Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils
Abstract
:Simple Summary
Abstract
1. Introduction
2. Neutrophils and Their Role in Cancer
3. Cytotoxic Effector Functions of Neutrophils
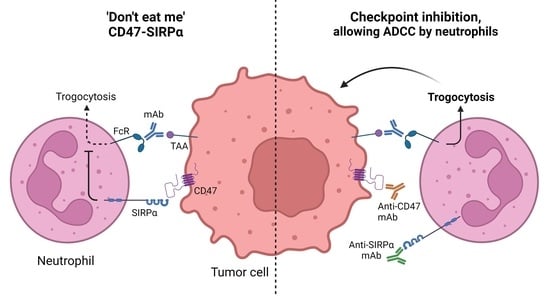
4. CD47-SIRPα as an Innate Immune Checkpoint in Neutrophil-Mediated Tumor Killing
4.1. SIRPα Signaling
4.2. Neutrophil Effector Functions Influenced by CD47-SIRPα
4.3. The Innate Immune Checkpoint CD47-SIRPα in Cancer
5. Targeting CD47-SIRPα to Potentiate Antibody Therapy
5.1. CD47-Targeting Agents
5.2. Anti-SIRPα mAbs
5.3. Alternative Ways to Disrupt CD47-SIRPα Interactions
6. Conclusions
| Anti-CD47 mAbs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Type | Phase | Trial Identifier | Name | Indication | Treatment | Results | Ref | |
| Toxicity (≥Grade 3) | ORR (DCR) | ||||||||
| Magrolimab/GS-4721/ (Hu5F9-G4) | anti-CD47 IgG4 | 1/1b | NCT02678338 | CAMELLIA | r/r AML and hrMDS | Mono | 72% anemia 6% lymphopenia | AML = 0% (56%) | [196,236] |
| 1 | NCT03248479 | AML (65% TP53 mut) | +Azacitidine | 31% anemia 19% hyperbilirubinemia 19% neutropenia 17% thrombocytopenia | 65% (97%) | [198,199,200] | |||
| int/high risk MDS (13% TP53 mut) | +Azacitidine | 38% anemia 19% neutropenia 18% thrombocytopenia | 91% (100%) | [198,199,200] | |||||
| 1 | NCT02216409 | Solid cancer | Mono | 18% lymphopenia 14% anemia 7% Hyperbilirubinemia | 2/44 (OvC and FTC) | [195] | |||
| 1b/2 | NCT02953782 | CRC KRAS wt/mut | +Cetuximab | 12% anemia 12% hyperbilirubinemia | wtKRAS = 6% (50%) mKRAS = 0% (38%) | [237] | |||
| 1b/2 | NCT02953509 | NHL | +Rituximab | 42% anemia 15% neutropenia 36% infusion reactions | NHL = 45% (62%) Indolent = 61% (85%) DLBCL = 36% (48%) | [193,194] | |||
| NHL | +Rituximab + Gemcitabine + Oxaliplatin | [192,193,194] | |||||||
| 1 | NCT03558139 | Ovarian cancer | +Avelumab | ||||||
| 1b/2 | NCT03869190 | MORPEUS-UC | Urothelial & Bladder cancer | +Atezolizumab | |||||
| 1b | NCT03922477 | AML | +Atezolizumab | ||||||
| 1 | NCT03527147 | PRISM | NHL | +Rituximab + Acalabrutinib | |||||
| 2 | NCT04788043 | HL | +Pembrolizumab | ||||||
| 1 | NCT04599634 | VENOM | r/r indNHL/CLL | +Obinutuzumab + Venetoclax | |||||
| 1 | NCT04751383 | High-risk & rrNeuroblastoma/rOsteosarcoma | +Dinutuximab | ||||||
| +Dinutuximab + surgery | |||||||||
| 1b/2 | NCT04541017 | CTCL (Myc. Fungoides & Sezary Syndr.) | +Docetaxel + Mogamilizumab | ||||||
| Mogamilizumab mono | |||||||||
| 2 | NCT04827576 | Solid (mNSCLC, mSCLC, mUC) | Mono | ||||||
| 3 | NCT04313881 | ENHANCE | Untreated hrMDS | +Azacitidine | |||||
| Azacitidine mono | |||||||||
| 3 | NCT05079230 | ENHANCE-3 | Untreated AML | +Azacitidine + Venetoclax | |||||
| Azacitidine + Venetoclax | |||||||||
| 1b/2 | NCT04435691 | r/r and nd AML | +Azacitidine + Venetoclax | ||||||
| 2 | NCT04958785 | tnmBC | +Paclitaxel- + Nab paclitaxel | ||||||
| +Sacituzumab govitecan | |||||||||
| 2 | NCT04892446 | r/r MM | +Daratumumab | ||||||
| +Pomalidomide + Dexamethasone | |||||||||
| +Bortezomib + Dexamethasone | |||||||||
| 3 | NCT04778397 | ENHANCE-2 | untreated T53mut AML | +Azacitidine | |||||
| phys choice | |||||||||
| 2 | NCT04854499 | untreated HNSCC | +Pembrolizumab | ||||||
| +Pembrolizumab + chemo/Docetaxel | |||||||||
| 2 | NCT04778410 | newly diagnosed or r/r AML | +Azacitidine + Venetoclax | ||||||
| +MEC chemo | |||||||||
| +CC-486 | |||||||||
| 1 | NCT05169944 | Malignant brain cancer (children & adults) | Mono | ||||||
| CC90002 | anti-CD47 IgG4PE | 1 | NCT02641002 | r/r AML and hrMDS | Mono | 36% feb neutropenia 32% thrombocytopenia 29% anemia 18% neutropenia 11% hypokalemia | AML = 0/14 (0/14) MDS = 0/3 (2/3) | [201,238] | |
| 1 | NCT02367196 | Solid cancer/MM/NHL | +/− Rituximab | 38% neutropenia | NHL = 13% (25%) | [203] | |||
| Letaplimab/IBI188 | anti-CD47 IgG4 | 1 | NCT03763149 | Solid tumors and lymphomas | Mono | 1/20 anemia 1/20 hyperbilirubinemia 1/20 thrombocytopenia | [205] | ||
| 1/1b | NCT03717103 | Solid tumors and lymphomas | Mono | ||||||
| +Rituximab | |||||||||
| 1b | NCT04861948 | Solid tumors (Lung adenocar., Osteosarc., Soft tissue sarc.) | +IBI188 (Anti-PD1)/chemo + Bevacizumab/GM-CSF | ||||||
| 1 | NCT04511975 | newly diagnosed hrMDS | +Azacitidine | ||||||
| 1b | NCT04485052 | newly diagnosed or rrAML | +Azacitidine | ||||||
| 1b | NCT04485065 | newly diagnosed hrMDS | +Azacitidine | ||||||
| IBI322 | CD47/PDL1 BsAb IgG4 | 1a | NCT04338659 | Solid tumors | Mono | ||||
| 1/1b | NCT04328831 | Solid tumors | Mono | ||||||
| 1/1b | NCT04912466 | Hematologic cancer | Mono | ||||||
| 1a/1b | NCT04795128 | Hematologic cancer | Mono | ||||||
| 1a/1b | NCT05148442 | r/r AML and r/r MDS | +Azacitidine | ||||||
| AO176 | anti-CD47 IgG2 | 1/2 | NCT03834948 | Solid tumors | Mono | ||||
| +Paclitaxel/Pembrolizumab | |||||||||
| 1/2 | NCT04445701 | Multiple myeloma | Mono | ||||||
| +DEX/Bortezomib | |||||||||
| SRF231 | anti-CD47 IgG4 | 1/1b | NCT03512340 | Solid and hematologic cancer | Mono | 1/25 feb neutropenia 1/25 hemolysis 1/21 thrombocytopenia 1/21 elevated lipase/amylase | 0% | [239] | |
| TG1801/NI-1701 | CD47/CD19 BsAb IgG4 | 1 | NCT03804996 | B lymphoma | Mono | ||||
| +Ublituximab | |||||||||
| 1/1b | NCT04806035 | CD20+ NHL and CLL | Mono | ||||||
| +Ublituximab | |||||||||
| Lemzoparlimab/TJ011133/ TJC4 | anti-CD47 IgG4 | 1 | NCT03934814 | Melanoma | Mono | 5% elevated lipase | 1/20 (4/20) | [206] | |
| NHL | +Rituximab | 11% neutropenia | NHL = 71% Indolent = 75% DLBCL = 50% | [207] | |||||
| Solid and hematologic cancer | Mono | ||||||||
| +Rituximab | |||||||||
| +Pembrolizumab | |||||||||
| 1/2a | NCT04202003 | AML and ir/hrMDS | +Azacitidine | 1/5 thrombocytopenia | 1/5 | [208] | |||
| 1 | NCT04912063 | untreated AML and hrMDS | +Azacitidine + Venetoclax | ||||||
| 1 | NCT04895410 | r/r MM | Mono | ||||||
| +Pomalidomide + Dexamethasone | |||||||||
| +Carfilzomib + Dexamethasone | |||||||||
| +Daratumumab + Dexamethasone | |||||||||
| 1/2 | NCT05148533 | advanced, melanoma, GC, HNSCC | +Toripalimab | ||||||
| ZL1201 | anti-CD47 IgG4 | 1 | NCT04257617 | Solid cancer and lymphoma | Mono | ||||
| HX009 | CD47/PD1 BsAb IgG4 | 1 | NCT04097769 | Solid cancer | Mono | ||||
| 2 | NCT04886271 | Solid cancer | Mono | ||||||
| 1/2 | NCT05189093 | r/r Lymphoma (NHL, Hodgkin, PTCL) | Mono | ||||||
| AK117 | anti-CD47 IgG4 | 1 | NCT04349969 | Solid cancer and NHL | Mono | ||||
| 1/2 | NCT04900350 | MDS | +Azacitidine | ||||||
| 1 | NCT04728334 | r/r Solid cancer and NHL | Mono | ||||||
| 1b/2 | NCT04980885 | AML | +Azacitidine | ||||||
| 1b/2 | NCT05214482 | adv. Solid cancer | +AK112 (anti-PD-1/VEGF BsAb) +/− chemo | ||||||
| 1b/2 | NCT05229497 | adv. Solid cancer (Ph2: Adv HNSCC) | + AK112 (anti-PD-1/VEGF BsAb) + chemo | ||||||
| 1b/2 | NCT05235542 | adv. solid cancer (Ph2: Adv GEJ or Esoph Cancer) | + AK104 (anti-PD-1/CTLA4 BsAb) +/− chemo | ||||||
| IMC-002 | anti-CD47 IgG4 | 1 | NCT04306224 | Solid and hematological | Mono | ||||
| 1 | NCT05276310 | adv. solid cancer | |||||||
| SGN-CD47M | anti-CD47 ADC | 1 | NCT03957096 | Solid cancer | Mono | ||||
| PF-07257876 | CD47/PDL1 BsAb IgG4 | 1 | NCT04881045 | NSCLC, SCCHN, ovarian cancer | Mono | ||||
| TQB2928 | CD47-SIRPα NME | 1 | NCT04854681 | Solid and hematological cancer | Mono | ||||
| H4C1/ SHR-1603 | anti-CD47 | 1/2 | NCT04588324 | Solid cancer | +SHR2150 (TLR7 agonist) + chemo + Anti-PD-1 | ||||
| STI-6643 | anti-CD47 IgG4 (228P) | 1 | NCT04900519 | Solid cancer | Mono | ||||
| sB24M | anti-CD47/TNF-α | 1 | NCT04895566 | Severe Pyoderma | Mono (local application) | ||||
| IMM0306 | CD47/CD20 BsAb IgG1 | 1 | NCT04746131 | NHL | Mono | ||||
| SHR-1603 | anti-CD47 IgG4 | 1 | NCT03722186 | Solid cancer and rrLymphoma | Mono | ||||
| anti-CD47 | anti-CD47 | 1 | NCT05266274 | recurr AML after transplantation | +Azacitidine | ||||
| Gentulizumab | anti-CD47 | 1 | NCT05221385 | Adv solid cancer and NHL | Mono | ||||
| 1a | NCT05263271 | r/r AML and MDS | Mono | ||||||
| TQB2928 | anti-CD47 | 1 | NCT05192512 | Adv solid cancer and lymphoma | Mono | ||||
| BAT7104 | CD47/PDL1 BsAb | 1 | NCT05200013 | Adv solid cancer | Mono | ||||
| SG2501 | CD47/CD38 BsAb | 1 | NCT05293912 | r/r hematologic malignancies | Mono | ||||
| SIRPα-Fc | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Type | Phase | Trial Identifier | Name | Indication | Treatment | Results | Ref | |
| Toxicity (≥Grade 3) | ORR (DCR) | ||||||||
| TTI-621 | SIRPα-IgG1Fc | 1/1b | NCT02663518 | Lymphoma | Mono | 9% neutropenia 20% thrombocytopenia 9% anemia | NHL = 10% (62%) Indolent = 0% (78%) DLBCL = 29% (29–50%) | [211,240] | |
| +Rituximab | 9% neutropenia 20% thrombocytopenia 9% anemia | NHL = 23% (62%) Indolent = 50% (100%) DLBCL = 20% (57%) CTCL = 18% PTCL = 26% Hodgkin = 13% (63%) | [211,241] | ||||||
| +Nivolumab | 9% neutropenia 20% thrombocytopenia 9% anemia | NHL = 50% (50%) | [211] | ||||||
| 1 | NCT02890368 | CTCL | Mono | None reported | CTCL = 90% (34%) | [212] | |||
| Solid tumors, melanoma, breast ca, soft tissue sarcoma, CTCL | Mono | ||||||||
| +Anti-PD-1/PD-L1 (nivolumab, pembrolizumab, durvalumab, avelumab, or atezolizumab) | |||||||||
| +PEG-IFN-α2a | |||||||||
| +T-VEC | |||||||||
| +radiation | |||||||||
| 1/2 | NCT04996004 | Leiomyosarcoma | +Doxorubicin | ||||||
| TTI-622 | SIRPα-IgG4Fc | 1/1b | NCT03530683 | r/r NHL | Mono | 9% neutropenia 5% thrombocytopenia 2% anemia | NHL = 24% (53%) Indolent = 33% (33%) DLBCL = 21% (43%) | ||
| r/r NHL, r/r MM, newly diagnosed AML T53wt/mut | Mono | ||||||||
| +Azacitidine +/− Venetoclax | |||||||||
| +Carfilzomib + Dexamethasone | |||||||||
| 1/2 | NCT05261490 | OvC | +Pegylated Doxoribicin | ||||||
| Both TTI-621 and TTI-622 | 1 | NCT05139225 | r/r MM | +Daratumumab Hyaloronidase-fihj | |||||
| ALX-148/Evorpacept | affinity-enhanced SIRPα-FcDEAD | 1 | NCT03013218 | ASPEN-1 | Hematologic and SCLC | +Rituximab | 6% neutropenia 3% anemia | NHL = 48% (60%) Indolent = 61% (91%) DLBCL = 38% (48%) | [242,243] |
| Mono | |||||||||
| +Nivolumab | |||||||||
| Gastric cancer | +Trastuzumab + Ramucirumab + Paclitaxel | 44% neutropenia 33% hypertension 22% anemia | 72% (89%) | [244,245,246] | |||||
| +Trastuzumab | 6% thrombocytopenia 6% neutropenia <5% feb neutropenia WBC count | 21% (47%) | [217,244,245] | ||||||
| HNSCC | +Pembrolizumab + 5FU/Platinum | 38% neutropenia 31% anemia | 39% (89%) | [245,246] | |||||
| +Pembrolizumab | Rare (<5%) anemia, thrombocytopenia, AIHA, neutropenia, pancytopenia | 20–40% | [217,245,246] | ||||||
| NSCLC | +Pembrolizumab | Rare (<5%) anemia, thrombocytopenia, AIHA, neutropenia, pancytopenia | 5% (40%) | [217] | |||||
| 1/2 | NCT04417517 | ASPEN-2 | untreated or r/r hrMDS | +Azacitidine | 18% neutropenia 18% feb neutropenia 14% thrombocytopenia 9% anemia | 55% (80%) | [247] | ||
| 2/3 | NCT05002127 | ASPEN-6 | HER-2+ GC/GJC | +Trastuzumab + Ramucirumab + Paclitaxel | |||||
| Trastuzumab + Ramucirumab + Paclitaxel | |||||||||
| Ramucirumab + Paclitaxel | |||||||||
| 1/2 | NCT04755244 | ASPEN-5 | AML | +Venetoclax + Azacitidine | |||||
| 2 | NCT04675333 | ASPEN-4 | HNSCC | +Pembrolizumab + 5FU/Platinum | |||||
| 2 | NCT04675294 | ASPEN-3 | HNSCC | +Pembrolizumab | |||||
| Pembrolizumab mono | |||||||||
| 2 | NCT05167409 | Microsatellite stable rCRC | +Cetuximab + Pembrolizumab | ||||||
| 1/2 | NCT05025800 | NHL | +Rituximab + Lenolidamide | ||||||
| 1/2 | NCT05027139 | metastatic/inoperable HER-2+ BC/GEC cancer | +Zanidatamab (anti-HER-2 BsAb) | ||||||
| 2 | NCT05167409 | MSS CRC | +Cetuximab + Pembrolizumab | ||||||
| SL-172154 | SIRPα-Fc-CD40L | 1 | NCT04406623 | Ovarian, Fallopian tube, peritoneal cancer | Mono | ||||
| 1 | NCT04502888 | HNSCC & CSCC | Mono (intratumorally) | ||||||
| CPO107/JMT601 | SIRPα ECD/anti-CD20 BsAb IgG1 | 1/2 | NCT04853329 | NHL | Mono | ||||
| IMM01 | SIRPα-Fc | 1/2 | NCT05140811 | AML and MDS | +Azacitidine | ||||
| IMM2902 | SIRPαECD/anti-HER-2 | 1 | NCT05076591 | HER-2+ BC and GC | Mono | ||||
| DSP107 | SIRPα-41BB fusion | 1/2 | NCT04440735 | Solid/NSCLC | Mono | ||||
| +Atezolizumab | |||||||||
| Anti-SIRPα mAbs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Type | Phase | Trial Identifier | Name | Indication | Treatment | Results | Ref | |
| Toxicity (≥Grade 3) | ORR (DCR) | ||||||||
| GS-0189 | anti-SIRPα IgG1 N297A | 1 | NCT04502706 | NHL | Mono | ||||
| +Rituximab | |||||||||
| CC-95251 | anti-SIRPα IgG1 K322A | 1 | NCT03783403 | Solid and hematologic cancer | Mono | 53% neutropenia 6% thrombocytopenia | 56% (56%) | [219] | |
| +Cetuximab/Rituximab | |||||||||
| 1 | NCT05168202 | r/r AML/MDS | Mono | ||||||
| +azacitidine | |||||||||
| BI765063/ OSE-172 | anti-SIRPαBIT IgG4 S229P-L445P | 1 | NCT03990233 | Solid cancer | Mono | None reported | 1/50 PR (HCC) | [220] | |
| +BI754091 (anti-PD-1) | 1 rash maculo-papular | 19% (25%) | [220] | ||||||
| 1 | NCT04653142 | Solid cancer | Mono | ||||||
| +BI754091 (anti-PD-1) | |||||||||
| 1 | NCT05068102 | Melanoma, HNSCC, NSCLC | Mono (Biodistribution, imaging with 89Zr-BI765063) | ||||||
| 1 | NCT05249426 | HNSCC | +BI754091 (anti-PD-1) +/− Cetuximab/chemo | ||||||
| HCC | +BI754091 (anti-PD-1) +/− BI836880 (anti-VEGF/Ang2 BsAb) | ||||||||
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.d.S.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.d.B.V.; da Silva, I.J., Jr.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, Z.; Huber, J.; Bassi, R.; Finnerty, B.; Corcoran, E.; Li, H.; Navarro, E.; Balderes, P.; Jimenez, X.; et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin. Cancer Res. 2006, 12, 6573–6584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.M.; Veeramani, S.; Weiner, G.J. Complement in monoclonal antibody therapy of cancer. Immunol. Res. 2014, 59, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, B.S.; Ackerman, M.E. Antibody-mediated complement activation in pathology and protection. Immunol. Cell Biol. 2020, 98, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Gül, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bögels, M.; Braster, R.; Vidarsson, G.; ten Hagen, T.L.M.; Kubes, P.; van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Investig. 2014, 124, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, N.; van Egmond, M. Monoclonal antibody-mediated killing of tumour cells by neutrophils. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12962. [Google Scholar] [CrossRef] [Green Version]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Mayes, P.A.; Hance, K.W.; Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov. 2018, 17, 509–527. [Google Scholar] [CrossRef]
- French, R.R.; Chan, H.T.; Tutt, A.L.; Glennie, M.J. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 1999, 5, 548–553. [Google Scholar] [CrossRef]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Tanaka, J.; Kim, J.A.; Rothchild, K.; Weinberg, A.; Shu, S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000, 60, 5514–5521. [Google Scholar] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campesato, L.F.; Weng, C.H.; Merghoub, T. Innate immune checkpoints for cancer immunotherapy: Expanding the scope of non T cell targets. Ann. Transl. Med. 2020, 8, 1031. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, M.; Mancardi, D.A.; Jonsson, F.; Iannascoli, B.; Fiette, L.; Di Santo, J.P.; Lowell, C.A.; Bruhns, P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 2013, 122, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Dancey, J.T.; Deubelbeiss, K.A.; Harker, L.A.; Finch, C.A. Neutrophil kinetics in man. J. Clin. Investig. 1976, 58, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Treffers, L.W.; Hiemstra, I.H.; Kuijpers, T.W.; Berg, T.K.; Matlung, H.L. Neutrophils in cancer. Immunol. Rev. 2016, 273, 312–328. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Mukaida, N.; Sasaki, S.-I.; Baba, T. Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 3457. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. The dual role of neutrophils in cancer. Semin. Immunol. 2021, 57, 101582. [Google Scholar] [CrossRef]
- Siwicki, M.; Pittet, M.J. Versatile neutrophil functions in cancer. Semin. Immunol. 2021, 57, 101538. [Google Scholar] [CrossRef]
- Taucher, E.; Taucher, V.; Fink-Neuboeck, N.; Lindenmann, J.; Smolle-Juettner, F.-M. Role of Tumor-Associated Neutrophils in the Molecular Carcinogenesis of the Lung. Cancers 2021, 13, 5972. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [Green Version]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Dysthe, M.; Parihar, R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Botta, C.; Zabaleta, A.; Puig, N.; Cedena, M.T.; Goicoechea, I.; Alameda, D.; San Jose-Eneriz, E.; Merino, J.; Rodriguez-Otero, P.; et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood 2020, 136, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Golay, J.; Valgardsdottir, R.; Musaraj, G.; Giupponi, D.; Spinelli, O.; Introna, M. Human neutrophils express low levels of FcgammaRIIIA, which plays a role in PMN activation. Blood 2019, 133, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, S.Q.; Kuijpers, T.W. Immunomodulation by IVIg and the Role of Fc-Gamma Receptors: Classic Mechanisms of Action after all? Front. Immunol. 2014, 5, 674. [Google Scholar] [CrossRef] [Green Version]
- Tsang, A.S.M.W.; Nagelkerke, S.Q.; Bultink, I.E.; Geissler, J.; Tanck, M.W.; Tacke, C.E.; Ellis, J.A.; Zenz, W.; Bijl, M.; Berden, J.H.; et al. Fc-gamma receptor polymorphisms differentially influence susceptibility to systemic lupus erythematosus and lupus nephritis. Rheumatology 2016, 55, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Futosi, K.; Fodor, S.; Mocsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Valerius, T.; Repp, R.; de Wit, T.P.; Berthold, S.; Platzer, E.; Kalden, J.R.; Gramatzki, M.; van de Winkel, J.G. Involvement of the high-affinity receptor for IgG (Fc gamma RI.; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood 1993, 82, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Treffers, L.W.; Van Houdt, M.; Bruggeman, C.W.; Heineke, M.H.; Zhao, X.W.; Van Der Heijden, J.; Nagelkerke, S.Q.; Verkuijlen, P.J.J.H.; Geissler, J.; Lissenberg-Thunnissen, S.; et al. FcγRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front. Immunol. 2019, 10, 3124. [Google Scholar] [CrossRef]
- Treffers, L.W.; Zhao, X.W.; van der Heijden, J.; Nagelkerke, S.Q.; van Rees, D.J.; Gonzalez, P.; Geissler, J.; Verkuijlen, P.; van Houdt, M.; de Boer, M.; et al. Genetic variation of human neutrophil Fcγ receptors and SIRPα in antibody-dependent cellular cytotoxicity towards cancer cells. Eur. J. Immunol. 2018, 48, 344–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- Valerius, T.; Wurflein, D.; Stockmeyer, B.; Repp, R.; Kalden, J.R.; Gramatzki, M. Activated neutrophils as effector cells for bispecific antibodies. Cancer Immunol. Immunother. 1997, 45, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, D.J.; Brinkhaus, M.; Klein, B.; Verkuijlen, P.; Tool, A.T.J.; Schornagel, K.; Treffers, L.W.; van Houdt, M.; Kater, A.P.; Vidarsson, G.; et al. Sodium stibogluconate and CD47-SIRPalpha blockade overcome resistance of anti-CD20-opsonized B cells to neutrophil killing. Blood Adv. 2022, 6, 2156–2166. [Google Scholar] [CrossRef]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef]
- Otten, M.A.; Rudolph, E.; Dechant, M.; Tuk, C.W.; Reijmers, R.M.; Beelen, R.H.; van de Winkel, J.G.; van Egmond, M. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J. Immunol. 2005, 174, 5472–5480. [Google Scholar] [CrossRef] [Green Version]
- Dechant, M.; Beyer, T.; Schneider-Merck, T.; Weisner, W.; Peipp, M.; van de Winkel, J.G.; Valerius, T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J. Immunol. 2007, 179, 2936–2943. [Google Scholar] [CrossRef] [Green Version]
- Bakema, J.E.; van Egmond, M. The human immunoglobulin A Fc receptor FcalphaRI: A multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011, 4, 612–624. [Google Scholar] [CrossRef] [Green Version]
- Bakema, J.E.; van Egmond, M. Immunoglobulin A: A next generation of therapeutic antibodies? mAbs 2011, 3, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, A.M.; Bondza, S.; Evers, M.; Koutstaal, R.; Nederend, M.; Jansen, J.H.M.; Rosner, T.; Valerius, T.; Leusen, J.H.W.; Ten Broeke, T. Potent Fc Receptor Signaling by IgA Leads to Superior Killing of Cancer Cells by Neutrophils Compared to IgG. Front. Immunol. 2019, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Otten, M.A.; Bakema, J.E.; Tuk, C.W.; Glennie, M.J.; Tutt, A.L.; Beelen, R.H.; van de Winkel, J.G.; van Egmond, M. Enhanced FcalphaRI-mediated neutrophil migration towards tumour colonies in the presence of endothelial cells. Eur. J. Immunol. 2012, 42, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Van der Steen, L.; Tuk, C.W.; Bakema, J.E.; Kooij, G.; Reijerkerk, A.; Vidarsson, G.; Bouma, G.; Kraal, G.; de Vries, H.E.; Beelen, R.H.; et al. Immunoglobulin A: Fc(alpha)RI interactions induce neutrophil migration through release of leukotriene B4. Gastroenterology 2009, 137, 2018–2029.e3. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Heitzmann, A.; Viel, S.; Nicolas, A.; Sastre-Garau, X.; Oppezzo, P.; Pritsch, O.; Osinaga, E.; Amigorena, S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011, 71, 5134–5143. [Google Scholar] [CrossRef] [Green Version]
- Van Spriel, A.B.; Leusen, J.H.; van Egmond, M.; Dijkman, H.B.; Assmann, K.J.; Mayadas, T.N.; van de Winkel, J.G. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 2001, 97, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, M.; van Vuuren, A.J.; Morton, H.C.; van Spriel, A.B.; Shen, L.; Hofhuis, F.M.; Saito, T.; Mayadas, T.N.; Verbeek, J.S.; van de Winkel, J.G. Human immunoglobulin A receptor (FcalphaRI, CD89) function in transgenic mice requires both FcR gamma chain and CR3 (CD11b/CD18). Blood 1999, 93, 4387–4394. [Google Scholar] [CrossRef]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef]
- Sengelov, H.; Kjeldsen, L.; Diamond, M.S.; Springer, T.A.; Borregaard, N. Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils. J. Clin. Investig. 1993, 92, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Olsson, I.; Klebanoff, S.J. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J. Cell Biol. 1976, 70, 719–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J. Clin. Investig. 1991, 88, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.; Seignez, C.; Racoeur, C.; Isambert, N.; Mabrouk, N.; Scagliarini, A.; Reveneau, S.; Arnould, L.; Bettaieb, A.; Jeannin, J.F.; et al. Tumor-derived granzyme B-expressing neutrophils acquire antitumor potential after lipid A treatment. Oncotarget 2018, 9, 28364–28378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.F.; Blank, A.; Reardon, C.A.; et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 2021, 184, 3163–3177.e21. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Treffers, L.W.; Ten Broeke, T.; Rosner, T.; Jansen, J.H.M.; van Houdt, M.; Kahle, S.; Schornagel, K.; Verkuijlen, P.; Prins, J.M.; Franke, K.; et al. IgA-Mediated Killing of Tumor Cells by Neutrophils Is Enhanced by CD47-SIRPalpha Checkpoint Inhibition. Cancer Immunol. Res. 2020, 8, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.; Blau, H.M. Death of solid tumor cells induced by Fas ligand expressing primary myoblasts. Somat. Cell Mol. Genet. 1997, 23, 249–257. [Google Scholar] [CrossRef]
- Liles, W.C.; Kiener, P.A.; Ledbetter, J.A.; Aruffo, A.; Klebanoff, S.J. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: Implications for the regulation of apoptosis in neutrophils. J. Exp. Med. 1996, 184, 429–440. [Google Scholar] [CrossRef]
- Sun, B.; Qin, W.; Song, M.; Liu, L.; Yu, Y.; Qi, X.; Sun, H. Neutrophil Suppresses Tumor Cell Proliferation via Fas/Fas Ligand Pathway Mediated Cell Cycle Arrested. Int. J. Biol. Sci. 2018, 14, 2103–2113. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef] [Green Version]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kuhn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front. Immunol. 2013, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 2016, 7, 11037. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Roberts, R.E.; Hallett, M.B. Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis. Int. J. Mol. Sci. 2019, 20, 1383. [Google Scholar] [CrossRef] [Green Version]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Bologna, L.; Gotti, E.; Da Roit, F.; Intermesoli, T.; Rambaldi, A.; Introna, M.; Golay, J. Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J. Immunol. 2013, 190, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouti, P.; Zhao, X.W.; Verkuijlen, P.J.J.H.; Tool, A.T.J.; van Houdt, M.; Köker, N.; Köker, M.Y.; Keskin, O.; Akbayram, S.; van Bruggen, R.; et al. Kindlin3-dependent CD11b/CD18-integrin activation is required for potentiation of neutrophil cytotoxicity by CD47-SIRPa checkpoint disruption. Cancer Immunol. Res. 2021, 9, 147–155. [Google Scholar] [CrossRef]
- Van Rees, D.J.; Bouti, P.; Klein, B.; Verkuijlen, P.J.H.; van Houdt, M.; Schornagel, K.; Tool, A.T.J.; Venet, D.; Sotiriou, C.; El-Abed, S.; et al. Cancer cells resist antibody-mediated destruction by neutrophils through activation of the exocyst complex. J. Immunother. Cancer 2022, 10, e004820. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Chtanova, T.; Schaeffer, M.; Han, S.J.; van Dooren, G.G.; Nollmann, M.; Herzmark, P.; Chan, S.W.; Satija, H.; Camfield, K.; Aaron, H.; et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 2008, 29, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Lore, K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Meinderts, S.M.; Baker, G.; van Wijk, S.; Beuger, B.M.; Geissler, J.; Jansen, M.H.; Saris, A.; Ten Brinke, A.; Kuijpers, T.W.; van den Berg, T.K.; et al. Neutrophils acquire antigen-presenting cell features after phagocytosis of IgG-opsonized erythrocytes. Blood Adv. 2019, 3, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, E.M.; Cochrane, F.; Barclay, A.N.; van den Berg, T.K. Signal Regulatory Proteins in the Immune System. J. Immunol. 2005, 175, 7781–7787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; van der Laan, L.J.; Vernon-Wilson, E.; Renardel de Lavalette, C.; Dopp, E.A.; Dijkstra, C.D.; Simmons, D.L.; van den Berg, T.K. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 1998, 161, 1853–1859. [Google Scholar] [PubMed]
- Van den Berg, T.K.; Yoder, J.A.; Litman, G.W. On the origins of adaptive immunity: Innate immune receptors join the tale. Trends Immunol. 2004, 25, 11–16. [Google Scholar] [CrossRef]
- Hatherley, D.; Harlos, K.; Dunlop, D.C.; Stuart, D.I.; Barclay, A.N. The structure of the macrophage signal regulatory protein alpha (SIRPalpha) inhibitory receptor reveals a binding face reminiscent of that used by T cell receptors. J. Biol. Chem. 2007, 282, 14567–14575. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, M.I.; Lindberg, F.P.; Plas, D.; Reynolds, S.; Peters, M.G.; Brown, E.J. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J. Cell Sci. 1995, 108, 3419–3425. [Google Scholar] [CrossRef]
- Brown, E.J.; Frazier, W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001, 11, 130–135. [Google Scholar] [CrossRef]
- Brown, E.; Hooper, L.; Ho, T.; Gresham, H. Integrin-associated protein: A 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990, 111, 2785–2794. [Google Scholar] [CrossRef]
- Campbell, I.G.; Freemont, P.S.; Foulkes, W.; Trowsdale, J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992, 52, 5416–5420. [Google Scholar]
- Oldenborg, P.A. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013, 2013, 614619. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Lagenaur, C.F.; Narayanan, V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 1999, 274, 559–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiffert, M.; Cant, C.; Chen, Z.; Rappold, I.; Brugger, W.; Kanz, L.; Brown, E.J.; Ullrich, A.; Buhring, H.J. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999, 94, 3633–3643. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Wilson, E.F.; Kee, W.J.; Willis, A.C.; Barclay, A.N.; Simmons, D.L.; Brown, M.H. CD47 is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPalpha 1. Eur. J. Immunol. 2000, 30, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Hatherley, D.; Graham, S.C.; Turner, J.; Harlos, K.; Stuart, D.I.; Barclay, A.N. Paired Receptor Specificity Explained by Structures of Signal Regulatory Proteins Alone and Complexed with CD47. Mol. Cell 2008, 31, 266–277. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Jansen, J.H.M.; Raaben, M.; Toebes, M.; Franke, K.; Brandsma, A.M.; Matlung, H.L.; Fauster, A.; Gomez-Eerland, R.; Bakker, N.A.M.; et al. Glutaminyl cyclase is an enzymatic modifier of the CD47-SIRPα axis and a target for cancer immunotherapy. Nat. Med. 2019, 25, 612–619. [Google Scholar] [CrossRef]
- Hatherley, D.; Lea, S.M.; Johnson, S.; Barclay, A.N. Polymorphisms in the human inhibitory signal-regulatory protein alpha do not affect binding to its ligand CD47. J. Biol. Chem. 2014, 289, 10024–10028. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.W.; Van Beek, E.M.; Schornagel, K.; Van Der Maaden, H.; Van Houdt, M.; Otten, M.A.; Finetti, P.; Van Egmond, M.; Matozaki, T.; Kraal, G.; et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. USA 2011, 108, 18342–18347. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, M.A.; Kern, N.; Vale, R.D. CD47 Ligation Repositions the Inhibitory Receptor SIRPA to Suppress Integrin Activation and Phagocytosis. Immunity 2020, 53, 290–302.e6. [Google Scholar] [CrossRef]
- Tsai, R.K.; Discher, D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008, 180, 989–1003. [Google Scholar] [CrossRef] [Green Version]
- Kharitonenkov, A.; Chen, Z.; Sures, I.; Wang, H.; Schilling, J.; Ullrich, A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997, 386, 181–186. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Gresham, H.D.; Lindberg, F.P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, U. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 2009, 228, 342–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Suk, K.; Lee, W.H. SHPS-1 and a synthetic peptide representing its ITIM inhibit the MyD88, but not TRIF, pathway of TLR signaling through activation of SHP and PI3K in THP-1 cells. Inflamm. Res. 2013, 62, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Thibaudeau, E.; Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 1998, 273, 22719–22728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Costell, M.; Fassler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Franke, K.; Pillai, S.Y.; Hoogenboezem, M.; Gijbels, M.J.J.; Matlung, H.L.; Geissler, J.; Olsman, H.; Pottgens, C.; van Gorp, P.J.; Ozsvar-Kozma, M.; et al. SIRPalpha on Mouse B1 Cells Restricts Lymphoid Tissue Migration and Natural Antibody Production. Front. Immunol. 2020, 11, 570963. [Google Scholar] [CrossRef]
- Cooper, D.; Lindberg, F.P.; Gamble, J.R.; Brown, E.J.; Vadas, M.A. Transendothelial migration of neutrophils involves integrin-associated protein (CD47). Proc. Natl. Acad. Sci. USA 1995, 92, 3978–3982. [Google Scholar] [CrossRef] [Green Version]
- Parkos, C.A.; Colgan, S.P.; Liang, T.W.; Nusrat, A.; Bacarra, A.E.; Carnes, D.K.; Madara, J.L. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J. Cell Biol. 1996, 132, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, F.P.; Bullard, D.C.; Caver, T.E.; Gresham, H.D.; Beaudet, A.L.; Brown, E.J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 1996, 274, 795–798. [Google Scholar] [CrossRef]
- Meinderts, S.M.; Oldenborg, P.A.; Beuger, B.M.; Klei, T.R.L.; Johansson, J.; Kuijpers, T.W.; Matozaki, T.; Huisman, E.J.; de Haas, M.; van den Berg, T.K.; et al. Human and murine splenic neutrophils are potent phagocytes of IgG-opsonized red blood cells. Blood Adv. 2017, 1, 875–886. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Merlin, D.; Burst, S.L.; Pochet, M.; Madara, J.L.; Parkos, C.A. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem. 2001, 276, 40156–40166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zen, K.; Liu, Y. Role of different protein tyrosine kinases in fMLP-induced neutrophil transmigration. Immunobiology 2008, 213, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Buhring, H.J.; Zen, K.; Burst, S.L.; Schnell, F.J.; Williams, I.R.; Parkos, C.A. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 2002, 277, 10028–10036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; O’Connor, M.B.; Mandell, K.J.; Zen, K.; Ullrich, A.; Buhring, H.J.; Parkos, C.A. Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J. Immunol. 2004, 172, 2578–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Zarate, J.; Matlung, H.L.; Matozaki, T.; Kuijpers, T.W.; Maridonneau-Parini, I.; van den Berg, T.K. Regulation of Phagocyte Migration by Signal Regulatory Protein-Alpha Signaling. PLoS ONE 2015, 10, e0127178. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Ishikawa-Sekigami, T.; Kaneko, Y.; Okazawa, H.; Tomizawa, T.; Okajo, J.; Saito, Y.; Okuzawa, C.; Sugawara-Yokoo, M.; Nishiyama, U.; Ohnishi, H.; et al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 2006, 107, 341–348. [Google Scholar] [CrossRef]
- Olsson, M.; Bruhns, P.; Frazier, W.A.; Ravetch, J.V.; Oldenborg, P.A. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood 2005, 105, 3577–3582. [Google Scholar] [CrossRef] [Green Version]
- Yamao, T.; Noguchi, T.; Takeuchi, O.; Nishiyama, U.; Morita, H.; Hagiwara, T.; Akahori, H.; Kato, T.; Inagaki, K.; Okazawa, H.; et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 2002, 277, 39833–39839. [Google Scholar] [CrossRef] [Green Version]
- Blazar, B.R.; Lindberg, F.P.; Ingulli, E.; Panoskaltsis-Mortari, A.; Oldenborg, P.A.; Iizuka, K.; Yokoyama, W.M.; Taylor, P.A. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J. Exp. Med. 2001, 194, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Madariaga, M.L.; Wang, S.; Van Rooijen, N.; Oldenborg, P.A.; Yang, Y.G. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc. Natl. Acad. Sci. USA 2007, 104, 13744–13749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007, 8, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.K.; van der Schoot, C.E. Innate immune ‘self’ recognition: A role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008, 29, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.S.; Brown, M.H.; Barclay, A.N.; Hatherley, D. Signal-regulatory protein alpha from the NOD mouse binds human CD47 with an exceptionally high affinity—Implications for engraftment of human cells. Immunology 2014, 143, 61–67. [Google Scholar] [CrossRef]
- Yamauchi, T.; Takenaka, K.; Urata, S.; Shima, T.; Kikushige, Y.; Tokuyama, T.; Iwamoto, C.; Nishihara, M.; Iwasaki, H.; Miyamoto, T.; et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 2013, 121, 1316–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Hu, H.; Chen, H. Combination of CD47 and CD68 expression predicts survival in eastern-Asian patients with non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 739–747. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van Den Berg, T.K. The interaction between signal regulatory protein alpha (SIRPα) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.P.; Liu, J.; Lim, J.S.; et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef]
- Li, F.; Lv, B.; Liu, Y.; Hua, T.; Han, J.; Sun, C.; Xu, L.; Zhang, Z.; Feng, Z.; Cai, Y.; et al. Blocking the CD47-SIRPalpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 2018, 7, e1391973. [Google Scholar] [CrossRef] [Green Version]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edris, B.; Weiskopf, K.; Weissman, I.L.; van de Rijn, M. Flipping the script on macrophages in leiomyosarcoma. Oncoimmunology 2012, 1, 1202–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.F.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; Xia, B.; et al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015, 6, 23662–23670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Dai, M.; Xu, Q.; Zhu, X.; Zhou, Y.; Jiang, S.; Wang, Y.; Ai, Z.; Ma, L.; Zhang, Y.; et al. SRSF10-mediated IL1RAP alternative splicing regulates cervical cancer oncogenesis via mIL1RAP-NF-kappaB-CD47 axis. Oncogene 2018, 37, 2394–2409. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Zhu, L.; Zhuang, H.; Hao, Y.; Gao, S.; Liu, S.; Liu, Q.; Liu, D.; Liu, J.; Lin, B. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am. J. Cancer Res. 2015, 5, 2777–2787. [Google Scholar]
- Chao, M.P.; Tang, C.; Pachynski, R.K.; Chin, R.; Majeti, R.; Weissman, I.L. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011, 118, 4890–4901. [Google Scholar] [CrossRef]
- Chao, M.P.; Takimoto, C.H.; Feng, D.D.; McKenna, K.; Gip, P.; Liu, J.; Volkmer, J.P.; Weissman, I.L.; Majeti, R. Therapeutic Targeting of the Macrophage Immune Checkpoint CD47 in Myeloid Malignancies. Front. Oncol. 2019, 9, 1380. [Google Scholar] [CrossRef]
- Martinez-Sanz, P.; Hoogendijk, A.J.; Verkuijlen, P.; Schornagel, K.; van Bruggen, R.; van den Berg, T.K.; Tytgat, G.A.M.; Franke, K.; Kuijpers, T.W.; Matlung, H.L. CD47-SIRPalpha Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells. Cancers 2021, 13, 4261. [Google Scholar] [CrossRef]
- Zhao, X.W.; Kuijpers, T.W.; van den Berg, T.K. Is targeting of CD47-SIRPalpha enough for treating hematopoietic malignancy? Blood 2012, 119, 4333–4334. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.W.; Matlung, H.L.; Kuijpers, T.W.; van den Berg, T.K. On the mechanism of CD47 targeting in cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E2843. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.C.; Chen, A.; Harrabi, O.; Sockolosky, J.T.; Zhang, A.; Sangalang, E.; Doyle, L.V.; Kauder, S.E.; Fontaine, D.; Bollini, S.; et al. Targeting the myeloid checkpoint receptor SIRPalpha potentiates innate and adaptive immune responses to promote anti-tumor activity. J. Hematol. Oncol. 2020, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [Green Version]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosner, T.; Kahle, S.; Montenegro, F.; Matlung, H.L.; Jansen, J.H.M.; Evers, M.; Beurskens, F.; Leusen, J.H.W.; van den Berg, T.K.; Valerius, T. Immune Effector Functions of Human IgG2 Antibodies against EGFR. Mol. Cancer Ther. 2019, 18, 75–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Sanz, P.; van Rees, D.J.; van Zogchel, L.M.J.; Klein, B.; Bouti, P.; Olsman, H.; Schornagel, K.; Kok, I.; Sunak, A.; Leeuwenburg, K.; et al. G-CSF as a suitable alternative to GM-CSF to boost dinutuximab-mediated neutrophil cytotoxicity in neuroblastoma treatment. J. Immunother. Cancer 2021, 9, e002259. [Google Scholar] [CrossRef]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Dev. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Li, Y.; Liu, J.; Chen, W.; Wei, Z.; Luo, Y.; Liu, H.; Qi, Y.; Wang, F.; Sui, J. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol. Ther. 2021, 29, 1572–1584. [Google Scholar] [CrossRef]
- Hendriks, M.; Ploeg, E.M.; Koopmans, I.; Britsch, I.; Ke, X.; Samplonius, D.F.; Helfrich, W. Bispecific antibody approach for EGFR-directed blockade of the CD47-SIRPalpha “don’t eat me” immune checkpoint promotes neutrophil-mediated trogoptosis and enhances antigen cross-presentation. Oncoimmunology 2020, 9, 1824323. [Google Scholar] [CrossRef]
- Hernandez-Ilizaliturri, F.J.; Jupudy, V.; Ostberg, J.; Oflazoglu, E.; Huberman, A.; Repasky, E.; Czuczman, M.S. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin. Cancer Res. 2003, 9, 5866–5873. [Google Scholar]
- Siders, W.M.; Shields, J.; Garron, C.; Hu, Y.; Boutin, P.; Shankara, S.; Weber, W.; Roberts, B.; Kaplan, J.M. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leuk. Lymphoma 2010, 51, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, E.F.; Gai, S.A.; Opel, C.F.; Kwan, B.H.; Surana, R.; Mihm, M.C.; Kauke, M.J.; Moynihan, K.D.; Angelini, A.; Williams, R.T.; et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 2015, 27, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, D.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.X.; Xu, M.M. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S.I.; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPalpha antibodies as a potential new tool for cancer immunotherapy. JCI Insight 2017, 2, e89140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, R.C.; Krishnan, S.; Wu, R.C.; Boda, A.R.; Liu, A.; Winkler, M.; Hsu, W.H.; Lin, S.H.; Hung, M.C.; Chan, L.C.; et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci. Immunol. 2022, 7, eabl9330. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef] [Green Version]
- Gauttier, V.; Pengam, S.; Durand, J.; Biteau, K.; Mary, C.; Morello, A.; Neel, M.; Porto, G.; Teppaz, G.; Thepenier, V.; et al. Selective SIRP alpha blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J. Clin. Investig. 2020, 130, 6109–6123. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Deng, Y.; Wei, F.; Zhao, Q.; Liu, Y.; Liu, Z.; Yu, B.; Huang, Z. Delivery of CD47 blocker SIRPalpha-Fc by CAR-T cells enhances antitumor efficacy. J. Immunother. Cancer 2022, 10, e003737. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef] [Green Version]
- Upton, R.; Banuelos, A.; Feng, D.; Biswas, T.; Kao, K.; McKenna, K.; Willingham, S.; Ho, P.Y.; Rosental, B.; Tal, M.C.; et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026849118. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.K.; Valerius, T. Myeloid immune-checkpoint inhibition enters the clinical stage. Nat. Rev. Clin. Oncol. 2019, 16, 275–276. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Roschewski, M. The First-In-Class Anti-CD47 Antibody HU5F9-G4 with Rituximab induces Durable Responses in Relapsed/Refractory DLBCL and Indolent Lymphoma: Interim Phase 1b/2 Results; EHA Meeting Report; EHA: Hague, The Netherlands, 2019. [Google Scholar]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Knapper, S.; Kelly, R.; Salim, R.; Lubowiecki, M.; Royston, D.; Johnson, H.; Roberts, C.; Chen, J.; Agoram, B.; et al. Initial Phase 1 Results of the First-In-Class Anti-CD47 Antibody HU5F9-G4 in Relapsed/Refractory Acute Myeloid Leukemia Patients; EHA Meeting Report; EHA: Hague, The Netherlands, 2018. [Google Scholar]
- Sallman, D.A.; Donnellan, W.B.; Asch, A.S.; Lee, D.J.; Al Malki, M.; Marcucci, G.; Pollyea, D.A.; Kambhampati, S.; Komrokji, R.S.; Van Elk, J.; et al. The first-in-class anti-CD47 antibody Hu5F9-G4 is active and well tolerated alone or with azacitidine in AML and MDS patients: Initial phase 1b results. J. Clin. Oncol. 2019, 37, 7009. [Google Scholar] [CrossRef]
- Sallman, D.A.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569. [Google Scholar] [CrossRef]
- Sallman, D.A.; Al Malki, M.; Asch, A.S.; Lee, D.J.; Kambhampati, S.; Donnellan, W.B.; Bradley, T.J.; Vyas, P.; Jeyakumar, D.; Marcucci, G.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results. J. Clin. Oncol. 2020, 38, 7507. [Google Scholar] [CrossRef]
- Sallman, D.; Asch, A.; Kambhampati, S.; Al Malki, M.; Zeidner, J.; Donnellan, W.; Lee, D.; Vyas, P.; Jeyakumar, D.; Mannis, G.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab in Combination with Azacitidine Is Well Tolerated and Effective in AML Patients: Phase 1b Results. Clin. Lymph. Myeloma Leuk. 2021, 21, S290. [Google Scholar] [CrossRef]
- Zeidan, A.M.; DeAngelo, D.J.; Palmer, J.; Seet, C.S.; Tallman, M.S.; Wei, X.; Raymon, H.; Sriraman, P.; Kopytek, S.; Bewersdorf, J.P.; et al. Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann. Hematol. 2022, 101, 557–569. [Google Scholar] [CrossRef]
- Narla, R.K.; Modi, H.; Wong, L.; Abassian, M.; Bauer, D.; Desai, P.; Gaffney, B.; Jackson, P.; Leisten, J.; Liu, J.; et al. The humanized anti-CD47 monclonal antibody, CC-90002, has antitumor activity in vitro and in vivo. Cancer Res. 2017, 77, 4694. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Sancho, J.M.; Cordoba, R.; Persky, D.O.; Andreadis, C.; Huntington, S.F.; Carpio, C.; Giles, D.M.; Wei, X.; Li, Y.F.; et al. Anti-CD47 Antibody, CC-90002, in Combination with Rituximab in Subjects with Relapsed and/or Refractory Non-Hodgkin Lymphoma (R/R NHL). Blood 2019, 134, 4089. [Google Scholar] [CrossRef]
- Ni, H.; Cao, L.; Wu, Z.; Wang, L.; Zhou, S.; Guo, X.; Gao, Y.; Jing, H.; Wu, M.; Liu, Y.; et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol. Immunother. 2022, 71, 353–363. [Google Scholar] [CrossRef]
- Lakhani, N.; Orloff, M.; Fu, S.Q.; Liu, Y.; Wang, Y.; Zhou, H.; Lin, K.D.; Liu, F.; Yan, S.L.; Patnaik, A. First-in-Human Phase I Trial of Ibi188, an Anti-Cd47 Targeting Monoclonal Antibody, in Patients with Advanced Solid Tumors and Lymphomas. J. Immunother. Cancer 2020, 8, A180. [Google Scholar] [CrossRef]
- Berlin, J.; Harb, W.; Adjei, A.; Xing, Y.; Swiecicki, P.; Seetharam, M.; Nandagopal, L.; Gopal, A.; Xu, C.; Meng, Y.; et al. A First-in-Human Study of Lemzoparlimab, a Differentiated Anti-Cd47 Antibody, in Subjects with Relapsed/Refractory Malignancy: Initial Monotherapy Results. J. Immunother. Cancer 2020, 8, A233–A234. [Google Scholar] [CrossRef]
- Mehta, A.; Harb, W.; Xu, C.; Meng, Y.; Lee, L.; Yuan, V.; Wang, Z.; Song, P.; Shen, J.H.; Gopal, A.K. Lemzoparlimab, a Differentiated Anti-CD47 Antibody in Combination with Rituximab in Relapsed and Refractory Non-Hodgkin’s Lymphoma: Initial Clinical Results. Blood 2021, 138, 3542. [Google Scholar] [CrossRef]
- Qi, J.Y.; Li, J.; Jiang, B.; Jiang, B.; Liu, H.Z.; Cao, X.X.; Zhang, M.X.; Meng, Y.; Ma, X.Y.; Jia, Y.M.; et al. A Phase I/IIa Study of Lemzoparlimab, a Monoclonal Antibody Targeting CD47, in Patients with Relapsed and/or Refractory Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS): Initial Phase I Results. Blood 2020, 136, 30–31. [Google Scholar] [CrossRef]
- Petrova, P.S.; Viller, N.N.; Wong, M.; Pang, X.; Lin, G.H.; Dodge, K.; Chai, V.; Chen, H.; Lee, V.; House, V.; et al. TTI-621 (SIRPalphaFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin. Cancer Res. 2017, 23, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.H.Y.; Chai, V.; Lee, V.; Dodge, K.; Truong, T.; Wong, M.; Johnson, L.D.; Linderoth, E.; Pang, X.; Winston, J.; et al. TTI-621 (SIRPalphaFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS ONE 2017, 12, e0187262. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.A.; Taylor, M.H.; DeSimone, J.A.; Zain, J.M.; Shustov, A.R.; Johns, C.; McCann, S.; Lin, G.H.Y.; Petrova, P.S.; et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sezary syndrome: A multicentre, phase 1 study. Lancet Haematol. 2021, 8, e808–e817. [Google Scholar] [CrossRef]
- Patel, K.; Ramchandren, R.; Maris, M.; Lesokhin, A.M.; von Keudell, G.R.; Cheson, B.D.; Zonder, J.; Seymour, E.K.; Catalano, T.; Lin, G.H.Y.; et al. Investigational CD47-Blocker TTI-622 Shows Single-Agent Activity in Patients with Advanced Relapsed or Refractory Lymphoma: Update from the Ongoing First-in-Human Dose Escalation Study. Blood 2020, 136, 46–47. [Google Scholar] [CrossRef]
- Patel, K.; Zonder, J.A.; Sano, D.; Maris, M.; Lesokhin, A.; von Keudell, G.R.; Lai, C.; Ramchandren, R.; Catalano, T.; Lin, G.H.Y.; et al. CD47-Blocker TTI-622 Shows Single-Agent Activity in Patients with Advanced Relapsed or Refractory Lymphoma: Update from the Ongoing First-in-Human Dose Escalation Study. Blood 2021, 138, 3560. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauder, S.E.; Kuo, T.C.; Harrabi, O.; Chen, A.; Sangalang, E.; Doyle, L.; Rocha, S.S.; Bollini, S.; Han, B.; Sim, J.; et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 2018, 13, e0201832. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, N.J.; Chow, L.Q.M.; Gainor, J.F.; LoRusso, P.; Lee, K.W.; Chung, H.C.; Lee, J.; Bang, Y.J.; Hodi, F.S.; Kim, W.S.; et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): A first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021, 22, 1740–1751. [Google Scholar] [CrossRef]
- Chan, H.; Trout, C.; Mikolon, D.; Adams, P.; Guzman, R.; Fenalti, G.; Mavrommatis, K.; Abbasian, M.; Dearth, L.; Fox, B.; et al. Discovery and Preclinical Characterization of CC-95251, an Anti-SIRPα Antibody That Enhances Macrophage-Mediated Phagocytosis of Non-Hodgkin Lymphoma (NHL) Cells When Combined with Rituximab. Blood 2021, 138, 2271. [Google Scholar] [CrossRef]
- Strati, P.; Hawkes, E.; Ghosh, N.; Tuscano, J.M.; Chu, Q.; Anderson, M.A.; Patel, A.; Burgess, M.R.; Hege, K.; Chhagan, S.; et al. Interim Results from the First Clinical Study of CC-95251, an Anti-Signal Regulatory Protein-Alpha (SIRPα) Antibody, in Combination with Rituximab in Patients with Relapsed and/or Refractory Non-Hodgkin Lymphoma (R/R NHL). Blood 2021, 183, 2493. [Google Scholar] [CrossRef]
- Champiat, S.; Cassier, P.A.; Kotecki, N.; Korakis, I.; Vinceneux, A.; Jungels, C.; Blatchford, J.; Elgadi, M.M.; Clarke, N.; Fromond, C.; et al. Safety, Pharmacokinetics, Efficacy, and Preliminary Biomarker Data of First-In-Class BI 765063, a Selective SIRPα Inhibitor: Results of Monotherapy Dose Escalation in Phase 1 Study in Patients with Advanced Solid Tumors; ASCO Meeting Report; ASCO: Alexandria, VA, USA, 2021. [Google Scholar]
- Kotecki, N.; Champiat, S.; Delord, J.; Vinceneux, A.; Jungels, C.; Marabelle, A.; Korakis, I.; Wojciekowski, S.; Block, E.; Clarke, N.; et al. Phase I Dose Escalation Study in Patients (pts) with Advanced Solid Tumours Receiving First-In-Class BI 765063, a Selective Signal-Regulatory Protein α (SIRPα) Inhibitor, in Combination with Ezabenlimab (BI 754091), a Programmed Cell Death Protein 1 (PD-1) Inhibitor; EMSO Meeting Report; EMSO: London, UK, 2021. [Google Scholar]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef] [Green Version]
- Ustyanovska Avtenyuk, N.; Choukrani, G.; Ammatuna, E.; Niki, T.; Cendrowicz, E.; Lourens, H.J.; Huls, G.; Wiersma, V.R.; Bremer, E. Galectin-9 Triggers Neutrophil-Mediated Anticancer Immunity. Biomedicines 2021, 10, 66. [Google Scholar] [CrossRef]
- Oronsky, B.; Guo, X.; Wang, X.; Cabrales, P.; Sher, D.; Cannizzo, L.; Wardle, B.; Abrouk, N.; Lybeck, M.; Caroen, S.; et al. Discovery of RRx-001, a Myc and CD47 Downregulating Small Molecule with Tumor Targeted Cytotoxicity and Healthy Tissue Cytoprotective Properties in Clinical Development. J. Med. Chem. 2021, 64, 7261–7271. [Google Scholar] [CrossRef]
- Oronsky, B.; Cabrales, P.; Caroen, S.; Guo, X.; Scribner, C.; Oronsky, A.; Reid, T.R. RRx-001, a downregulator of the CD47- SIRPalpha checkpoint pathway, does not cause anemia or thrombocytopenia. Expert Opin. Drug Metab. Toxicol. 2021, 17, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P. RRx-001 Acts as a Dual Small Molecule Checkpoint Inhibitor by Downregulating CD47 on Cancer Cells and SIRP-alpha on Monocytes/Macrophages. Transl. Oncol. 2019, 12, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Oronsky, B.; Scicinski, J.; Scribner, C.L.; Knox, S.J.; Ning, S.; Peehl, D.M.; Korn, R.; Stirn, M.; Carter, C.A.; et al. Safety and activity of RRx-001 in patients with advanced cancer: A first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 2015, 16, 1133–1142. [Google Scholar] [CrossRef]
- Tomita, Y.; Oronsky, B.; Abrouk, N.; Cabrales, P.; Reid, T.R.; Lee, M.J.; Yuno, A.; Baker, J.; Lee, S.; Trepel, J.B. In small cell lung cancer patients treated with RRx-001, a downregulator of CD47, decreased expression of PD-L1 on circulating tumor cells significantly correlates with clinical benefit. Transl. Lung Cancer Res. 2021, 10, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Das, D.S.; Ray, A.; Das, A.; Song, Y.; Tian, Z.; Oronsky, B.; Richardson, P.; Scicinski, J.; Chauhan, D.; Anderson, K.C. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells. Leukemia 2016, 30, 2187–2197. [Google Scholar] [CrossRef] [Green Version]
- Oronsky, B.; Reid, T.R.; Larson, C.; Caroen, S.; Quinn, M.; Burbano, E.; Varner, G.; Thilagar, B.; Brown, B.; Coyle, A.; et al. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 2019, 15, 3427–3433. [Google Scholar] [CrossRef]
- Wu, Z.; Weng, L.; Zhang, T.; Tian, H.; Fang, L.; Teng, H.; Zhang, W.; Gao, J.; Hao, Y.; Li, Y.; et al. Identification of Glutaminyl Cyclase isoenzyme isoQC as a regulator of SIRPalpha-CD47 axis. Cell Res. 2019, 29, 502–505. [Google Scholar] [CrossRef]
- Baumann, N.; Rosner, T.; Jansen, J.H.M.; Chan, C.; Marie Eichholz, K.; Klausz, K.; Winterberg, D.; Muller, K.; Humpe, A.; Burger, R.; et al. Enhancement of epidermal growth factor receptor antibody tumor immunotherapy by glutaminyl cyclase inhibition to interfere with CD47/signal regulatory protein alpha interactions. Cancer Sci. 2021, 112, 3029–3040. [Google Scholar] [CrossRef]
- Burgess, T.L.; Amason, J.D.; Rubin, J.S.; Duveau, D.Y.; Lamy, L.; Roberts, D.D.; Farrell, C.L.; Inglese, J.; Thomas, C.J.; Miller, T.W. A homogeneous SIRPalpha-CD47 cell-based, ligand-binding assay: Utility for small molecule drug development in immuno-oncology. PLoS ONE 2020, 15, e0226661. [Google Scholar] [CrossRef] [Green Version]
- Evers, M.; Rosner, T.; Dunkel, A.; Jansen, J.H.M.; Baumann, N.; Ten Broeke, T.; Nederend, M.; Eichholz, K.; Klausz, K.; Reiding, K.; et al. The selection of variable regions affects effector mechanisms of IgA antibodies against CD20. Blood Adv. 2021, 5, 3807–3820. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Rao, D.; Lu, M.; Wen, J.; Chen, X.; Wang, H.; Cui, X.; Tang, W.; Xu, S.; et al. Luteolin promotes macrophage-mediated phagocytosis by inhibiting CD47 pyroglutamation. Transl. Oncol. 2021, 14, 101129. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.A.; Lakhani, N.J.; Eng, C.; Hecht, J.R.; Bendell, J.C.; Philip, P.A.; O’Dwyer, P.J.; Johnson, B.; Kardosh, A.; Ippolito, T.M.; et al. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J. Clin. Oncol. 2020, 38, 114. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Wang, R.; Wang, X.; Shallis, R.M.; Podoltsev, N.A.; Bewersdorf, J.P.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Clinical Outcomes of Older Patients (pts) with Acute Myeloid Leukemia (AML) Receiving Hypomethylating Agents (HMAs): A Large Population-Based Study in the United States. Blood 2019, 134, 646. [Google Scholar] [CrossRef]
- Patnaik, A.; Spreafico, A.; Paterson, A.M.; Peluso, M.O.; Chung, J.; Bowers, B.; Niforos, D.; O’Neill, A.M.; Beeram, M.; Iafolla, M.; et al. Results of a first-in-human phase I study of SRF231, a fully human, high-affinity anti-CD47 antibody. J. Clin. Oncol. 2020, 38, 3064. [Google Scholar] [CrossRef]
- Johnson, L.D.S.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C.; et al. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, S.M.; Foran, J.M.; Maris, M.; Lue, J.K.; Sawas, A.; Okada, C.; Feldman, T.A.; Sokol, L.; Mei, M.; Flinn, I.W.; et al. Updates from Ongoing, First-in-Human Phase 1 Dose Escalation and Expansion Study of TTI-621, a Novel Biologic Targeting CD47, in Patients with Relapsed or Refractory Hematologic Malignancies. Blood 2021, 138, 2448. [Google Scholar] [CrossRef]
- Kim, T.M.; Lakhani, N.; Gainor, J.F.; Kamdar, M.; Fanning, P.; Squifflet, P.; Jin, F.; Forgie, A.J.; Wan, H.I.; Pons, J.; et al. ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma; ASPEN-1. Blood 2020, 136, 13–14. [Google Scholar]
- Kim, T.M.; Lakhani, N.; Gainor, J.F.; Kamdar, M.; Fanning, P.; Squifflet, P.; Jin, F.; Forgie, A.J.; Wan, H.I.; Pons, J.; et al. ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Non-Hodgkin Lymphoma. In Proceedings of the 62nd ASH Annual Meeting and Exposition, Washington, DC, USA, 5–8 December 2020. [Google Scholar]
- Chung, H.C.; Lee, K.W.; Kim, W.S.; Gainor, J.F.; Lakhani, N.; Chow, L.Q.; Messersmith, W.A.; Fanning, P.; Squifflet, P.; Jin, F.; et al. ESMO meeting report: ASPEN-01: A phase 1 study of ALX148, a CD47 blocker, in combination with trastuzumab, ramucirumab and paclitaxel in patients with second-line HER2-positive advanced gastric or gastroesophageal junction cancer. In Proceedings of the Esmo World Congress on Gastrointestinal Cancer, Virtual Meeting, 30 June–3 July 2021. [Google Scholar]
- Lee, K.W.; Chung, H.C.; Kim, W.S.; Chow, L.Q.; Lakhani, N.; Messersmith, W.A.; Bang, Y.J.; LoRusso, P.; Fanning, P.; Squifflet, P.; et al. ALX148, a CD47 blocker, in combination with standard chemotherapy and antibody regimens in patients with gastric/gastroesophageal junction (GC) cancer and head and neck squamous cell carcinoma (HNSCC). J. Immunother. Cancer 2020, 8, A429. [Google Scholar]
- Lee, K.W.; Chung, H.C.; Kim, T.M.; Lakhani, N.; Messersmith, W.A.; Santana-Davila, R.; Kim, W.S.; LoRusso, P.; Bang, Y.J.; Chow, L.Q.; et al. Evorpacept (ALX148), a CD47 myeloid checkpoint inhibitor, in patients with head and neck squamous cell carcinoma (HNSCC) and with gastric/gastroesophageal cancer (GC); ASPEN-01. J. Immunother. Cancer 2021, 9, 498. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Erba, H.P.; Sanikommu, S.R.; Altman, J.K.; Sayar, H.; Scott, B.L.; Fong, A.P.; Guan, S.; Jin, F.; Forgie, A.J.; et al. Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine: A Phase 1/2 Study in Patients with Myelodysplastic Syndrome (ASPEN-02). Blood 2021, 138, 2601. [Google Scholar] [CrossRef]
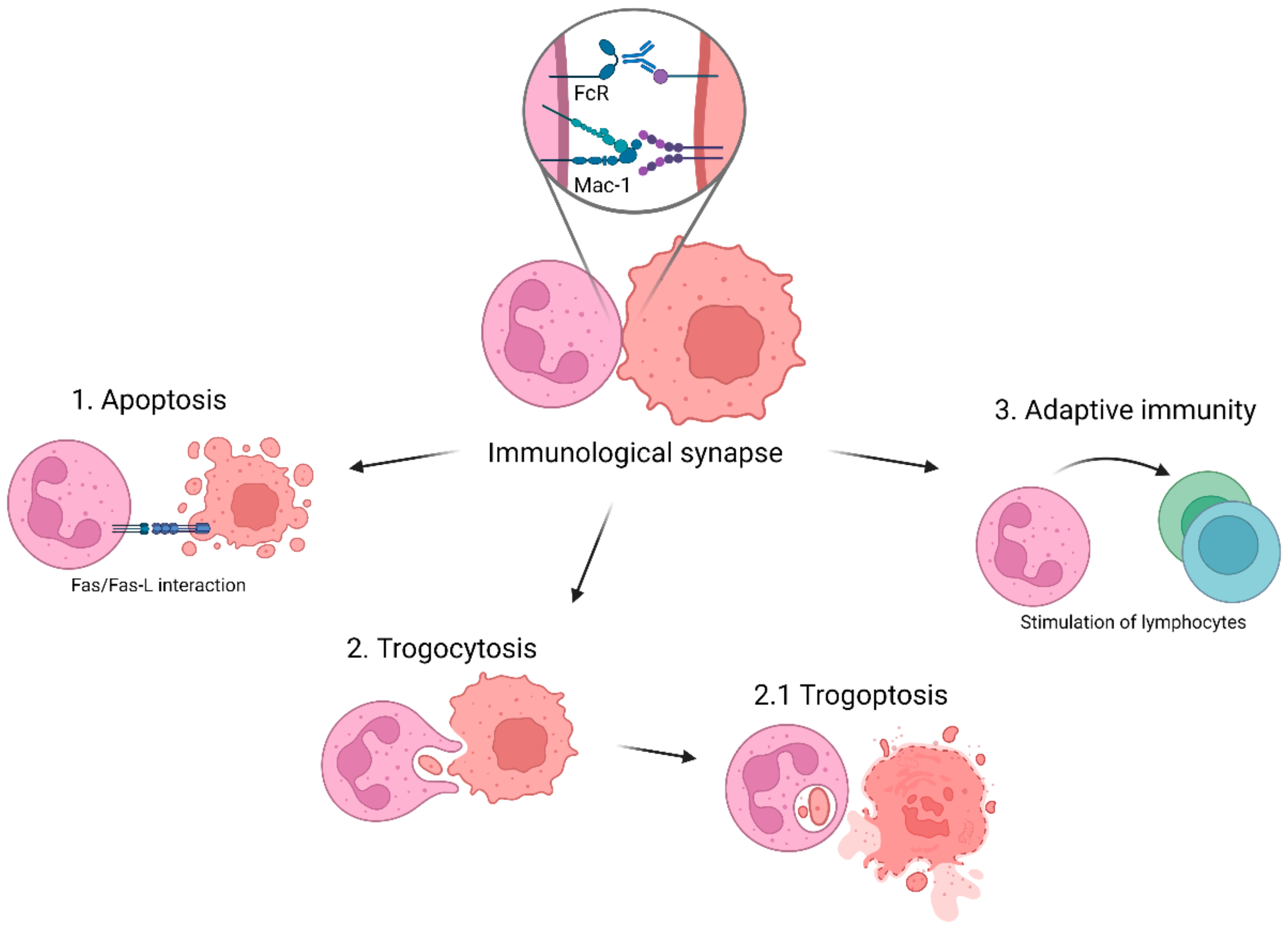
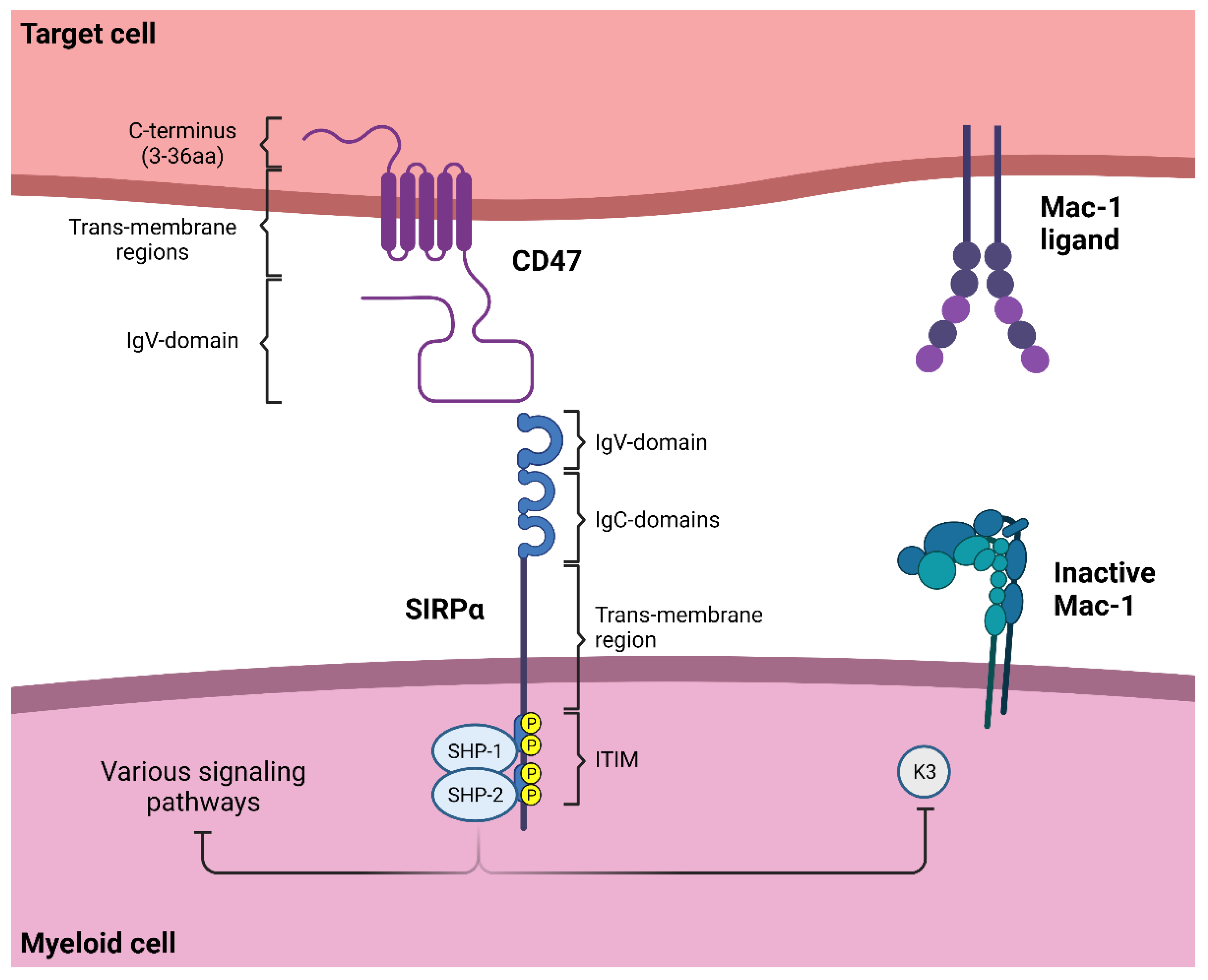
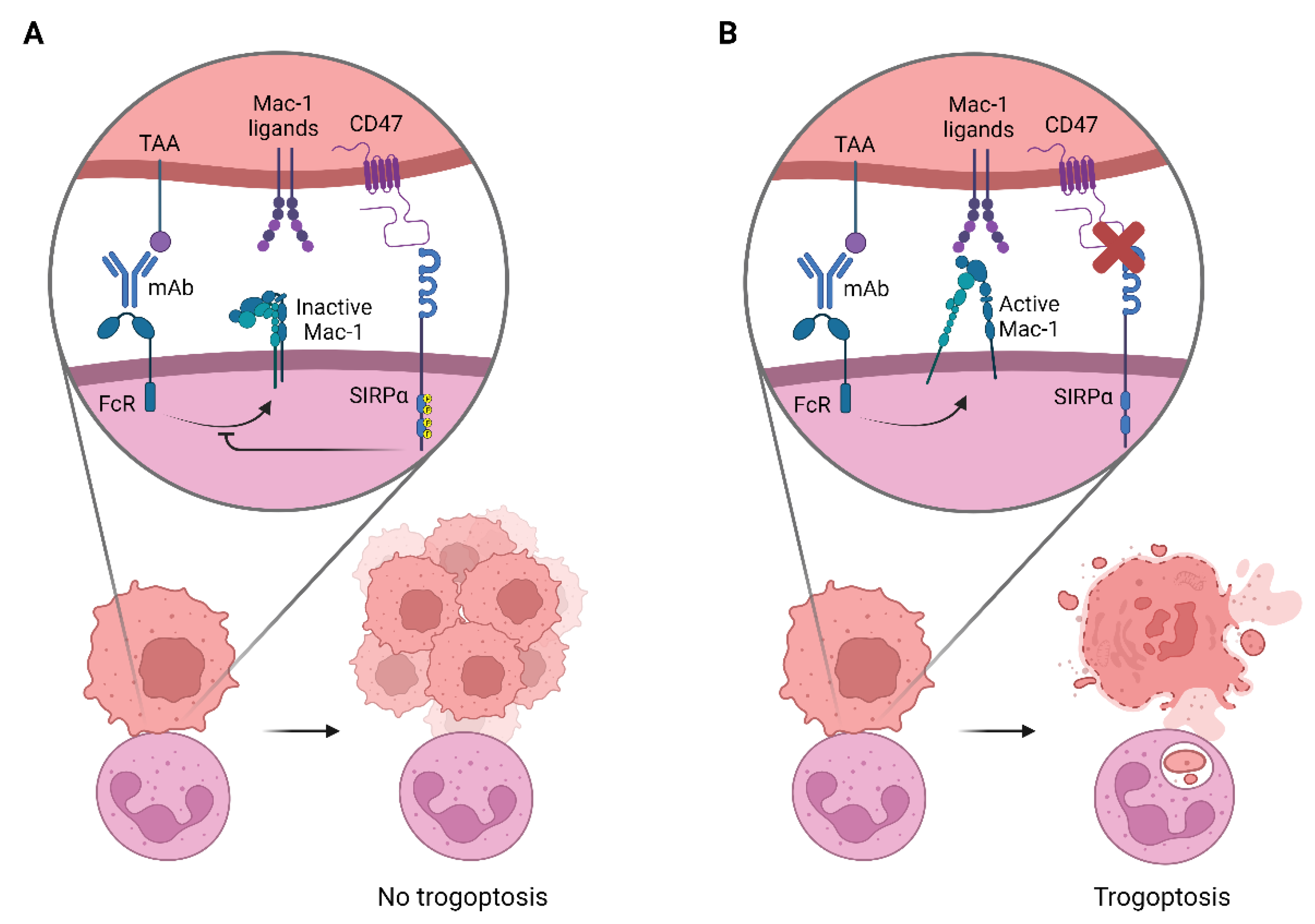
| Name | FcγRI (CD64) | FcγRIIa (CD32a) | FcγRIIb (CD32b) | FcγRIIc (CD32c) | FcγRIIIa (CD16a) | FcγRIIIb (CD16b) | FcαRI (CD89) |
|---|---|---|---|---|---|---|---|
| Structure | 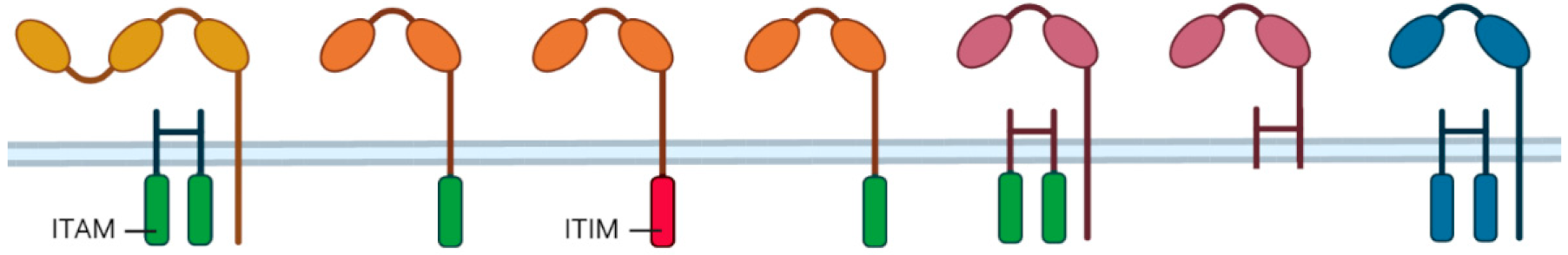 | ||||||
| Affinity | High | Low | Low | Low | Low | Low | High |
| Expression on neutrophils | Induced 1 | + | Genotype-dependent 2 | Genotype-dependent 3 | −/+ 4 | + | + |
| Class | Activation | Activation | Inhibition | Activation | Decoy | Activation | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrens, L.M.; van den Berg, T.K.; van Egmond, M. Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils. Cancers 2022, 14, 3366. https://doi.org/10.3390/cancers14143366
Behrens LM, van den Berg TK, van Egmond M. Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils. Cancers. 2022; 14(14):3366. https://doi.org/10.3390/cancers14143366
Chicago/Turabian StyleBehrens, Leonie M., Timo K. van den Berg, and Marjolein van Egmond. 2022. "Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils" Cancers 14, no. 14: 3366. https://doi.org/10.3390/cancers14143366
APA StyleBehrens, L. M., van den Berg, T. K., & van Egmond, M. (2022). Targeting the CD47-SIRPα Innate Immune Checkpoint to Potentiate Antibody Therapy in Cancer by Neutrophils. Cancers, 14(14), 3366. https://doi.org/10.3390/cancers14143366