Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Target Delineation
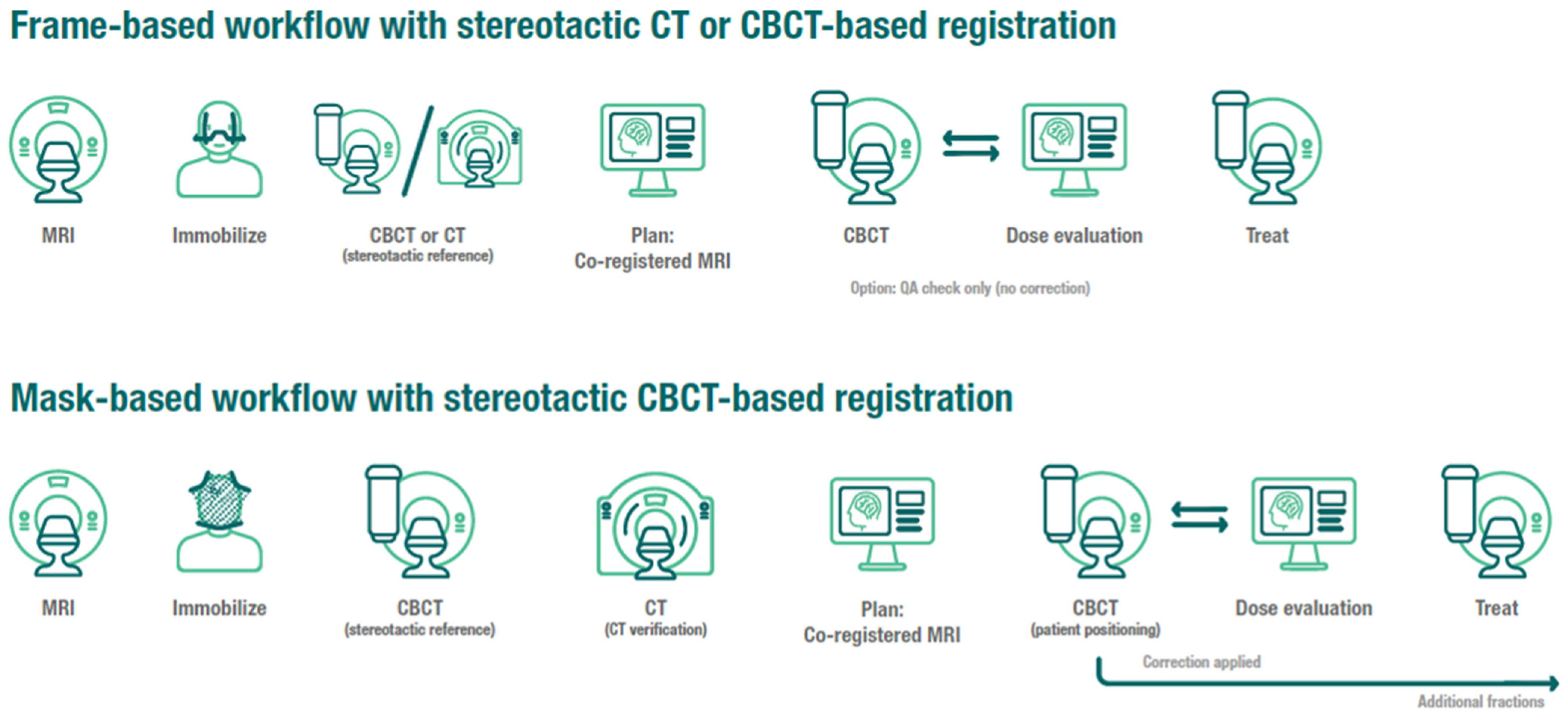
2.3. Frame Immobilization Workflow
2.4. Mask Immobilization Workflow and Treatment Delivery with Motion Management
2.5. Patient Follow-Up and Endpoints
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.; Xu, W.; Sahgal, A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012, 118, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients with 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta. Chir. Scand. 1951, 102, 316–319. [Google Scholar]
- Torrens, M.; Chung, C.; Chung, H.T.; Hanssens, P.; Jaffray, D.; Kemeny, A.; Larson, D.; Levivier, M.; Lindquist, C.; Lippitz, B.; et al. Standardization of terminology in stereotactic radiosurgery: Report from the Standardization Committee of the International Leksell Gamma Knife Society: Special topic. J. Neurosurg. 2014, 121, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Babic, S.; Lee, Y.; Ruschin, M.; Lochray, F.; Lightstone, A.; Atenafu, E.; Phan, N.; Mainprize, T.; Tsao, M.; Soliman, H.; et al. To frame or not to frame? Cone-beam CT-based analysis of head immobilization devices specific to linac-based stereotactic radiosurgery and radiotherapy. J. Appl. Clin. Med. Phys. 2018, 19, 111–120. [Google Scholar] [CrossRef]
- Vulpe, H.; Save, A.V.; Xu, Y.; Elliston, C.D.; Garrett, M.D.; Wu, C.C.; Cheng, S.K.; Jani, A.H.; Bruce, J.N.; McKhann, G.M.; et al. Frameless Stereotactic Radiosurgery on the Gamma Knife Icon: Early Experience From 100 Patients. Neurosurgery 2020, 86, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Park, W.Y.; Kim, T.H.; Kim, Y.K.; Chun, K.J. Assessment of the accuracy and stability of frameless gamma knife radiosurgery. J. Appl. Clin. Med. Phys. 2018, 19, 148–154. [Google Scholar] [CrossRef]
- AlDahlawi, I.; Prasad, D.; Podgorsak, M.B. Evaluation of stability of stereotactic space defined by cone-beam CT for the Leksell Gamma Knife Icon. J. Appl. Clin. Med. Phys. 2017, 18, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corns, R.; Mehta, D.; Massock, W.; Roberts, L. A quality assurance tool for daily checks of the gamma knife high definition motion management system. J. Appl. Clin. Med. Phys. 2020, 21, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Han, E.Y.; Luo, D.; Kim, J.O.; Tharp, K.; Wen, Z.; Briere, T.M. Dosimetric validation of the Gamma Knife((R)) Icon(TM) plan adaptation and high-definition motion management system with a motorized anthropomorphic head phantom. J. Radiosurg. SBRT 2019, 6, 217–226. [Google Scholar]
- Lunsford, L.D.; Niranjan, A.; Fallon, K.; Kim, J.O. Frame versus Frameless Leksell Stereotactic Radiosurgery. Prog. Neurol. Surg. 2019, 34, 19–27. [Google Scholar] [CrossRef]
- Pavlica, M.; Dawley, T.; Goenka, A.; Schulder, M. Frame-Based and Mask-Based Stereotactic Radiosurgery: The Patient Experience, Compared. Stereotact. Funct. Neurosurg. 2021, 99, 241–249. [Google Scholar] [CrossRef]
- Carminucci, A.; Nie, K.; Weiner, J.; Hargreaves, E.; Danish, S.F. Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell Gamma Knife Icon radiosurgery system. J. Neurosurg. 2018, 129, 133–139. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, R.L.; Lee, Y.; Schasfoort, J.; Soliman, H.; Sahgal, A.; Ruschin, M. Real-Time Infrared Motion Tracking Analysis for Patients Treated With Gated Frameless Image Guided Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Wegner, R.E.; Horne, Z.D.; Liang, Y.; Goss, M.; Yu, A.; Pace, J.; Williamson, R.W.; Leonardo, J.; Karlovits, S.M.; Fuhrer, R. Single Fraction Frameless Stereotactic Radiosurgery on the Gamma Knife Icon for Patients with Brain Metastases: Time to Abandon the Frame? Adv. Radiat. Oncol. 2021, 6, 100736. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, T.; Abrams, K.J.; Tom, M.C.; Rubens, M.; Appel, H.; Sidani, C.; Hall, M.D.; Tolakanahalli, R.; Wieczorek, D.J.J.; Gutierrez, A.N.; et al. Dedicated isotropic 3-D T1 SPACE sequence imaging for radiosurgery planning improves brain metastases detection and reduces the risk of intracranial relapse. Radiother. Oncol. 2022, 173, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.A.; Koppen, U.; Stieler, F.; Welzel, G.; Ruder, A.M.; Polednik, M.; Wenz, F.; Mai, S.K.; Giordano, F.A. Prospective assessment of mask versus frame fixation during Gamma Knife treatment for brain metastases. Radiother. Oncol. 2020, 147, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Angelov, L.; Lee, S.Y.; Li, L.; Barnett, G.H.; Suh, J.H. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J. Neurosurg. 2006, 104, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, T.; Tolakanahalli, R.; Williams, A.; Tom, M.C.; Vadhan, J.D.; Appel, H.; Hall, M.D.; Wieczorek, D.J.J.; Davis, S.; McDermott, M.W.; et al. Impact of MRI timing on tumor volume and anatomic displacement for brain metastases undergoing stereotactic radiosurgery. Neurooncol. Pract. 2021, 8, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Duggar, W.N.; Morris, B.; Fatemi, A.; Bonds, J.; He, R.; Kanakamedala, M.; Rey-Dios, R.; Vijayakumar, S.; Yang, C. Gamma Knife(®) icon CBCT offers improved localization workflow for frame-based treatment. J. Appl. Clin. Med. Phys. 2019, 20, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Miller, J.A.; Bennett, E.E.; Xiao, R.; Kotecha, R.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Angelov, L.; Murphy, E.S.; Yu, J.S.; et al. Association between Radiation Necrosis and Tumor Biology after Stereotactic Radiosurgery for Brain Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1060–1069. [Google Scholar] [CrossRef]
- Grunert, P. Accuracy of stereotactic coordinate transformation using a localisation frame and computed tomographic imaging. Part II. Analysis of matrix-based coordinate transformation. Neurosurg. Rev. 1999, 22, 188–203; discussion 204. [Google Scholar] [CrossRef]
- Sedrak, M.; Alaminos-Bouza, A.L.; Bruna, A.; Brown, R.A. Monte Carlo Simulation of Errors for N-localizer Systems in Stereotactic Neurosurgery: Novel Proposals for Improvements. Cureus 2021, 13, e13393. [Google Scholar] [CrossRef] [PubMed]

| Total Cohort (% or Range) | Frame Immobilization Cohort (% or Range) | Mask Immobilization Cohort (% or Range) | p-Value | |
|---|---|---|---|---|
| Number of patients | 150 | 93 | 57 | |
| Median age, years | 65 (28–90) | 65 (28–90) | 66 (28–89) | 0.876 |
| Sex | 0.398 | |||
| Female | 86 (57.3) | 56 (60.2) | 30 (52.6) | |
| Male | 64 (42.7) | 37 (39.8) | 27 (47.4) | |
| Race | 0.052 | |||
| White | 137 (91.3) | 89 (95.7) | 48 (84.2) | |
| African American | 10 (6.7) | 3 (3.2) | 7 (12.3) | |
| Other | 3 (2.0) | 1 (1.1) | 2 (4.5) | |
| Extracranial disease | 0.113 | |||
| No | 109 (24.1) | 71 (21.9) | 38 (29.4) | |
| Yes | 344 (75.9) | 253 (78.1) | 91 (80.6) | |
| Status of extracranial disease | 0.143 | |||
| Stable | 201 (44.4) | 151 (46.6) | 50 (38.8) | |
| Progressive | 252 (55.6) | 173 (53.4) | 79 (61.2) | |
| Median KPS | 90 (60–100) | 90 (60–100) | 90 (60–100) | 0.184 |
| Primary tumor histology | 0.024 | |||
| Lung | 252 (55.6) | 176 (54.3) | 76 (58.9) | |
| Breast | 82 (18.1) | 54 (16.7) | 28 (21.7) | |
| Gastrointestinal | 43 (9.5) | 39 (12.0) | 4 (3.1) | |
| Other | 76 (16.8) | 55 (16.9) | 21 (16.3) | |
| Number of SRS courses | 189 | 115 | 74 | |
| Number of brain metastases | 453 | 324 | 129 | |
| Median number of brain metastases treated per SRS course | 6 (1–23) | 7 (1–23) | 3 (1–14) | <0.001 |
| SRS prescription dose | 0.086 | |||
| 22 Gy | 107 (23.6) | 84 (25.9) | 23 (17.8) | |
| 24 Gy | 346 (76.4) | 240 (74.1) | 106 (82.2) | |
| Prescription Isodose Line (%) | 56 (50–94) | 56 (50–94) | 55 (50–94) | 0.860 |
| Median maximal tumor diameter, cm | 0.8 (0.5–1.95) | 0.73 (0.5–1.95) | 0.8 (0.5–1.95) | 0.068 |
| Median tumor volume, cm3 | 0.18 (0.01–3.5) | 0.16 (0.01–3.5) | 0.18 (0.02–2.47) | 0.054 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age | 1.02 (0.99, 1.04) | 0.156 | ||
| Sex | 0.022 | 0.008 | ||
| Male | Reference | Reference | ||
| Female | 0.64 (0.43, 0.94) | 0.35 (0.16, 0.76) | ||
| Primary tumor histology | 0.411 | |||
| Other | Reference | |||
| Lung | 1.60 (0.84, 3.04) | |||
| Breast | 1.28 (0.53, 3.14) | |||
| Gastroinestinal | 0.81 (0.26, 2.50) | |||
| Total number of brain metastases treated per SRS course | 0.97 (0.90, 1.04) | 0.374 | ||
| Extracranial disease | 0.006 | 0.016 | ||
| Yes | Reference | Reference | ||
| No | 0.45 (0.22, 0.92) | 0.17 (0.40, 0.72) | ||
| Status of extracranial disease | 0.251 | |||
| Stable | Reference | |||
| Progressive | 1.24 (0.86, 1.80) | |||
| KPS | 1.01 (0.97, 1.05) | 0.773 | ||
| Immobilization method | 0.290 | 0.518 | ||
| Mask | Reference | Reference | ||
| Frame | 0.81 (0.55, 1.19) | 0.52 (0.24, 1.14) | ||
| SRS prescription dose | 0.336 | |||
| 24 Gy | Reference | |||
| 22 Gy | 1.26 (0.77, 2.04) | |||
| Tumor maximal diameter | 1.33 (0.52, 3.38) | 0.561 | ||
| Tumor volume | 1.16 (0.63, 2.14) | 0.636 | ||
| Patient Number | Lesion Number | Median SRS Dose | Setup Margin | HDMM Threshold | Median Follow-Up | Local Control Rate | Overall Survival Rate | Radiation Necrosis Rate | |
|---|---|---|---|---|---|---|---|---|---|
| Grimm et al. [23] | Mask:17 Frame: 59 | Mask: 28 Frame: 169 | 22 Gy (10 patients FSRT, 24 patients had prior WBRT) | Mask: 1 mm Frame: Zero | 1 mm | Mask: 10.4 months Frame: 9 months | Mask: 6-month and 1-year 100% Frame: 6-month 92.6%, 1-year 80.9% (p = 0.03) | Mask: not reached Frame: 16.9 months (p = 0.999) | Mask: Zero event Frame: 3 events (p = 0.67) |
| Wegner et al. [21] | Mask: 56 Frame: 39 | Mask: 80 Frame: 80 (after propensity matching) | 20 Gy (20 patients had prior WBRT) | Mask: Zero for intact metastases, 1 mm for postoperative cavities Frame: Zero for intact metastases, 1 mm for postoperative cavities | 1 mm | Entire cohort: 5 months clinical follow-up 6 months imaging follow-up | Mask: 1-year 85% Frame: 1-year 96% (p = 0.07) | Mask: not reached, 1-year 75% Frame: 8 months, 1-year: 48% (p = 0.12) | NA |
| Kutuk et al. | Mask: 57 Frame: 93 | Mask: 129 Frame: 324 | 24 Gy | Mask: Zero Frame: Zero | 1.5 mm | Mask: 17.4 months Frame: 15 months | Mask: 6-month 92.2% and 1-year 90.5% Frame: 6-month 94.3%, 1-year 92% (p = 0.272) | Mask: 10.4 months Frame: 12 months (p = 0.796) | Mask: 6-month 2.9% and 1-year 12.5% Frame: 6-month 1.5% and 1-year 4.1% (p = 0.502) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutuk, T.; Kotecha, R.; Tolakanahalli, R.; Wieczorek, D.J.J.; Lee, Y.C.; Ahluwalia, M.S.; Hall, M.D.; McDermott, M.W.; Appel, H.; Gutierrez, A.N.; et al. Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases. Cancers 2022, 14, 3392. https://doi.org/10.3390/cancers14143392
Kutuk T, Kotecha R, Tolakanahalli R, Wieczorek DJJ, Lee YC, Ahluwalia MS, Hall MD, McDermott MW, Appel H, Gutierrez AN, et al. Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases. Cancers. 2022; 14(14):3392. https://doi.org/10.3390/cancers14143392
Chicago/Turabian StyleKutuk, Tugce, Rupesh Kotecha, Ranjini Tolakanahalli, D Jay J. Wieczorek, Yongsook C. Lee, Manmeet S. Ahluwalia, Matthew D. Hall, Michael W. McDermott, Haley Appel, Alonso N. Gutierrez, and et al. 2022. "Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases" Cancers 14, no. 14: 3392. https://doi.org/10.3390/cancers14143392
APA StyleKutuk, T., Kotecha, R., Tolakanahalli, R., Wieczorek, D. J. J., Lee, Y. C., Ahluwalia, M. S., Hall, M. D., McDermott, M. W., Appel, H., Gutierrez, A. N., Mehta, M. P., & Tom, M. C. (2022). Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases. Cancers, 14(14), 3392. https://doi.org/10.3390/cancers14143392








