Insomnia Symptoms and Daytime Fatigue Co-Occurrence in Adolescent and Young Adult Childhood Cancer Patients in Follow-Up after Treatment: Prevalence and Associated Risk Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
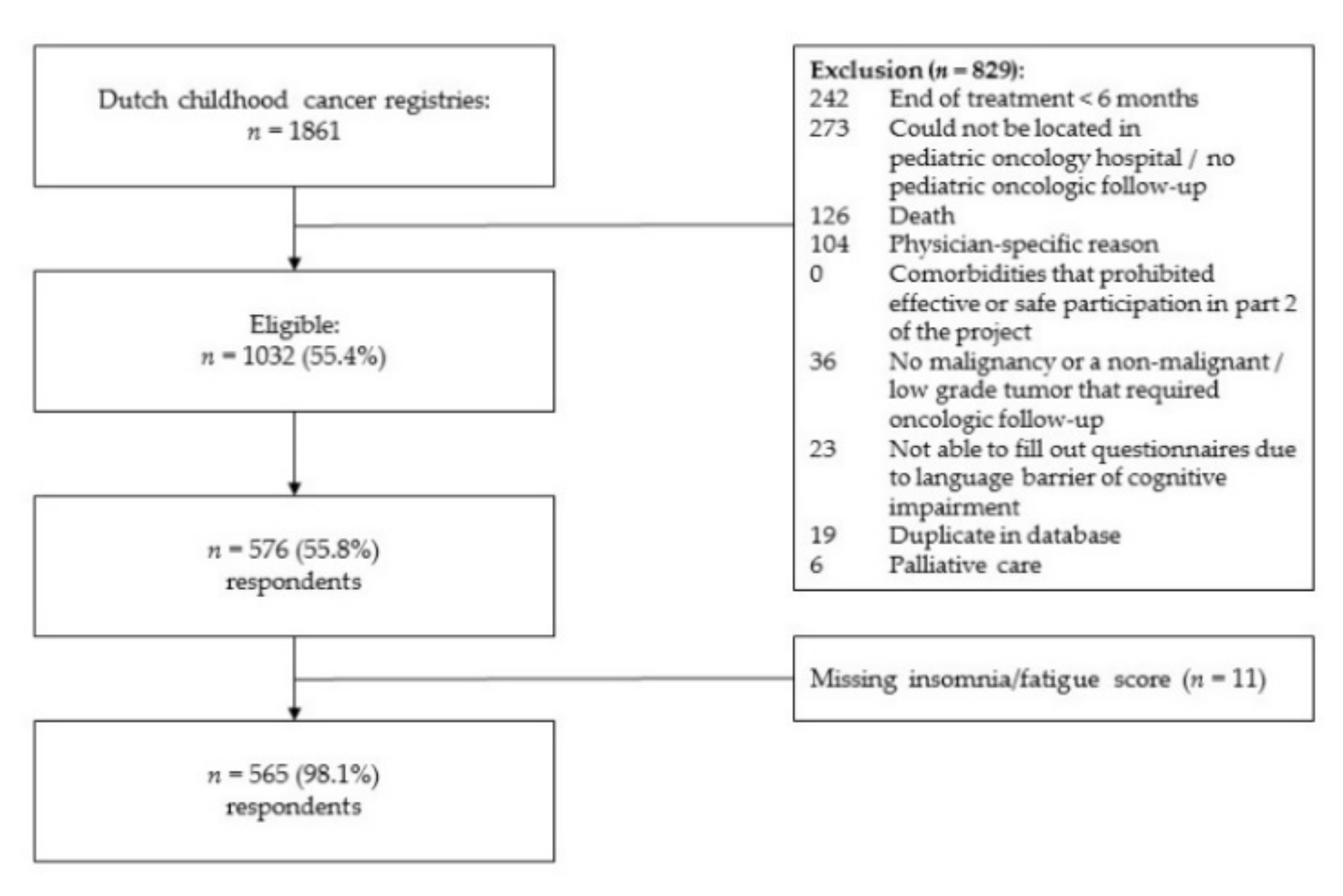
2.1. Study Participants
2.2. Procedures
2.3. Measures
2.3.1. Insomnia Severity Index (ISI)
2.3.2. Daytime Fatigue
2.3.3. Risk Factors
2.4. Statistical Analyses
3. Results
3.1. Sample Characteristics
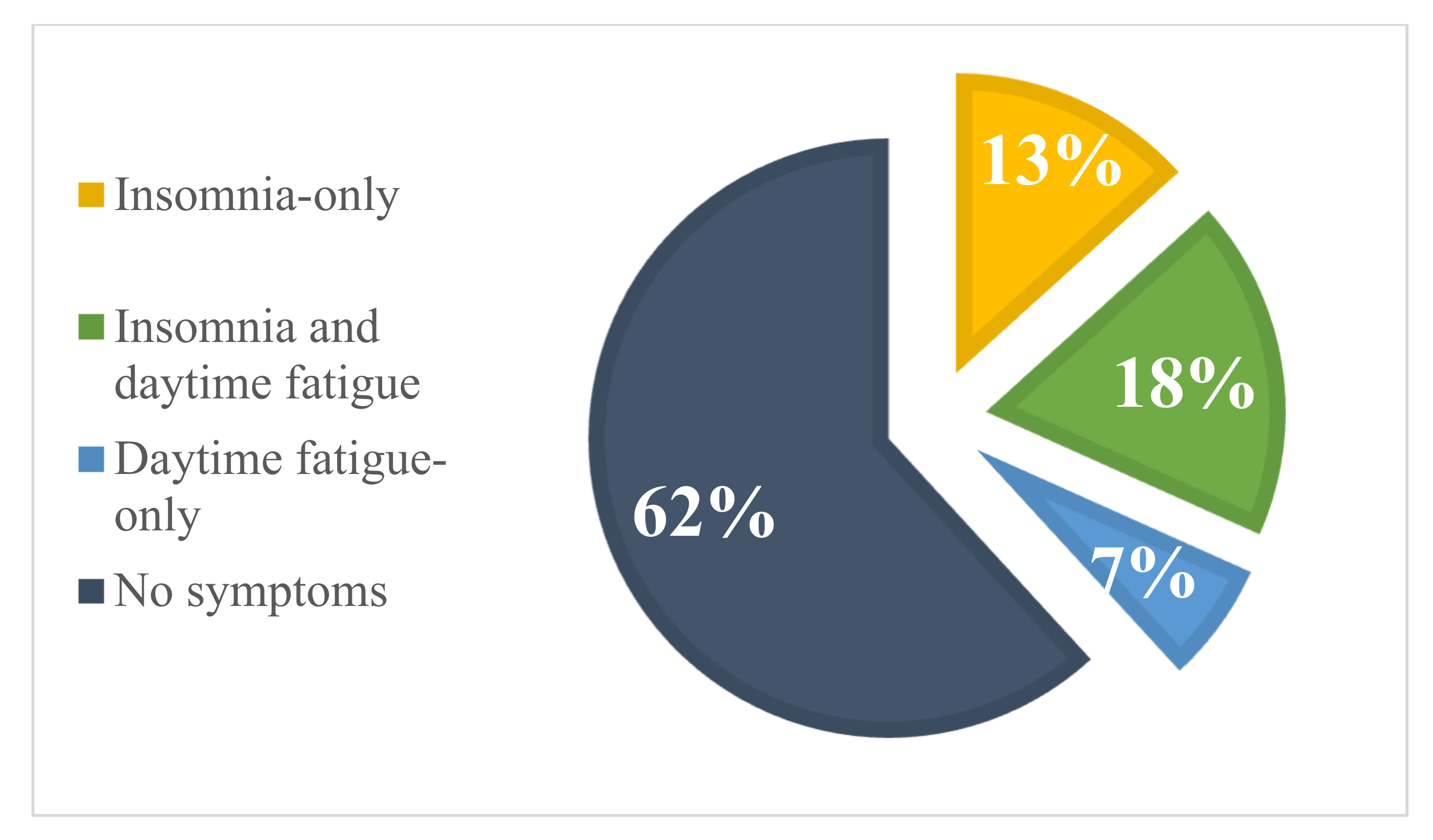
3.2. Prevalence Rates of Insomnia and Daytime Fatigue
3.3. Clinical Severity Ranges of Insomnia in the Total and Subgroups
3.4. Risk Factors of Insomnia and Daytime Fatigue Groups
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, L.C.; Aggarwal, R.; Schwartz, L.A. Sleep in adolescents and young adults in the year after cancer treatment. J. Adolesc. Young Adult Oncol. 2017, 6, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.C.; Wang, M.; Mulrooney, D.A.; Srivastava, D.K.; Schwartz, L.A.; Edelstein, K.; Brinkman, T.M.; Zhou, E.S.; Howell, R.M.; Gibson, T.M. Sleep, emotional distress, and physical health in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Psycho-Oncology 2019, 28, 903–912. [Google Scholar] [CrossRef]
- Zhou, E.S.; Recklitis, C.J. Insomnia in adult survivors of childhood cancer: A report from project REACH. Supportive Care Cancer 2014, 22, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Peersmann, S.H.; Grootenhuis, M.A.; van Straten, A.; Kerkhof, G.A.; Tissing, W.J.; Abbink, F.; de Vries, A.C.; Loonen, J.; Kremer, L.; Kaspers, G.J. Prevalence of Sleep Disorders, Risk Factors and Sleep Treatment Needs of Adolescents and Young Adult Childhood Cancer Patients in Follow-Up after Treatment. Cancers 2022, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; Volume 21, pp. 591–643. [Google Scholar]
- Espie, C.A.; Kyle, S.D.; Hames, P.; Cyhlarova, E.; Benzeval, M. The daytime Impactof DSM-5 insomnia disorder: Comparative analysis of insomnia subtypes from the great British sleep survey. J. Clin. Psychiatry 2012, 73, 4027. [Google Scholar] [CrossRef]
- Benjamins, J.S.; Migliorati, F.; Dekker, K.; Wassing, R.; Moens, S.; Blanken, T.F.; Te Lindert, B.H.; Mook, J.S.; Van Someren, E.J. Insomnia heterogeneity: Characteristics to consider for data-driven multivariate subtyping. Sleep Med. Rev. 2017, 36, 71–81. [Google Scholar] [CrossRef]
- Blanken, T.F.; Benjamins, J.S.; Borsboom, D.; Vermunt, J.K.; Paquola, C.; Ramautar, J.; Dekker, K.; Stoffers, D.; Wassing, R.; Wei, Y. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry 2019, 6, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Fortier-Brochu, É.; Beaulieu-Bonneau, S.; Ivers, H.; Morin, C.M. Relations between sleep, fatigue, and health-related quality of life in individuals with insomnia. J. Psychosom. Res. 2010, 69, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Ortuño, M.M.; Edinger, J.D.; Wyatt, J.K. Daytime symptom patterns in insomnia sufferers: Is there evidence for subtyping insomnia? J. Sleep Res. 2011, 20, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Christen, S.; Roser, K.; Mulder, R.L.; Ilic, A.; Lie, H.C.; Loonen, J.J.; Mellblom, A.V.; Kremer, L.; Hudson, M.M.; Constine, L.S. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. J. Cancer Surviv. 2020, 14, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Van Deuren, S.; Boonstra, A.; van Dulmen-den Broeder, E.; Blijlevens, N.; Knoop, H.; Loonen, J. Severe fatigue after treatment for childhood cancer. Cochrane Database Syst. Rev. 2020, CD012681. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, N.; Ubbink, M.; Smets, E.; Dutch Late Effects Study Group. ‘I don’t have any energy’: The experience of fatigue in young adult survivors of childhood cancer. Eur. J. Oncol. Nurs. 2000, 4, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nap-van der Vlist, M.M.; Dalmeijer, G.W.; Grootenhuis, M.A.; van der Ent, C.K.; van den Heuvel-Eibrink, M.M.; Wulffraat, N.M.; Swart, J.F.; Van Litsenburg, R.R.; Van De Putte, E.M.; Nijhof, S.L. Fatigue in childhood chronic disease. Arch. Dis. Child. 2019, 104, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Nap-van der Vlist, M.M.; Dalmeijer, G.W.; Grootenhuis, M.A.; van der Ent, K.; van den Heuvel-Eibrink, M.M.; Swart, J.F.; van de Putte, E.M.; Nijhof, S.L. Fatigue among children with a chronic disease: A cross-sectional study. BMJ Paediatr. Open 2021, 5, e000958. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Walter, L.M.; Nixon, G.M.; Davey, M.J.; Downie, P.A.; Horne, R.S. Sleep and fatigue in pediatric oncology: A review of the literature. Sleep Med. Rev. 2015, 24, 71–82. [Google Scholar] [CrossRef]
- Werker, C.L.; Nijhof, S.L.; van de Putte, E.M. Clinical practice: Chronic fatigue syndrome. Eur. J. Pediatrics 2013, 172, 1293–1298. [Google Scholar] [CrossRef]
- Penson, A.; van Deuren, S.; Bronkhorst, E.; Keizer, E.; Heskes, T.; Coenen, M.J.; Rosmalen, J.G.; Tissing, W.J.; van Der Pal, H.J.; de Vries, A.C. Methodology of the DCCSS later fatigue study: A model to investigate chronic fatigue in long-term survivors of childhood cancer. BMC Med. Res. Methodol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Steur, L.; Kaspers, G.; Van Someren, E.; Van Eijkelenburg, N.; Van der Sluis, I.; Dors, N.; van den Bos, C.; Tissing, W.; Grootenhuis, M.; Van Litsenburg, R. The impact of maintenance therapy on sleep-wake rhythms and cancer-related fatigue in pediatric acute lymphoblastic leukemia. Supportive Care Cancer 2020, 28, 5983–5993. [Google Scholar] [CrossRef] [Green Version]
- Steur, L.M.; Kaspers, G.J.; Van Someren, E.J.; Van Eijkelenburg, N.K.; Van der Sluis, I.M.; Dors, N.; Van den Bos, C.; Tissing, W.J.; Grootenhuis, M.A.; Van Litsenburg, R.R. Sleep–wake rhythm disruption is associated with cancer-related fatigue in pediatric acute lymphoblastic leukemia. Sleep 2020, 43, zsz320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, L.C.; Meltzer, L.J.; Gross, J.Y.; Flannery, J.L.; Forrest, C.B.; Barakat, L.P. Sleep practices in pediatric cancer patients: Indirect effects on sleep disturbances and symptom burden. Psycho-Oncology 2021, 30, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Brinkman, T.M.; Mulrooney, D.A.; Mzayek, Y.; Liu, W.; Banerjee, P.; Panoskaltsis-Mortari, A.; Srivastava, D.; Pui, C.H.; Robison, L.L. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017, 123, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Clanton, N.R.; Klosky, J.L.; Li, C.; Jain, N.; Srivastava, D.K.; Mulrooney, D.; Zeltzer, L.; Stovall, M.; Robison, L.L.; Krull, K.R. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2011, 117, 2559–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rach, A.M.; Crabtree, V.M.; Brinkman, T.M.; Zeltzer, L.; Marchak, J.G.; Srivastava, D.; Tynes, B.; Lai, J.-S.; Robison, L.L.; Armstrong, G.T. Predictors of fatigue and poor sleep in adult survivors of childhood Hodgkin’s lymphoma: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2017, 11, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Zeller, B.; Loge, J.H.; Kanellopoulos, A.; Hamre, H.; Wyller, V.B.; Ruud, E. Chronic fatigue in long-term survivors of childhood lymphomas and leukemia: Persistence and associated clinical factors. J. Pediatric Hematol. Oncol. 2014, 36, 438–444. [Google Scholar] [CrossRef]
- Gordijn, M.S.; van Litsenburg, R.R.; Gemke, R.J.; Huisman, J.; Bierings, M.B.; Hoogerbrugge, P.M.; Kaspers, G.J. Sleep, fatigue, depression, and quality of life in survivors of childhood acute lymphoblastic leukemia. Pediatric Blood Cancer 2013, 60, 479–485. [Google Scholar] [CrossRef]
- Vasquez, P.; Escalante, J.; Raghubar, K.P.; Kahalley, L.S.; Taylor, O.A.; Moore, I.K.; Hockenberry, M.J.; Scheurer, M.E.; Brown, A.L. Association between fatigue and sleep disturbances during treatment for pediatric acute lymphoblastic leukemia and posttreatment neurocognitive performance. Pediatric Blood Cancer 2021, 69, e29507. [Google Scholar] [CrossRef]
- Meeske, K.A.; Siegel, S.E.; Globe, D.R.; Mack, W.J.; Bernstein, L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J. Clin. Oncol. 2005, 23, 5501–5510. [Google Scholar] [CrossRef]
- Haque, R.; Hsu, J.W.; Avila, C.; Olmstead, R.; Carroll, J.E.; Irwin, M.R. Insomnia and susceptibility to depressive symptoms and fatigue in diverse breast cancer survivors. J. Women’s Health 2021, 30, 1604–1615. [Google Scholar] [CrossRef]
- Legg, M.; Meertens, R.M.; van Roekel, E.; Breukink, S.O.; Janssen, M.L.; Keulen, E.T.; Steindorf, K.; Weijenberg, M.P.; Bours, M. The Association between Sleep Quality and Fatigue in Colorectal Cancer Survivors up until Two Years after Treatment: A Cross-Sectional and Longitudinal Analysis. Cancers 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Twomey, R.; Medysky, M.E.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. The Relationship between Fatigue and Actigraphy-Derived Sleep and Rest—Activity Patterns in Cancer Survivors. Curr. Oncol. 2021, 28, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Oberoi, S.; Tomlinson, D.; Duong, N.; Davis, H.; Cataudella, D.; Culos-Reed, N.; Gibson, F.; Götte, M.; Hinds, P. Management of fatigue in children and adolescents with cancer and in paediatric recipients of haemopoietic stem-cell transplants: A clinical practice guideline. Lancet Child Adolesc. Health 2018, 2, 371–378. [Google Scholar] [CrossRef]
- Baglioni, C.; Altena, E.; Bjorvatn, B.; Blom, K.; Bothelius, K.; Devoto, A.; Espie, C.A.; Frase, L.; Gavriloff, D.; Tuuliki, H. The European Academy for Cognitive Behavioural Therapy for Insomnia: An initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J. Sleep Res. 2020, 29, e12967. [Google Scholar] [CrossRef]
- Kallestad, H.; Jacobsen, H.B.; Landrø, N.I.; Borchgrevink, P.C.; Stiles, T.C. The role of insomnia in the treatment of chronic fatigue. J. Psychosom. Res. 2015, 78, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Vethe, D.; Kallestad, H.; Jacobsen, H.B.; Landrø, N.I.; Borchgrevink, P.C.; Stiles, T.C. The relationship between improvement in insomnia severity and long-term outcomes in the treatment of chronic fatigue. Front. Psychol. 2018, 9, 1764. [Google Scholar] [CrossRef] [Green Version]
- Peersmann, S.H.; van Straten, A.; Kaspers, G.J.; Thano, A.; van den Bergh, E.; Grootenhuis, M.A.; van Litsenburg, R.R. Does the guided online cognitive behavioral therapy for insomnia “i-Sleep youth” improve sleep of adolescents and young adults with insomnia after childhood cancer?(MICADO-study): Study protocol of a randomized controlled trial. Trials 2021, 22, 307. [Google Scholar] [CrossRef]
- Manzar, M.D.; Jahrami, H.A.; Bahammam, A.S. Structural validity of the Insomnia Severity Index: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 60, 101531. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Michaud, A.L.; Zhou, E.S.; Chang, G.; Recklitis, C.J. Validation of the Insomnia Severity Index (ISI) for identifying insomnia in young adult cancer survivors: Comparison with a structured clinical diagnostic interview of the DSM-5 (SCID-5). Sleep Med. 2021, 81, 80–85. [Google Scholar] [CrossRef]
- Chung, K.-F.; Kan, K.K.-K.; Yeung, W.-F. Assessing insomnia in adolescents: Comparison of insomnia severity index, Athens insomnia scale and sleep quality index. Sleep Med. 2011, 12, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.A. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.A.; Geuke, M.E.; Brouwer, A.; Rijsman, R.M.; Schimsheimer, R.J.; Van Kasteel, V. Holland Sleep Disorders Questionnaire: A new sleep disorders questionnaire based on the International Classification of Sleep Disorders-2. J. Sleep Res. 2013, 22, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Onderwijsindeling, S. Standard Educational Classification; Centraal Bureau voor de Statistiek [Statistics Netherlands]: Den Haag, The Netherlands, 2016. [Google Scholar]
- Meltzer, L.J.; Forrest, C.B.; de la Motte, A.; Mindell, J.A.; Bevans, K.B. Development and validation of the pediatric sleep practices questionnaire: A self-report measure for youth ages 8–17 years. Behav. Sleep Med. 2021, 19, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, S.; Jeon, S.; Leary, E.B.; Barwick, F.; Mignot, E. Factors associated with fatigue in patients with insomnia. J. Psychiatr. Res. 2019, 117, 24–30. [Google Scholar] [CrossRef]
- Sivertsen, B.; Lallukka, T.; Salo, P.; Pallesen, S.; Hysing, M.; Krokstad, S.; Øverland, S. Insomnia as a risk factor for ill health: Results from the large population-based prospective HUNT Study in Norway. J. Sleep Res. 2014, 23, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Barone, R.; Gulisano, M.; Cannata, E.; Padalino, S.; Saia, F.; Maugeri, N.; Pettinato, F.; Lo Nigro, L.; Casabona, A.; Russo, G. Self-and Parent-Reported psychological symptoms in young cancer survivors and control Peers: Results from a clinical center. J. Clin. Med. 2020, 9, 3444. [Google Scholar] [CrossRef]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of insomnia, depression, and anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef]
- Van Deuren, S.; Penson, A.; van Dulmen-den Broeder, E.; Grootenhuis, M.A.; van der Heiden-van der Loo, M.; Bronkhorst, E.; Blijlevens, N.M.; Streefkerk, N.; Teepen, J.C.; Tissing, W.J. Prevalence and risk factors of cancer-related fatigue in childhood cancer survivors: A DCCSS LATER study. Cancer 2022, 128, 1110–1121. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.; Beaulieu-Bonneau, S.; Mérette, C.; Savard, J.; Ivers, H.; Morin, C.M. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J. Psychosom. Res. 2007, 63, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Léger, D.; Scheuermaier, K.; Philip, P.; Paillard, M.; Guilleminault, C. SF-36: Evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom. Med. 2001, 63, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, D.; Eley, T.C.; Rijsdijk, F.; Zavos, H.M.; Keers, R.; Espie, C.A.; Luik, A.I.; Badini, I.; Derveeuw, S.; Hodsoll, J. Is digital cognitive behavioural therapy for insomnia effective in treating sub-threshold insomnia: A pilot RCT. Sleep Med. 2020, 66, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, M.J.; Harju, E.; Michel, G. The unmet needs of childhood cancer survivors in long-term follow-up care: A qualitative study. Psycho-Oncology 2021, 30, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.; Martins, A.; Fern, L.A.; Bassett, M.; Cable, M.; Doig, G.; Morgan, S.; Soanes, L.; Whelan, M.; Taylor, R.M. The support and information needs of adolescents and young adults with cancer when active treatment ends. BMC Cancer 2020, 20, 697. [Google Scholar] [CrossRef]
- Cook, G.; Appleton, J.V.; Wiggs, L. Parentally reported barriers to seeking help and advice for child sleep from healthcare professionals. Child Care Health Dev. 2020, 46, 513–521. [Google Scholar] [CrossRef]
- Daniel, L.C.; van Litsenburg, R.R.; Rogers, V.E.; Zhou, E.S.; Ellis, S.J.; Wakefield, C.E.; Stremler, R.; Walter, L.; Crabtree, V.M.; International Psycho-Oncology Society Pediatrics Special Interest Group. A call to action for expanded sleep research in pediatric oncology: A position paper on behalf of the International Psycho-Oncology Society Pediatrics Special Interest Group. Psycho-Oncology 2020, 29, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Howell, D.; Molloy, S.; Wilkinson, K.; Green, E.; Orchard, K.; Wang, K.; Liberty, J. Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. Ann. Oncol. 2015, 26, 1846–1858. [Google Scholar] [CrossRef]
- Blumenstein, K.; Brose, A.; Kemp, C.; Meister, D.; Walling, E.; DuVall, A.; Zhang, A. Effectiveness of Cognitive Behavioral Therapy in Improving Functional Health in Cancer Survivors: A Systematic Review and Meta-analysis. Crit. Rev. Oncol. Hematol. 2022, 175, 103709. [Google Scholar] [CrossRef]
- Benz, F.; Knoop, T.; Ballesio, A.; Bacaro, V.; Johann, A.F.; Rücker, G.; Feige, B.; Riemann, D.; Baglioni, C. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: A systematic review and network meta-analysis. Clin. Psychol. Rev. 2020, 80, 101873. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Mallory, L.J.; Lichstein, K.L.; Durrence, H.H.; Riedel, B.W.; Bush, A.J. Comorbidity of chronic insomnia with medical problems. Sleep 2007, 30, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, B.L.; Sand, T.; Ødegård, S.S.; Hagen, K. Prevalence and associated factors of DSM-V insomnia in Norway: The Nord-Trøndelag Health Study (HUNT 3). Sleep Med. 2014, 15, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Marchak, J.G.; Christen, S.; Mulder, R.L.; Baust, K.; Blom, J.M.; Brinkman, T.M.; Elens, I.; Harju, E.; Kadan-Lottick, N.S.; Khor, J.W. Recommendations for the surveillance of mental health problems in childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2022, 23, e184–e196. [Google Scholar] [CrossRef]
- Soares, C.N. Insomnia in women: An overlooked epidemic? Arch. Women’s Ment. Health 2005, 8, 205–213. [Google Scholar] [CrossRef]
- Suh, S.; Cho, N.; Zhang, J. Sex differences in insomnia: From epidemiology and etiology to intervention. Curr. Psychiatry Rep. 2018, 20, 69. [Google Scholar] [CrossRef]
- Bhakta, N.; Liu, Q.; Ness, K.K.; Baassiri, M.; Eissa, H.; Yeo, F.; Chemaitilly, W.; Ehrhardt, M.J.; Bass, J.; Bishop, M.W. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017, 390, 2569–2582. [Google Scholar] [CrossRef]
- Brimeyer, C.; Adams, L.; Zhu, L.; Srivastava, D.K.; Wise, M.; Hudson, M.M.; Crabtree, V.M. Sleep complaints in survivors of pediatric brain tumors. Supportive Care Cancer 2016, 24, 23–31. [Google Scholar] [CrossRef]
- Van Kooten, J.A.; Maurice-Stam, H.; Schouten, A.Y.; van Vuurden, D.G.; Granzen, B.; Gidding, C.; de Ruiter, M.A.; van Litsenburg, R.R.; Grootenhuis, M.A. High occurrence of sleep problems in survivors of a childhood brain tumor with neurocognitive complaints: The association with psychosocial and behavioral executive functioning. Pediatric Blood Cancer 2019, 66, e27947. [Google Scholar] [CrossRef]
- Pickering, L.; Main, K.M.; Feldt-Rasmussen, U.; Klose, M.; Sehested, A.; Mathiasen, R.; Jennum, P. Brain tumours in children and adolescents may affect the circadian rhythm and quality of life. Acta Paediatr. 2021, 110, 3376–3386. [Google Scholar] [CrossRef]
- Pickering, L.; Main, K.M.; Sehested, A.; Mathiasen, R.; Feldt-Rasmussen, U.; Klose, M.; Kotagal, S.; Jennum, P.J. Brain tumours result in sleep disorders in children and adolescents. Sleep Med. 2021, 88, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Van Hulst, A.M.; Peersmann, S.H.; van den Akker, E.L.; Schoonmade, L.J.; van den Heuvel-Eibrink, M.M.; Grootenhuis, M.A.; van Litsenburg, R.R. Risk factors for steroid-induced adverse psychological reactions and sleep problems in pediatric acute lymphoblastic leukemia: A systematic review. Psycho-Oncology 2021, 30, 1009–1028. [Google Scholar] [CrossRef] [PubMed]
- Zupanec, S.; Jones, H.; Stremler, R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J. Pediatric Oncol. Nurs. 2010, 27, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Rensen, N.; Steur, L.; Grootenhuis, M.; Twisk, J.; van Eijkelenburg, N.; van der Sluis, I.; Dors, N.; van den Bos, C.; Tissing, W.; Kaspers, G. Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later. Cancers 2022, 14, 2779. [Google Scholar] [CrossRef]
- Broman, J.-E.; Lundh, L.-G.; Aleman, K.; Hetta, J. Subjective and objective performance in patients with persistent insomnia. Cogn. Behav. Ther. 1992, 21, 115–126. [Google Scholar] [CrossRef]
- Crowley, S.J.; Wolfson, A.R.; Tarokh, L.; Carskadon, M.A. An update on adolescent sleep: New evidence informing the perfect storm model. J. Adolesc. 2018, 67, 55–65. [Google Scholar] [CrossRef]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef]
- Pugh, G.; Gravestock, H.L.; Hough, R.E.; King, W.M.; Wardle, J.; Fisher, A. Health behavior change interventions for teenage and young adult cancer survivors: A systematic review. J. Adolesc. Young Adult Oncol. 2016, 5, 91–105. [Google Scholar] [CrossRef]
- Touyz, L.M.; Cohen, J.; Cohn, R.J.; Garnett, S.P.; Anazodo, A.; Gohil, P.; Grech, A.M.; Ng, A.; Wakefield, C.E. Childhood cancer survivors report preferring lifestyle interventions delivered in person rather than online: An adolescent and parent perspective. Pediatric Blood Cancer 2019, 66, e27922. [Google Scholar] [CrossRef]
- Kirsh, K.L.; Passik, S.; Holtsclaw, E.; Donaghy, K.; Theobald, D. I get tired for no reason: A single item screening for cancer-related fatigue. J. Pain Symptom Manag. 2001, 22, 931–937. [Google Scholar] [CrossRef]
- Van Hooff, M.L.; Geurts, S.A.; Kompier, M.A.; Taris, T.W. “How fatigued do you currently feel?” Convergent and discriminant validity of a single-item fatigue measure. J. Occup. Health 2007, 49, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.R.; Chordas, C.; Liptak, C.; Manley, P.; Recklitis, C. Screening for fatigue in adolescent and young adult pediatric brain tumor survivors: Accuracy of a single-item screening measure. Supportive Care Cancer 2016, 24, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Bastiani, J.; Williams, L.K. Are parenting behaviors associated with child sleep problems during treatment for acute lymphoblastic leukemia? Cancer Med. 2016, 5, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rensen, N.; Steur, L.M.; Schepers, S.A.; Merks, J.H.; Moll, A.C.; Grootenhuis, M.A.; Kaspers, G.J.; van Litsenburg, R.R. Concurrence of sleep problems and distress: Prevalence and determinants in parents of children with cancer. Eur. J. Psychotraumatology 2019, 10, 1639312. [Google Scholar] [CrossRef] [Green Version]
- Steur, L.M.; Grootenhuis, M.A.; Van Someren, E.J.; Van Eijkelenburg, N.K.; Van der Sluis, I.M.; Dors, N.; Van den Bos, C.; Tissing, W.J.; Kaspers, G.J.; Van Litsenburg, R.R. High prevalence of parent-reported sleep problems in pediatric patients with acute lymphoblastic leukemia after induction therapy. Pediatric Blood Cancer 2020, 67, e28165. [Google Scholar] [CrossRef] [Green Version]
| Total Group (n = 565) | Insomnia-Daytime Fatigue Subgroups | ||||
|---|---|---|---|---|---|
| Insomnia and Daytime Fatigue (n = 104) | Insomnia Only (n = 75) | Daytime Fatigue Only (n = 37) | No Symptoms (n = 349) | ||
| Age at study invitation (y), mean (SD) Age group, % Adolescents (12–17y) Young adult (18–26y) | 17.0 (2.9) 57.3 42.8 | 17.8 (2.7) 40.4 59.6 | 16.6 (2.0) 68.0 32.0 | 18.5 (2.7) 35.1 64.9 | 16.7 (2.9) 62.2 37.8 |
| Sex, % Female | 49.6 | 66.3 | 57.3 | 78.4 | 39.8 |
| Current educational level, % Low Middle High | 23.9 56.6 18.2 | 27.9 51.9 17.3 | 25.3 64.0 10.7 | 24.3 40.5 32.4 | 22.3 58.2 18.6 |
| Country of birth, % The Netherlands Other | 95.2 3.4 | 90.4 7.7 | 94.7 5.3 | 94.6 2.7 | 96.8 1.7 |
| Age at diagnosis (y), mean (SD) | 12.8 (3.2) | 13.5 (3.3) | 13.0 (2.9) | 13.9 (2.6) | 12.5 (3.2) |
| Time since diagnosis in years, mean (SD) | 4.0 (2.4) | 4.1 (2.4) | 3.5 (1.9) | 4.3 (2.5) | 4.0 (2.4) |
| Diagnosis groups, % Hemato-oncology Neuro-oncology Solid | 44.1 23.7 32.2 | 45.2 28.8 26.0 | 49.3 13.3 37.3 | 43.2 21.6 35.1 | 42.7 24.6 32.7 |
| Time since end of treatment in years, mean (SD) | 3.2 (2.2) | 3.3 (2.1) | 2.7 (1.8) | 3.2 (2.4) | 3.3 (2.3) |
| Type of oncologic treatment, % No treatment Chemotherapy Radiation Surgery SCT Mixed/Other | 1.4 73.1 25.5 54.7 6.2 4.8 | 0.9 67.3 24.0 63.5 9.6 6.7 | - 73.3 21.3 54.7 6.7 6.7 | 2.7 73.0 24.3 54.1 5.4 - | 2.0 74.8 26.9 52.1 5.2 4.0 |
| Comorbid health problems, % Yes | 17.9 | 31.7 | 22.7 | 32.4 | 11.2 |
| Sleeping accompanied, % Yes | 7.4 | 15.4 | 6.7 | 8.3 | 5.2 |
| Sleep behaviors, mean (SD) Needing someone else to fall asleep Bedtime routine Trying to fall asleep every night at the same time Waking up every morning at the same time Bedtime technology TV Games Phone/computer | 1.18 (.67) 3.43 (1.44) 3.35 (1.20) 3.43 (1.12) 3.60 (1.20) 2.10 (1.28) 3.83 (1.31) | 1.52 (1.08) 3.75 (1.15) 3.64 (1.04) 3.13 (1.14) 3.66 (1.03) 2.07 (1.22) 4.04 (1.07) | 1.25 (.81) 3.43 (1.31) 3.43 (1.27) 3.33 (1.08) 3.65 (1.07) 2.25 (1.35) 3.69 (1.38) | 1.05 (.23) 3.59 (1.46) 3.46 (1.10) 3.24 (1.09) 3.46 (1.32) 1.68 (1.18) 4.27 (1.05) | 1.08 (.44) 3.32 (1.53) 3.24 (1.23) 3.57 (1.11) 3.58 (1.26) 2.13 (1.29) 3.74 (1.36) |
| Total Group (n = 565) | Insomnia-Daytime Fatigue Subgroups | ||||
|---|---|---|---|---|---|
| Insomnia and Daytime Fatigue (n = 104) | Insomnia Only (n = 75) | Daytime Fatigue Only (n = 37) | No Symptoms (n = 349) | ||
| Insomnia categories (n, %): No clinically significant insomnia (ISI: 0–7) Subthreshold insomnia (ISI: 8–14) Clinical insomnia (moderate severity, ISI: 15–21) Clinical insomnia (severe, ISI: 22–28) | 386 (68.3) 115 (20.4) 56 (9.9) 8 (1.4) | - 49 (47.1) 47 (45.1) 8 (7.7) | - 66 (88.0) 9 (12.0) - | 37 (100.0) - - - | 349 (100.0) - - - |
| ISI score, mean (SD) | 6.15 (5.84) | 14.89 (4.72) | 11.41 (2.96) | 4.59 (1.69) | 2.58 (2.18) |
| Reference: No Symptoms (n = 349) | Insomnia and Daytime Fatigue (n = 104) | Insomnia Only (n = 75) | Daytime Fatigue Only (n = 37) |
|---|---|---|---|
| Female | 3.08 (1.75–5.41) *** | 2.41 (1.34–4.35) ** | 6.38 (2.44–16.70) *** |
| Age group: young adults (ref: adolescents) | 3.11 (1.75–5.54) *** | 1.09 (0.55–2.14) | 3.95 (1.58–9.90) ** |
| High educational level (ref: low/middle) | 0.36 (0.17–0.76) ** | 0.58 (0.22–1.50) | 0.95 (0.37–2.47) |
| Having a comorbid health condition | 4.53 (2.47–8.31) *** | 3.00 (1.50–5.98) ** | 4.32 (1.79–10.44) ** |
| Time since end of treatment (in years) | 0.93 (0.83–1.05) | 0.86 (0.75–0.99) * | 0.85 (0.70–1.02) |
| Needing someone else to fall asleep | 1.79 (1.25–2.56) ** | 1.56 (1.04–2.33) * | 0.63 (0.20–1.97) |
| Trying to fall asleep every night at the same time | 1.40 (1.10–1.77)** | 1.19 (0.93–1.51) | 1.10 (0.77–1.56) |
| Waking up every morning at the same time | 0.65 (0.51–0.82) *** | 0.77 (0.60–1.00) * | 0.72 (0.51–1.03) |
| Bedtime technology use-gaming | 1.17 (0.94–1.45) | 1.27 (1.03–1.59) * | 0.98 (0.67–1.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peersmann, S.H.M.; Grootenhuis, M.A.; van Straten, A.; Tissing, W.J.E.; Abbink, F.; de Vries, A.C.H.; Loonen, J.; van der Pal, H.J.H.; Kaspers, G.J.L.; van Litsenburg, R.R.L. Insomnia Symptoms and Daytime Fatigue Co-Occurrence in Adolescent and Young Adult Childhood Cancer Patients in Follow-Up after Treatment: Prevalence and Associated Risk Factors. Cancers 2022, 14, 3316. https://doi.org/10.3390/cancers14143316
Peersmann SHM, Grootenhuis MA, van Straten A, Tissing WJE, Abbink F, de Vries ACH, Loonen J, van der Pal HJH, Kaspers GJL, van Litsenburg RRL. Insomnia Symptoms and Daytime Fatigue Co-Occurrence in Adolescent and Young Adult Childhood Cancer Patients in Follow-Up after Treatment: Prevalence and Associated Risk Factors. Cancers. 2022; 14(14):3316. https://doi.org/10.3390/cancers14143316
Chicago/Turabian StylePeersmann, Shosha H. M., Martha A. Grootenhuis, Annemieke van Straten, Wim J. E. Tissing, Floor Abbink, Andrica C. H. de Vries, Jacqueline Loonen, Helena J. H. van der Pal, Gertjan J. L. Kaspers, and Raphaële R. L. van Litsenburg. 2022. "Insomnia Symptoms and Daytime Fatigue Co-Occurrence in Adolescent and Young Adult Childhood Cancer Patients in Follow-Up after Treatment: Prevalence and Associated Risk Factors" Cancers 14, no. 14: 3316. https://doi.org/10.3390/cancers14143316
APA StylePeersmann, S. H. M., Grootenhuis, M. A., van Straten, A., Tissing, W. J. E., Abbink, F., de Vries, A. C. H., Loonen, J., van der Pal, H. J. H., Kaspers, G. J. L., & van Litsenburg, R. R. L. (2022). Insomnia Symptoms and Daytime Fatigue Co-Occurrence in Adolescent and Young Adult Childhood Cancer Patients in Follow-Up after Treatment: Prevalence and Associated Risk Factors. Cancers, 14(14), 3316. https://doi.org/10.3390/cancers14143316







