Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
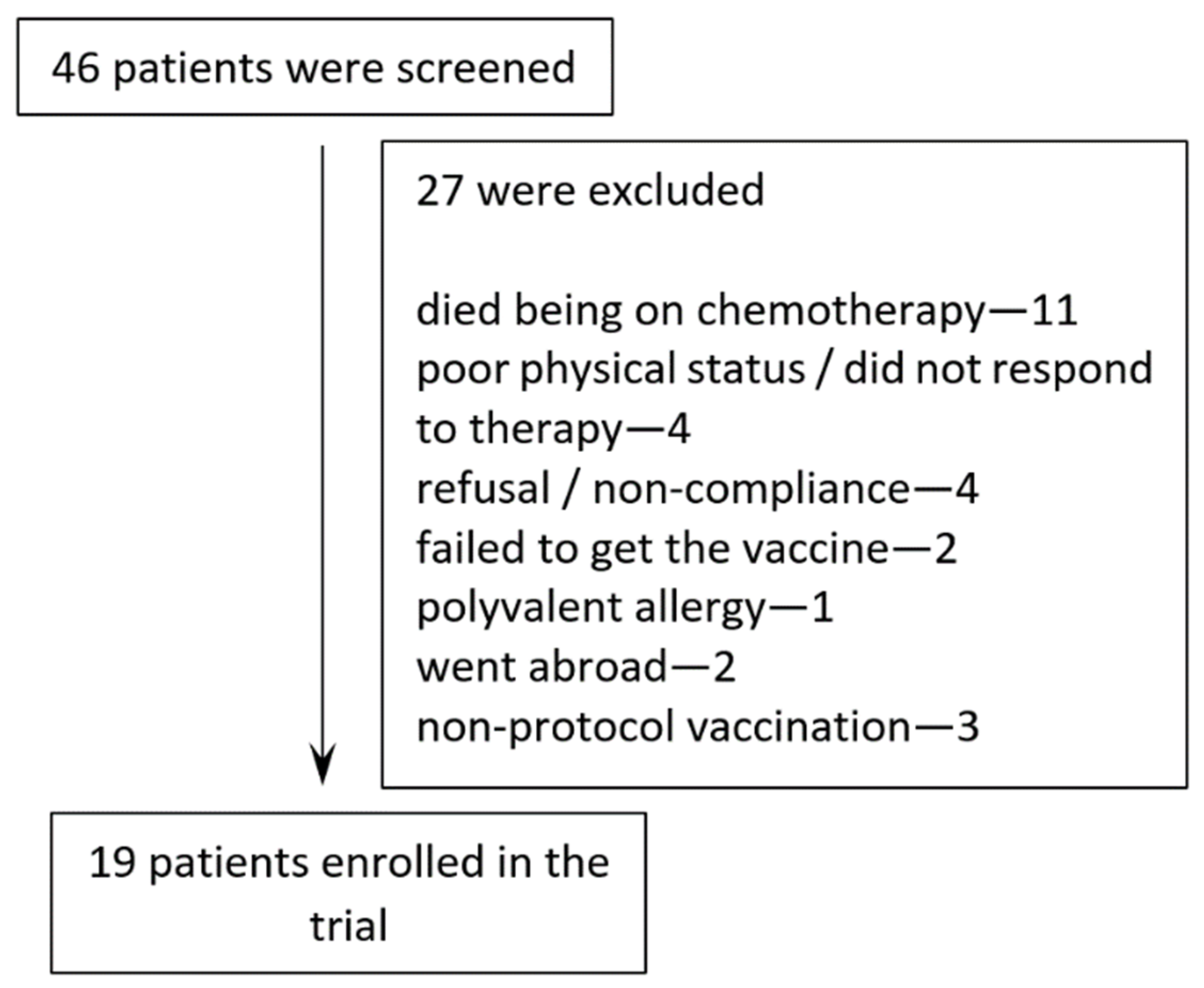
2.1. Patients
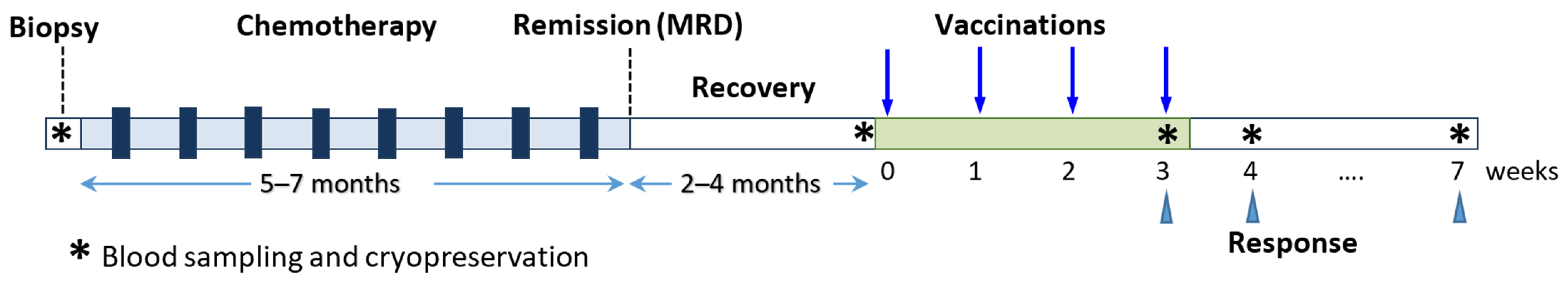
2.2. Clinical Protocol
2.3. Vaccine Production
2.4. Minimal Residual Disease Monitoring
2.5. Immunological Evaluation
3. Results
3.1. Patient Characteristics and Treatment Administration
3.2. Safety and Adverse Events
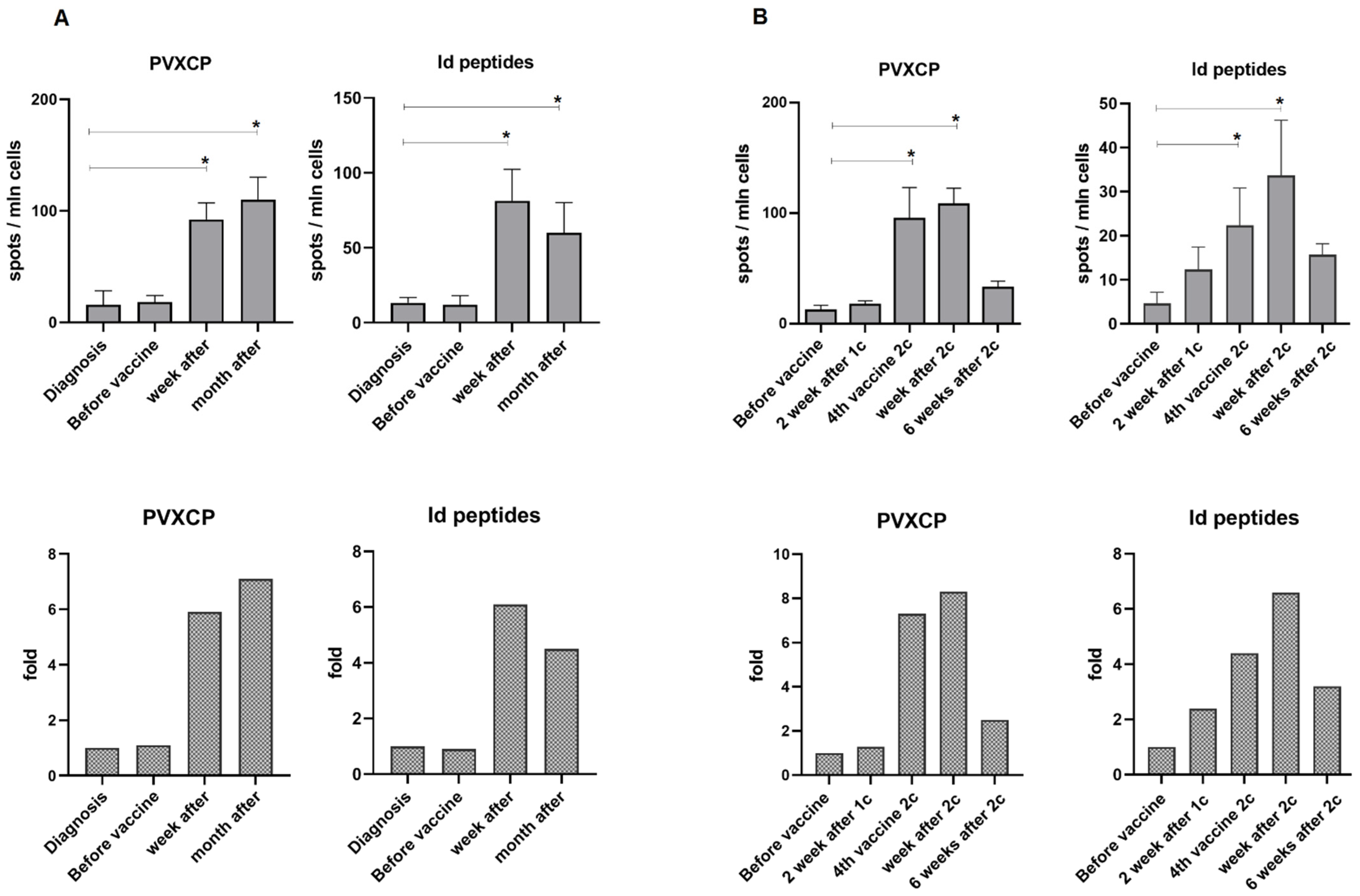
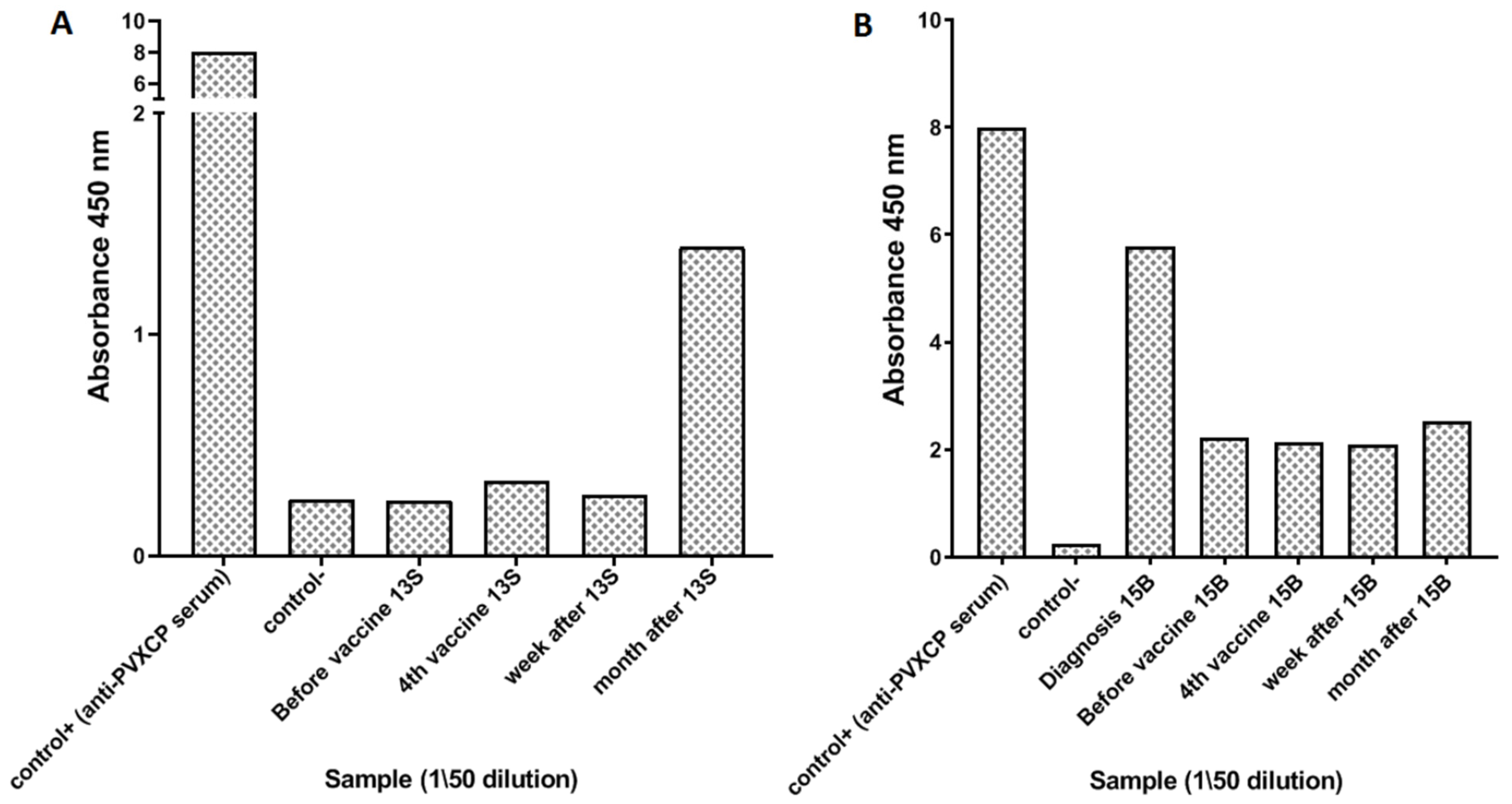
3.3. Immune Responses
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oceanov, A.I.; Moiseev, P.I.; Levin, L.F.; Evmenenko, A.A.; Ipatiy, T.B. Cancer in Belarus: Figures and Facts: Analysis of Data of the Belarusian Cancer Register for 2010–2019; N. N. Alexandrov National Cancer Centre: Minsk, Belarus, 2020. [Google Scholar]
- Palomba, M.L. Active Immunotherapy: Current State of the Art in Vaccine Approaches for NHL. Curr. Oncol. Rep. 2012, 14, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Neelapu, S.S.; Gause, B.L.; Janik, J.E.; Muggia, F.M.; Gockerman, J.P.; Winter, J.N.; Flowers, C.R.; Nikcevich, D.A.; Sotomayor, E.M.; et al. Vaccination with Patient-Specific Tumor-Derived Antigen in First Remission Improves Disease-Free Survival in Follicular Lymphoma. J. Clin. Oncol. 2011, 29, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Bertinetti, C.; Zirlik, K.; Heining-Mikesch, K.; Ihorst, G.; Dierbach, H.; Waller, C.F.; Veelken, H. Phase I Trial of a Novel Intradermal Idiotype Vaccine in Patients with Advanced B-Cell Lymphoma: Specific Immune Responses despite Profound Immunosuppression. Cancer Res. 2006, 66, 4496–4502. [Google Scholar] [CrossRef][Green Version]
- Inogés, S.; de Cerio, A.L.-D.; Villanueva, H.; Soria, E.; Pastor, F.; Bendandi, M. Idiotype Vaccines for Lymphoma Therapy. Expert Rev. Vaccines 2011, 10, 801–809. [Google Scholar] [CrossRef]
- McCann, K.J.; Godeseth, R.; Chudley, L.; Mander, A.; Di Genova, G.; Lloyd-Evans, P.; Kerr, J.P.; Malykh, V.B.; Jenner, M.W.; Orchard, K.H.; et al. Idiotypic DNA Vaccination for the Treatment of Multiple Myeloma: Safety and Immunogenicity in a Phase I Clinical Study. Cancer Immunol. Immunother. 2015, 64, 1021–1032. [Google Scholar] [CrossRef]
- Thomas, S.K.; Cha, S.-C.; Smith, D.L.; Kim, K.H.; Parshottam, S.R.; Rao, S.; Popescu, M.; Lee, V.Y.; Neelapu, S.S.; Kwak, L.W. Phase I Study of an Active Immunotherapy for Asymptomatic Phase Lymphoplasmacytic Lymphoma with DNA Vaccines Encoding Antigen-Chemokine Fusion: Study Protocol. BMC Cancer 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, J.M.; Singh, G.; Hermanson, G.; Hobart, P.; Czerwinski, D.K.; Taidi, B.; Rajapaksa, R.; Caspar, C.B.; Van Beckhoven, A.; Levy, R. Immunogenicity of a Plasmid DNA Vaccine Encoding Chimeric Idiotype in Patients with B-Cell Lymphoma. Cancer Res. 2002, 62, 5845–5852. [Google Scholar] [PubMed]
- Grant, E.V.; Thomas, M.; Fortune, J.; Klibanov, A.M.; Letvin, N.L. Enhancement of Plasmid DNA Immunogenicity with Linear Polyethylenimine. Eur. J. Immunol. 2012, 42, 2937–2948. [Google Scholar] [CrossRef]
- Kimura, S.; Khalil, I.A.; Elewa, Y.H.A.; Harashima, H. Novel Lipid Combination for Delivery of Plasmid DNA to Immune Cells in the Spleen. J. Control. Release 2021, 330, 753–764. [Google Scholar] [CrossRef]
- Graham, B.S.; Enama, M.E.; Nason, M.C.; Gordon, I.J.; Peel, S.A.; Ledgerwood, J.E.; Plummer, S.A.; Mascola, J.R.; Bailer, R.T.; Roederer, M.; et al. DNA Vaccine Delivered by a Needle-Free Injection Device Improves Potency of Priming for Antibody and CD8+ T-Cell Responses after RAd5 Boost in a Randomized Clinical Trial. PLoS ONE 2013, 8, e59340. [Google Scholar] [CrossRef]
- Lin, I.Y.C.; Van, T.T.H.; Smooker, P.M. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef] [PubMed]
- Meleshko, A.N.; Petrovskaya, N.A.; Savelyeva, N.; Vashkevich, K.P.; Doronina, S.N.; Sachivko, N.V. Phase I Clinical Trial of Idiotypic DNA Vaccine Administered as a Complex with Polyethylenimine to Patients with B-Cell Lymphoma. Hum. Vaccin. Immunother. 2017, 13, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Savelyeva, N.; Munday, R.; Spellerberg, M.B.; Lomonossoff, G.P.; Stevenson, F.K. Plant Viral Genes in DNA Idiotypic Vaccines Activate Linked CD4+ T-Cell Mediated Immunity against B-Cell Malignancies. Nat. Biotechnol. 2001, 19, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Meleshko, A.N.; Vashkevich, K.P.; Fomina, E.G.; Scheslenok, E.P.; Schkolina, T.V.; Sergeev, G. V Cloning of Variable Fragments of Tumor Immunoglobulin, Assembling and Expressing of Human SCFV Protein in E. Coli for Anti-Idiotype Vaccination. Exp. Oncol. 2013, 35, 8–14. [Google Scholar]
- Whitlow, M.; Bell, B.A.; Feng, S.L.; Filpula, D.; Hardman, K.D.; Hubert, S.L.; Rollence, M.L.; Wood, J.F.; Schott, M.E.; Milenic, D.E.; et al. An Improved Linker for Single-Chain Fv with Reduced Aggregation and Enhanced Proteolytic Stability. Protein Eng. Des. Sel. 1993, 6, 989–995. [Google Scholar] [CrossRef]
- Wang, C.; Zainal, N.S.; Chai, S.J.; Dickie, J.; Gan, C.P.; Zulaziz, N.; Lye, B.K.W.; Sutavani, R.V.; Ottensmeier, C.H.; King, E.V.; et al. DNA Vaccines Targeting Novel Cancer-Associated Antigens Frequently Expressed in Head and Neck Cancer Enhance the Efficacy of Checkpoint Inhibitor. Front. Immunol. 2021, 12, 763086. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Shen, H.; Brandsma, J.L. DNA Vaccines: Methods and Protocols, 2nd ed.; Hackworth, J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; ISBN 978-1-58829-484-5. [Google Scholar]
- Oh, Y.K.; Kim, J.P.; Yoon, H.; Kim, J.M.; Yang, J.S.; Kim, C.K. Prolonged Organ Retention and Safety of Plasmid DNA Administered in Polyethylenimine Complexes. Gene Ther. 2001, 8, 1587–1592. [Google Scholar] [CrossRef][Green Version]
- Stegantseva, M.V.; Shinkevich, V.A.; Tumar, E.M.; Meleshko, A.N. Multi-Antigen DNA Vaccine Delivered by Polyethylenimine and Salmonella Enterica in Neuroblastoma Mouse Model. Cancer Immunol. Immunother. 2020, 69, 2613–2622. [Google Scholar] [CrossRef]
- Meleshko, A.N.; Savva, N.N.; Fedasenka, U.U.; Romancova, A.S.; Krasko, O.V.; Eckert, C.; von Stackelberg, A.; Aleinikova, O. V Prognostic Value of MRD-Dynamics in Childhood Acute Lymphoblastic Leukemia Treated According to the MB-2002/2008 Protocols. Leuk. Res. 2011, 35, 1312–1320. [Google Scholar] [CrossRef]
- van der Velden, V.H.J.; Cazzaniga, G.; Schrauder, A.; Hancock, J.; Bader, P.; Panzer-Grumayer, E.R.; Flohr, T.; Sutton, R.; Cave, H.; Madsen, H.O.; et al. Analysis of Minimal Residual Disease by Ig/TCR Gene Rearrangements: Guidelines for Interpretation of Real-Time Quantitative PCR Data. Leukemia 2007, 21, 604–611. [Google Scholar] [CrossRef]
- Smirnova, N.V.; Myakova, N.V.; Belogurova, M.B.; Ryskal, O.V.; Nikonova, O.E.; Sharapova, G.R.; Fedorova, A.S.; Grigorieva, N.A.; Shamardina, A.V.; Ponomareva, N.I.; et al. Treatment of B-Cells Non-Hodgkin Lymphomas with Combined Immunochemotherapy: Ability to Treatment Optimization. Oncohematology 2015, 10, 15–24. [Google Scholar] [CrossRef]
- Berger, E.; Soldati, R.; Huebener, N.; Hohn, O.; Stermann, A.; Durmus, T.; Lobitz, S.; Zenclussen, A.C.; Christiansen, H.; Lode, H.N.; et al. Salmonella SL7207 Application Is the Most Effective DNA Vaccine Delivery Method for Successful Tumor Eradication in a Murine Model for Neuroblastoma. Cancer Lett. 2013, 331, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Savelyeva, N.; Zhu, D.; Stevenson, F.K. Engineering DNA Vaccines That Include Plant Virus Coat Proteins. Biotechnol. Genet. Eng. Rev. 2003, 20, 101–116. [Google Scholar] [CrossRef][Green Version]
- Bolhassani, A.; Safaiyan, S.; Rafati, S. Improvement of Different Vaccine Delivery Systems for Cancer Therapy. Mol. Cancer 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Conese, M. Polyethylenimine-Mediated Gene Delivery to the Lung and Therapeutic Applications. Drug Des. Dev. Ther. 2009, 2, 163–188. [Google Scholar] [CrossRef][Green Version]
- Stegantseva, M.V.; Shinkevich, V.A.; Tumar, E.M.; Meleshko, A.N. Conjugation of New DNA Vaccine with Polyethylenimine Induces Cellular Immune Response and Tumor Regression in Neuroblastoma Mouse Model. Exp. Oncol. 2020, 42, 120–125. [Google Scholar] [CrossRef]
- Patnaik, S.; Gupta, K.C. Novel Polyethylenimine-Derived Nanoparticles for in Vivo Gene Delivery. Expert Opin. Drug Deliv. 2013, 10, 215–228. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Jin, C.G.; Zhou, Y.C.; Navab, R.; Jakobsen, K.R.; Chen, X.Q.; Li, J.; Li, T.T.; Luo, L.; et al. An Orally Administered DNA Vaccine Targeting Vascular Endothelial Growth Factor Receptor-3 Inhibits Lung Carcinoma Growth. Tumour Biol. 2016, 37, 2395–2404. [Google Scholar] [CrossRef]
- Zuo, S.G.; Chen, Y.; Wu, Z.P.; Liu, X.; Liu, C.; Zhou, Y.C.; Wu, C.L.; Jin, C.G.; Gu, Y.L.; Li, J.; et al. Orally Administered DNA Vaccine Delivery by Attenuated Salmonella Typhimurium Targeting Fetal Liver Kinase 1 Inhibits Murine Lewis Lung Carcinoma Growth and Metastasis. Biol. Pharm. Bull. 2010, 33, 174–182. [Google Scholar] [CrossRef]
- Niethammer, A.G.; Lubenau, H.; Mikus, G.; Hohmann, N.; Leowardi, C.; Beckhove, P.; Akhisaroglu, M.; Ge, Y.; Springer, M.; Grenacher, L.; et al. Double-Blind, Placebo-Controlled First in Human Study to Investigate an Oral Vaccine Aimed to Elicit an Immune Reaction against the VEGF-Receptor 2 in Patients with Stage IV and Locally Advanced Pancreatic Cancer. BMC Cancer 2012, 12, 361. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A Phase 1 Trial Extension to Assess Immunologic Efficacy and Safety of Prime-Boost Vaccination with VXM01, an Oral T Cell Vaccine against VEGFR2, in Patients with Advanced Pancreatic Cancer. Oncoimmunology 2018, 7, e1303584. [Google Scholar] [CrossRef] [PubMed]
- Cachot, A.; Bilous, M.; Liu, Y.-C.; Li, X.; Saillard, M.; Cenerenti, M.; Rockinger, G.A.; Wyss, T.; Guillaume, P.; Schmidt, J.; et al. Tumor-Specific Cytolytic CD4 T Cells Mediate Immunity against Human Cancer. Sci. Adv. 2021, 7, eabe3348. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age 1 | Diagnosis | Tumor Ig | Chemotherapy | Response to Chemo | Time from Last Cycle of Chemo (Month) | VC | Adverse Events | Immune-Response ELISpot (Max Vac/Prevac Fold)/ELISA | MRD Status Post-Chemo | MRD after 1 Course | MRD after 2 Courses | Post-Vaccine Status | Last Follow-Up | Time from Vaccination (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1F | M/57 | CLL | IgM,D/IgK | FCR | PR | 5 | 1 | Local Grade 1 | negative/n.d. | 0.76% | 0.05% | stabilization | remission | 34 | |
| 2K | M/60 | CLL | IgD/IgK | FCR | PR | 10 | 2 | Local Grade 1 | n.d. | 249% | 563% | n.d. | stabilization | remission | 33 |
| 3R | M/52 | MCL | IgM,D/IgK | EPOCH_BAC | CR | 12 | 2 | Flu-like syndrome, nausea (grade 2) | positive (10)/negative | 1.7% | 0.57% | 0.2% | stabilization | n.d. | 20 |
| 4D | M/67 | MCL | IgM,D/IgK | BCDP + R-CHOP + Benda | CR | 9 | 2 | Local Grade 1 | positive (5.4)/negative | 0.05% | 0 | 0 | remission | relapse 2 | 33 |
| 5S | F/43 | SLL | IgG/IgL | FCR | CR | 1 | 1 | Local Grade 1 | positive (7.6)/n.d. | n.d. | 0 | remission | remission | 22 | |
| 6L | M/63 | CLL | IgM,D/IgK | FCR | PR | 4 | 1 | Local Grade 1 | n.d. | 34% | 275% | stabilization | progression | 28 | |
| 7T | F/50 | MZL | IgM/IgKlow | FCR | CR | 5 | 2 | Diarrhea (1) | positive (8)/negative | 0 | 0 | 0 | remission | n.d. | 8 |
| 8S | F/58 | SLL | IgM/IgK | FCR | CR | 9 | 1 | Local Grade 1 | n.d./negative | n.d. | n.d. | remission | remission | 32 | |
| 9M | M/65 | SLL | IgM/IgL | FC | CR | 11 | 1 | Local Grade 1 | negative/negative | 0 | 0 | remission | n.d. | 5 | |
| 10S | M/70 | CLL | IgM,D/IgK | Benda + Ganziva | CR | 5 | 1 | Local Grade 1 | uncertain (2.4)/negative | 0.0175% | 0 | remission | remission | 33 | |
| 11K | F/63 | CLL/SLL | IgD,M/IgL | FCR | SD | 10 | 1 | Local Grade 1 | n.d./negative | 16% | 15% | stabilization | progression | 16 | |
| 12O | M/64 | FL | IgM/IgK | BR | CR | 6 | 1 | Local Grade 1 | positive (6)/negative | n.d. | 0 | remission | remission | 31 | |
| 13S | M/18 | DLBCL | IgG/IgK | B-NHL-M [23] | CR | 3 | 1 | Local Grade 1 | positive (3.6)/positive | 0 | 0 | remission | remission | 32 | |
| 14K | F/35 | FL | IgM/IgK | BR + R-CHOP | CR | 4 | 1 | Local Grade 1 | positive (14.3)/negative | 0 | 0 | remission | remission | 5 | |
| 15B | M/59 | FL | IgM/IgK | BR | CR | 7 | 1 | Local Grade 1 | positive (16.9)/positive | 0.024% | 0 | remission | remission | 28 | |
| 16B | M/69 | DLBCL | IgM/IgK | R-CHOP + radiotherapy | CR | 6 | 1 | Local Grade 1 | n.d./negative | 0 | 0 | remission | remission | 35 | |
| 17K | M/62 | MCL | IgM/IgK | BR plus rituximab | CR | 6 | 1 | Local Grade 1 | positive (4.9)/negative | n.d. | 0 | remission | remission | 24 | |
| 18B | F/39 | DLBCL | IgM/IgL | R-CHOP | CR | 5 | 1 | Local Grade 1 | positive (10.2)/negative | 0 | 0 | remission | remission | 24 | |
| 19V | F/18 | DLBCL | IgG/IgL | B-NHL-M [23] | CR | 6 | 1 | Local Grade 1 | positive (9.4)/negative | 0 | 0 | remission | remission | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleshko, A.; Piatrouskaya, N.; Vashkevich, K.; Lutskovich, D.; Wang, C.; Dormeshkin, D.; Savelyeva, N.; Katsin, M. Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study. Cancers 2022, 14, 3298. https://doi.org/10.3390/cancers14143298
Meleshko A, Piatrouskaya N, Vashkevich K, Lutskovich D, Wang C, Dormeshkin D, Savelyeva N, Katsin M. Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study. Cancers. 2022; 14(14):3298. https://doi.org/10.3390/cancers14143298
Chicago/Turabian StyleMeleshko, Alexander, Nadzeya Piatrouskaya, Katsiaryna Vashkevich, Dzmitry Lutskovich, Chuan Wang, Dmitri Dormeshkin, Natalia Savelyeva, and Mikalai Katsin. 2022. "Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study" Cancers 14, no. 14: 3298. https://doi.org/10.3390/cancers14143298
APA StyleMeleshko, A., Piatrouskaya, N., Vashkevich, K., Lutskovich, D., Wang, C., Dormeshkin, D., Savelyeva, N., & Katsin, M. (2022). Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study. Cancers, 14(14), 3298. https://doi.org/10.3390/cancers14143298






