RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Univariable Analysis
3.2. Multivariable Analysis
3.3. Scoring System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, J.; Ornstein, M.C. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. J. Oncol. Pract. 2021, 18, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Mazumdar, M.; Bacik, J.; Berg, W.; Amsterdam, A.; Ferrara, J. Survival and Prognostic Stratification of 670 Patients With Advanced Renal Cell Carcinoma. J. Clin. Oncol. 1999, 17, 2530–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Sherry, R.M.; Steinberg, S.M.; Topalian, S.L.; Schwartzentruber, D.J.; Hwu, P.; Seipp, C.A.; Rogers-Freezer, L.; Morton, K.E.; White, D.E.; et al. Randomized Study of High—Dose and Low—Dose Inter leukin—2 in Patients with Metastatic Renal Cancer. J. Clin. Oncol. 2003, 21, 3127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, D.F.; Regan, M.M.; Clark, J.I.; Flaherty, L.E.; Weiss, G.R.; Logan, T.F.; Kirkwood, J.M.; Gordon, M.S.; Sosman, J.A.; Ernstoff, M.S.; et al. Randomized Phase III Trial of High-Dose Interleukin-2 Versus Subcutaneous Interleukin-2 and Interferon in Patients With Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2005, 23, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasian, L.M.; Motzer, R.J.; Gluck, L.; Mazumdar, M.; Vlamis, V.; Krown, S.E. Interferon alfa-2a in advanced renal cell carcinoma: Treatment results and survival in 159 patients with long-term follow-up. J. Clin. Oncol. 1993, 11, 1368–1375. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R.J.; Munshi, N.; Crawford, E.D. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef]
- Mekhail, T.M.; Abou-Jawde, R.M.; BouMerhi, G.; Malhi, S.; Wood, L.; Elson, P.; Bukowski, R. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J. Clin. Oncol. 2005, 23, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Négrier, S.; Escudier, B.; Gomez, F.; Douillard, J.Y.; Ravaud, A.; Chevreau, C.; Buclon, M.; Pérol, D.; Lasset, C. Prognostic factors of survival and rapid progression in782 patients with metastatic renal carcinomas treated by cytokines: A report from the Groupe Français d’Immunothérapie. Ann. Oncol. 2002, 13, 1460–1468. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141. [Google Scholar] [CrossRef] [Green Version]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Manola, J.; Royston, P.; Elson, P.; McCormack, J.B.; Mazumdar, M.; Négrier, S.; Escudier, B.; Eisen, T.; Dutcher, J.; Atkins, M.; et al. Prognostic Model for Survival in Patients with Metastatic Renal Cell Carcinoma: Results from the International Kidney Cancer Working Group. Clin. Cancer Res. 2011, 17, 5443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.; Figlin, R.A.; Hutson, T.E.; Michaelson, M.D.; Négrier, S.; Kim, S.T.; Huang, X.; Motzer, R.J. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. 2011, 22, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef]
- Takagi, T.; Fukuda, H.; Kondo, T.; Ishihara, H.; Yoshida, K.; Kobayashi, H.; Iizuka, J.; Okumi, M.; Ishida, H.; Tanabe, K. Prognostic Markers for Refined Stratification of IMDC Intermediate-Risk Metastatic Clear Cell Renal Cell Carcinoma Treated with First-Line Tyrosine Kinase Inhibitor Therapy. Target Oncol. 2019, 14, 179–186. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [Green Version]
- Longo, N.; Capece, M.; Celentano, G.; La Rocca, R.; Califano, G.; Ruvolo, C.C.; Buonerba, C.; Esposito, F.; Napolitano, L.; Mangiapia, F.; et al. Clinical and Pathological Characteristics of Metastatic Renal Cell Carcinoma Patients Needing a Second-Line Therapy: A Systematic Review. Cancers 2020, 12, 3634. [Google Scholar] [CrossRef]
- Albiges, L.; Negrier, S.; Dalban, C.; Chevreau, C.; Gravis, G.; Oudard, S.; Laguerre, B.; Barthelemy, P.; Borchiellini, D.; Gross-Goupil, M.; et al. Safety and efficacy of nivolumab in metastatic renal cell carcinoma (mRCC): Final analysis from the NIVOREN GETUG AFU 26 study. J. Clin. Oncol. 2019, 37, 542. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Juárez, V.M.O.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Sharma, R.; Kadife, E.; Myers, M.; Kannourakis, G.; Prithviraj, P.; Ahmed, N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 186. [Google Scholar] [CrossRef]
- Motzer, R.J.; Banchereau, R.; Hamidi, H.; Powles, T.; McDermott, D.; Atkins, M.B.; Escudier, B.; Liu, L.F.; Leng, N.; Abbas, A.R.; et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell 2020, 38, 803.e4–817.e4. [Google Scholar] [CrossRef]
- Abdou, E.; Pedapenki, R.M.; Abouagour, M.; Zar, A.R.; Dawoud, E.; Elshourbagy, D.; Al-Shamsi, H.O.; Grande, E. Patient selection and risk factors in the changing treatment landscape of metastatic renal cell carcinoma. Expert Rev. Anticancer Ther. 2020, 20, 1810572. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Powles, T.; Donskov, F.; Plimack, E.R.; Barthélémy, P.; Hammers, H.J.; et al. Original research: Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 2020, 8, e000891. [Google Scholar] [CrossRef]
- Di Nunno, V.; Mollica, V.; Schiavina, R.; Nobili, E.; Fiorentino, M.; Brunocilla, E.; Ardizzoni, A.; Massari, F. Improving IMDC Prognostic Prediction Through Evaluation of Initial Site of Metastasis in Patients With Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2020, 18, e83–e90. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Di Nunno, V.; Guida, A.; Costa Silva, C.A.; Derosa, L.; Mollica, V.; Colomba, E.; Brandi, G.; Albiges, L. Addition of Primary Metastatic Site on Bone, Brain, and Liver to IMDC Criteria in Patients With Metastatic Renal Cell Carcinoma: A Validation Study. Clin. Genitourin. Cancer 2021, 19, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Tassinari, E.; Giunchi, F.; Schiavina, R.; Fiorentino, M.; Brunocilla, E.; Ardizzoni, A.; Massari, F. Prognostic and predictive factors to nivolumab in patients with metastatic renal cell carcinoma: A single center study. Anticancer Drugs 2021, 32, 74–81. [Google Scholar] [CrossRef]
- Park, S.Y.; Nam, J.S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569. [Google Scholar] [CrossRef] [Green Version]
- Sella, A.; Michaelson, M.D.; Matczak, E.; Simantov, R.; Lin, X.; Figlin, R.A. Heterogeneity of Patients With Intermediate-Prognosis Metastatic Renal Cell Carcinoma Treated With Sunitinib. Clin. Genitourin. Cancer 2017, 15, 291–299.e1. [Google Scholar] [CrossRef]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.l.; Rini, B.I.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T.; et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef]
- Yip, S.M.; Wells, C.; Moreira, R.; Wong, A.; Srinivas, S.; Beuselinck, B.; Porta, C.; Sim, H.W.; Ernst, D.S.; Rini, B.I.; et al. Checkpoint inhibitors in patients with metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer 2018, 124, 3677–3683. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Overall (n, %) | Univariable HR (CI, p-Value) |
|---|---|---|
| Gender, Male (%) | 91 (71.7) | 0.86 (0.48–1.54, p = 0.606) |
| Age at diagnosis (median [IQR]) | 62.00 [52.00, 69.00] | 1.00 (0.97–1.02, p = 0.833) |
| Smoking, yes (%) | 43 (35.0) | 1.03 (0.57–1.88, p = 0.912) |
| Sarcomatoid elements, yes (%) | 18 (17.6) | 1.32 (0.63–2.79, p = 0.462) |
| RCC subtype (%) | ||
| Clear cell | 107 (87.0) | |
| Chromophobe | 2 (1.6) | * 1.15 (0.58–2.28, p = 0.682) |
| Papillary | 10 (8.1) | |
| Other | 4 (3.2) | |
| Intact primary kidney tumor (%) | 27 (21.3) | 2.8 (1.58–4.92, p = 0.0004) |
| Number of metastatic sites (median [IQR]) | 2.00 [1.00, 3.00] | 1.55 (1.18–2.05, p = 0.002) |
| Number of metastatic sites (%) | ||
| 1 | 45 (35.4) | |
| 2 | 43 (33.9) | |
| 3 | 25 (19.7) | |
| 4 | 13 (10.2) | |
| Lymph node metastases, yes (%) | 44 (34.6) | 1.93 (1.10–3.38, p = 0.022) |
| Lung metastases, yes (%) | 83 (65.4) | 0.91 (0.51–1.63, p = 0.754) |
| Bone metastases, yes (%) | 41 (32.3) | 0.98 (0.54–1.79, p = 0.952) |
| Brain metastases, yes (%) | 7 (5.5) | 4.24 (1.27–14.12, p = 0.019) |
| Pancreas metastases, yes (%) | 7 (5.5) | 0.30 (0.04–2.15, p = 0.228) |
| Soft tissue metastases, yes (%) | 20 (15.7) | 1.55 (0.75–3.18, p = 0.237) |
| Liver metastases, yes (%) | 17 (13.4) | 3.55 (1.79–7.03, p < 0.001) |
| Adrenal metastases, yes (%) | 16 (12.6) | 1.15 (0.49–2.69, p = 0.754) |
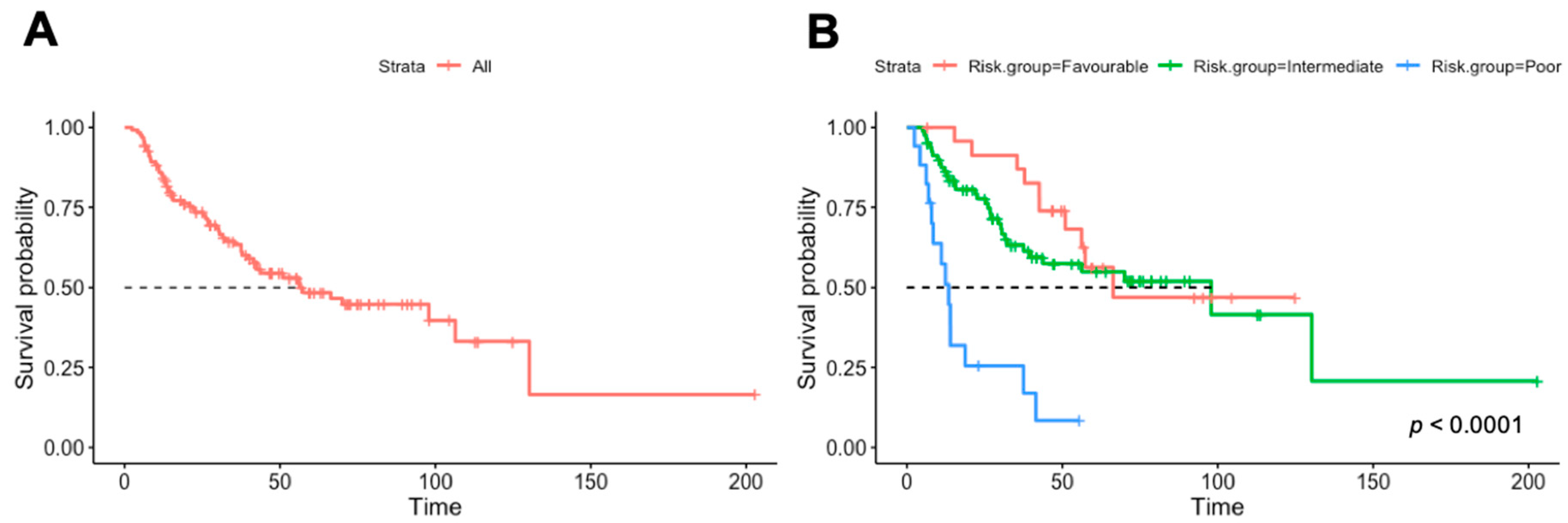
| Heng risk group (%) | ||
| Favorable | 25 (20.0) | |
| Intermediate | 83 (66.4) | 1.15 (0.57–2.34, p = 0.697) |
| Poor | 17 (13.6) | 6.25 (2.69–14.51, p < 0.001) |
| Time criteria, yes (%) | 66 (52.8) | 2.41 (1.36–4.27, p = 0.002) |
| Performance status criteria, yes (%) | 13 (10.5) | 3.29 (1.58–6.87, p = 0.001) |
| Hemoglobin criteria, yes (%) | 75 (61.0) | 1.85 (1.02–3.36, p = 0.044) |
| Neutrophil criteria, yes (%) | 8 (6.5) | 2.76 (1.08–7.03, p = 0.033) |
| Platelet criteria, yes (%) | 10 (8.1) | 5.08 (2.02–12.78, p = 0.001) |
| Calcium criteria, yes (%) | 3 (2.5) | 1.25 (0.17–9.41, p = 0.825) |
| Received VEGFRi before CPI, yes (%) | 67 (52.8) | 1.14 (0.64–2.03, p = 0.661) |
| Previous treatment lines (median (IQR)) | 1.00 [0.00, 1.00] | 0.90 (0.64–1.28, p = 0.565) |
| Number of treatment lines prior to CPI (%) | ||
| 0 | 59 (46.5) | |
| 1 | 51 (40.2) | |
| 2 | 14 (11.0) | |
| 3 or more | 3 (2.4) | |
| CPI response rate (complete and partial) (%) | 47 (38.8) | 0.2 (0.1–0.4, p < 0.001) |
| Variables | Final Multivariable Model | Bootstrap | ||||
|---|---|---|---|---|---|---|
| HR | 2.5% | 97.5% | p-Value | Percentage of Times Variable Entered Model | Bootstrap Parameter Means | |
| Intact primary kidney tumor | 2.33 | 1.202 | 4.502 | 0.012 | 94 | 2.35 |
| Liver metastases | 3.33 | 1.671 | 6.623 | 0.001 | 100 | 3.27 |
| Time criteria | 1.98 | 1.072 | 3.641 | 0.029 | 86 | 2.02 |
| Performance status criteria | 3.42 | 1.654 | 7.077 | 0.001 | 100 | 3.44 |
| Platelet criteria | 3.06 | 1.245 | 7.514 | 0.015 | 85 | 3.257 |
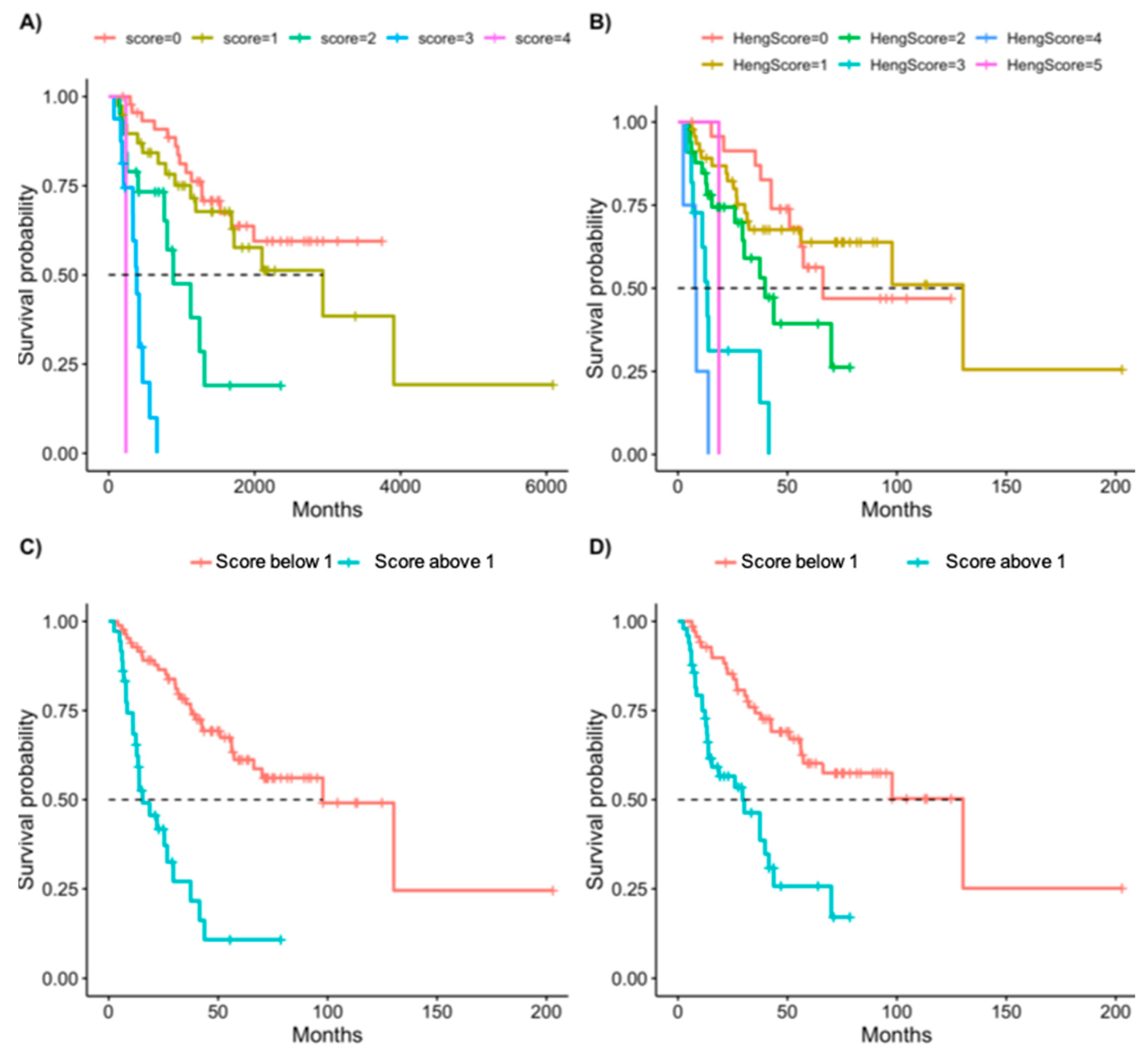
| New Score | Heng Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 2.50% | 97.50% | p-Value | HR | 2.50% | 97.50% | p-Value | ||
| One point | 1.36 | 0.67 | 2.75 | 0.40 | One point | 0.94 | 0.43 | 2.03 | 0.87 |
| Two points | 4.41 | 1.99 | 9.74 | 0.00 | Two points | 2.13 | 0.96 | 4.70 | 0.06 |
| Three points | 18.17 | 7.38 | 44.73 | 0.00 | Three points | 7.47 | 2.96 | 18.86 | 0.00 |
| Four points | 45.91 | 5.26 | 400.49 | 0.00 | Four points | 21.64 | 6.17 | 75.90 | 0.00 |
| Five points | 7.49 | 0.93 | 60.55 | 0.06 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagie, S.; Sarfaty, M.; Levartovsky, M.; Gantz Sorotsky, H.; Berger, R.; Percik, R.; Gadot, M. RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era. Cancers 2022, 14, 3127. https://doi.org/10.3390/cancers14133127
Sagie S, Sarfaty M, Levartovsky M, Gantz Sorotsky H, Berger R, Percik R, Gadot M. RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era. Cancers. 2022; 14(13):3127. https://doi.org/10.3390/cancers14133127
Chicago/Turabian StyleSagie, Shira, Michal Sarfaty, Meital Levartovsky, Hadas Gantz Sorotsky, Raanan Berger, Ruth Percik, and Moran Gadot. 2022. "RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era" Cancers 14, no. 13: 3127. https://doi.org/10.3390/cancers14133127
APA StyleSagie, S., Sarfaty, M., Levartovsky, M., Gantz Sorotsky, H., Berger, R., Percik, R., & Gadot, M. (2022). RCC Real-World Data: Prognostic Factors and Risk Stratification in the Immunotherapy Era. Cancers, 14(13), 3127. https://doi.org/10.3390/cancers14133127







