J. Biol. Chem. 2019, 294, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Broker, M.E.; Lalmahomed, Z.S.; Roest, H.P.; van Huizen, N.A.; Dekker, L.J.; Calame, W.; Verhoef, C.; Ijzermans, J.N.; Luider, T.M. Collagen peptides in urine: A new promising biomarker for the detection of colorectal liver metastases. PLoS ONE 2013, 8, e70918. [Google Scholar] [CrossRef] [Green Version]
- Nystrom, H.; Naredi, P.; Hafstrom, L.; Sund, M. Type IV collagen as a tumour marker for colorectal liver metastases. Eur. J. Surg. Oncol. 2011, 37, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Nystrom, H.; Tavelin, B.; Bjorklund, M.; Naredi, P.; Sund, M. Improved tumour marker sensitivity in detecting colorectal liver metastases by combined type IV collagen and CEA measurement. Tumor Biol. 2015, 36, 9839–9847. [Google Scholar] [CrossRef] [Green Version]
- Ambiru, S.; Miyazaki, M.; Nakagawa, K.; Nakajima, N. Increased serum type IV collagen 7-S levels in patients with hepatic metastasis. Am. J. Gastroenterol. 1995, 90, 783–787. [Google Scholar]
- Kim, M.S.; Ha, S.E.; Wu, M.; Zogg, H.; Ronkon, C.F.; Lee, M.Y.; Ro, S. Extracellular Matrix Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 9185. [Google Scholar] [CrossRef]
- Casey, T.; Bond, J.; Tighe, S.; Hunter, T.; Lintault, L.; Patel, O.; Eneman, J.; Crocker, A.; White, J.; Tessitore, J.; et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res. Treat. 2009, 114, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 2012, 11, M111.014647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasanen, I.; Lehtonen, S.; Sormunen, R.; Skarp, S.; Lehtilahti, E.; Pietila, M.; Sequeiros, R.B.; Lehenkari, P.; Kuvaja, P. Breast cancer carcinoma-associated fibroblasts differ from breast fibroblasts in immunological and extracellular matrix regulating pathways. Exp. Cell Res. 2016, 344, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Pankova, D.; Chen, Y.; Terajima, M.; Schliekelman, M.J.; Baird, B.N.; Fahrenholtz, M.; Sun, L.; Gill, B.J.; Vadakkan, T.J.; Kim, M.P.; et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol. Cancer Res. 2016, 14, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Ohlund, D.; Lundin, C.; Ardnor, B.; Oman, M.; Naredi, P.; Sund, M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br. J. Cancer 2009, 101, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Nystrom, H.; Naredi, P.; Berglund, A.; Palmqvist, R.; Tavelin, B.; Sund, M. Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour. Anticancer Res. 2012, 32, 5183–5191. [Google Scholar]
- Rolff, H.C.; Christensen, I.J.; Vainer, B.; Svendsen, L.B.; Eefsen, R.L.; Wilhelmsen, M.; Lund, I.K.; Hoyer-Hansen, G.; Nielsen, H.J.; Illemann, M.; et al. The Prognostic and Predictive Value of Soluble Type IV Collagen in Colorectal Cancer: A Retrospective Multicenter Study. Clin. Cancer Res. 2016, 22, 2427–2434. [Google Scholar] [CrossRef] [Green Version]
- Laurie, G.W.; Leblond, C.P.; Martin, G.R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J. Cell Biol. 1982, 95, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008, 71, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Burnier, J.V.; Wang, N.; Michel, R.P.; Hassanain, M.; Li, S.; Lu, Y.; Metrakos, P.; Antecka, E.; Burnier, M.N.; Ponton, A.; et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene 2011, 30, 3766–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehlet, S.N.; Sanz-Pamplona, R.; Brix, S.; Leeming, D.J.; Karsdal, M.A.; Moreno, V. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci. Rep. 2016, 6, 30599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledo, T.; Arriaga-Pizano, L.; Lopez-Perez, M.; Salazar, E.P. Type IV collagen induces STAT5 activation in MCF7 human breast cancer cells. Matrix Biol. 2005, 24, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in Oncogenesis. Biomedicines 2020, 8, 316. [Google Scholar] [CrossRef]
- Ohlund, D.; Franklin, O.; Lundberg, E.; Lundin, C.; Sund, M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer 2013, 13, 154. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, M.; Jansson, M.; Tavelin, B.; Dirix, L.; Vermeulen, P.; Nystrom, H. Type IV collagen as a potential biomarker of metastatic breast cancer. Clin. Exp. Metastasis 2021, 38, 175–185. [Google Scholar] [CrossRef]
- Mazouni, C.; Arun, B.; Andre, F.; Ayers, M.; Krishnamurthy, S.; Wang, B.; Hortobagyi, G.N.; Buzdar, A.U.; Pusztai, L. Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. Br. J. Cancer 2008, 99, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Vasterbotten Intervention Programme: Background, design and implications. Glob. Health Action 2010, 3, 4643. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Terajima, M.; Wang, A.Y.; Georges, P.C.; Janmey, P.A.; Yamauchi, M.; Wells, R.G. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G605–G614. [Google Scholar] [CrossRef]
- Wang, T.; Jin, H.; Hu, J.; Li, X.; Ruan, H.; Xu, H.; Wei, L.; Dong, W.; Teng, F.; Gu, J.; et al. COL4A1 promotes the growth and metastasis of hepatocellular carcinoma cells by activating FAK-Src signaling. J. Exp. Clin. Cancer Res. 2020, 39, 148. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, J.S.; Lin, H.C.; Shan, Y.S.; Cheng, Y.J.; Yang, B.C. Dependence of fibroblast infiltration in tumor stroma on type IV collagen-initiated integrin signal through induction of platelet-derived growth factor. Biochim. Biophys. Acta 2015, 1853, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, K.; Iyama, K.; Ishikawa, N.; Egami, H.; Nakao, M.; Sado, Y.; Ninomiya, Y.; Baba, H. Loss of expression of type IV collagen alpha5 and alpha6 chains in colorectal cancer associated with the hypermethylation of their promoter region. Am. J. Pathol. 2006, 168, 856–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borregaard, N.; Sehested, M.; Nielsen, B.S.; Sengelov, H.; Kjeldsen, L. Biosynthesis of granule proteins in normal human bone marrow cells. Gelatinase is a marker of terminal neutrophil differentiation. Blood 1995, 85, 812–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Crawford, H.C.; Matrisian, L.M. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 1996, 49, 20–37. [Google Scholar] [CrossRef]
- Liu, C.C.; Chang, T.C.; Lin, Y.T.; Yu, Y.L.; Ko, B.S.; Sung, L.Y.; Liou, J.Y. Paracrine regulation of matrix metalloproteinases contributes to cancer cell invasion by hepatocellular carcinoma-secreted 14-3-3sigma. Oncotarget 2016, 7, 36988–36999. [Google Scholar] [CrossRef]


| Characteristic | CLM Patients (n = 30) | CLM Patients for ISH Analysis * (n = 15) |
|---|---|---|
| Age CRC diagnosis (median, range) | 62 (40–80) | 68 (56–80) |
| Gender (M/F) | 16/14 | 7/8 |
| Primary tumor (colon/rectum/N.A.) | 20/9/1 | 10/5 |
| TNM stage | ||
| I | 3 | 3 |
| II | 6 | 5 |
| III | 8 | 3 |
| IV | 13 | 4 |
| Chemotherapy | ||
| Adjuvant | 3 | 3 |
| Preoperative chemotherapy | 7 | 1 |
| N.A. | 1 | 0 |
| Diagnosis metastasis | ||
| Synchronous (<1 month) | 13 | 4 |
| Metachronous | 17 | 11 |
| MMP Score 0–2 | Immune Cells n = 30, n (%) | Tumor Cells n = 30, n (%) | Hepatocytes n = 30, n (%) |
|---|---|---|---|
| MMP-2 | |||
| 0 | 1 (3.3) | 25 (83.3) | 1 (3.3) |
| 1 | 20 (66.7) | 5 (16.7) | 5 (16.7) |
| 2 | 9 (30) | 0 (0) | 24 (80) |
| MMP-7 | |||
| 0 | 4 (13.3) | 19 (63.3) | 2 (6.7) |
| 1 | 25 (83.3) | 10 (33.3) | 24 (80) |
| 2 | 1 (3.3) | 1 (3.3) | 3 (10) |
| N.A. | 0 (0) | 0 (0) | 1 (3.3) |
| MMP-9 | |||
| 0 | 0 (0) | 30 (100) | 13 (43.3) |
| 1 | 17 (56.7) | 0 (0) | 17 (56.7) |
| 2 | 13 (43.3) | 0 (0) | 0 (0) |
| MMP-13 | |||
| 0 | 8 (26.7) | 30 (100) | 3 (10) |
| 1 | 17 (56.7) | 0 (0) | 22 (73.3) |
| 2 | 5 (16.7) | 0 (0) | 5 (16.7) |
| Characteristic | CLM Patients (n = 138) | Healthy Controls (n = 118) | p-Value |
|---|---|---|---|
| cCOL IV (ng/mL; median, range) | 174.1 (69.9–483.1) | 101.2 (42.4–239.2) | <0.0001 |
| Age, blood sample (median, range) | 66 (37–84) | 60 (30–72) | <0.0001 |
| Gender (M/F) | 94/44 | 60/58 | 0.0049 |
| Primary tumor (colon/rectum) | 74/64 | ||
| TNM stage | |||
| I | 6 | ||
| II | 15 | ||
| III | 21 | ||
| IV | 91 | ||
| N.A. | 5 | ||
| N-positive primary tumor | |||
| Yes | 94 | ||
| No | 37 | ||
| N.A. | 7 | ||
| Primary tumor removed prior to liver surgery (no/yes) | 39/99 | ||
| Liver surgery | |||
| Liver resection | 102 | ||
| Ablation * | 13 | ||
| Resection + ablation * | 23 | ||
| Preoperative chemotherapy | |||
| Neoadjuvant | 62 | ||
| Conversion therapy | 20 | ||
| No treatment | 53 | ||
| N.A. | 3 | ||
| Liver metastasis > 1 (yes/no) | 96/42 | ||
| Size metastasis > 5 cm (yes/no) | 25/113 | ||
| Number of metastases (median, range) | 2 (1–30) | ||
| Extrahepatic metastases at resection | |||
| Lung | 4 | ||
| Carcinosis | 6 | ||
| Lymph nodes | 4 | ||
| Other | 2 | ||
| Interval CRC–CLM +, months (median, range) | 0 (0–57) |
| Marker | I-Specificity | Sensitivity | Cutoff (ng/mL) |
|---|---|---|---|
| cCOL IV | 16.1 | 74.6 | 135.2 |
| cCOL IV (age-corrected) | 19.5 | 83.3 | unapplicable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindgren, M.; Rask, G.; Jonsson, J.; Berglund, A.; Lundin, C.; Jonsson, P.; Ljuslinder, I.; Nyström, H. Type IV Collagen in Human Colorectal Liver Metastases—Cellular Origin and a Circulating Biomarker. Cancers 2022, 14, 3396. https://doi.org/10.3390/cancers14143396
Lindgren M, Rask G, Jonsson J, Berglund A, Lundin C, Jonsson P, Ljuslinder I, Nyström H. Type IV Collagen in Human Colorectal Liver Metastases—Cellular Origin and a Circulating Biomarker. Cancers. 2022; 14(14):3396. https://doi.org/10.3390/cancers14143396
Chicago/Turabian StyleLindgren, Moa, Gunilla Rask, Josefin Jonsson, Anette Berglund, Christina Lundin, Pär Jonsson, Ingrid Ljuslinder, and Hanna Nyström. 2022. "Type IV Collagen in Human Colorectal Liver Metastases—Cellular Origin and a Circulating Biomarker" Cancers 14, no. 14: 3396. https://doi.org/10.3390/cancers14143396
APA StyleLindgren, M., Rask, G., Jonsson, J., Berglund, A., Lundin, C., Jonsson, P., Ljuslinder, I., & Nyström, H. (2022). Type IV Collagen in Human Colorectal Liver Metastases—Cellular Origin and a Circulating Biomarker. Cancers, 14(14), 3396. https://doi.org/10.3390/cancers14143396






