Perioperative Carcinoid Crisis: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Selection Criteria
2.3. Data Extraction and Tabulation
2.4. Risk of Bias/Assessment of Confounding
2.5. Data Analysis and Statistical Methods
3. Results
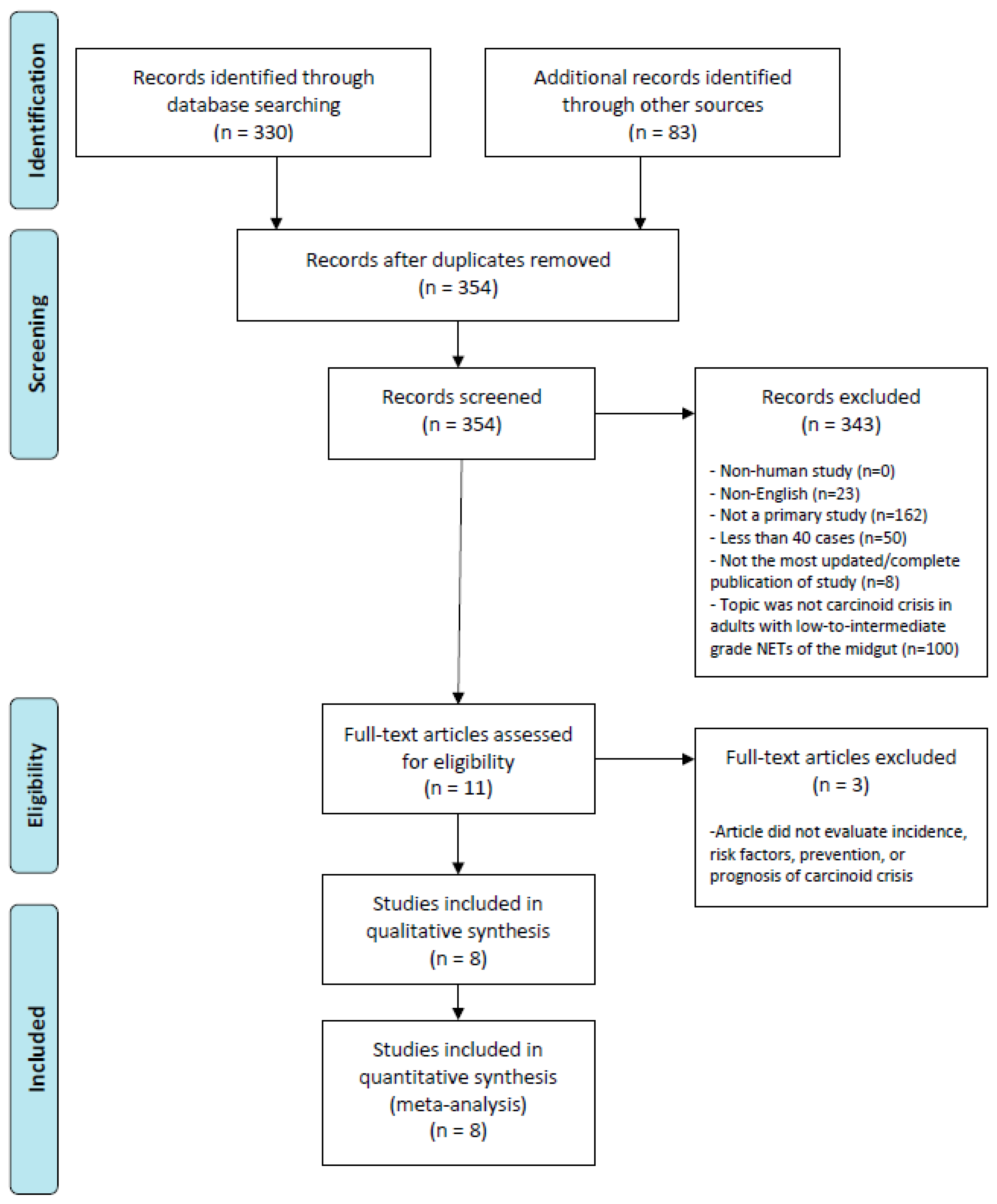
3.1. Search Result
3.2. Study and Patient Characteristics
3.3. Risk of Bias of Studies Included
3.4. Outcomes
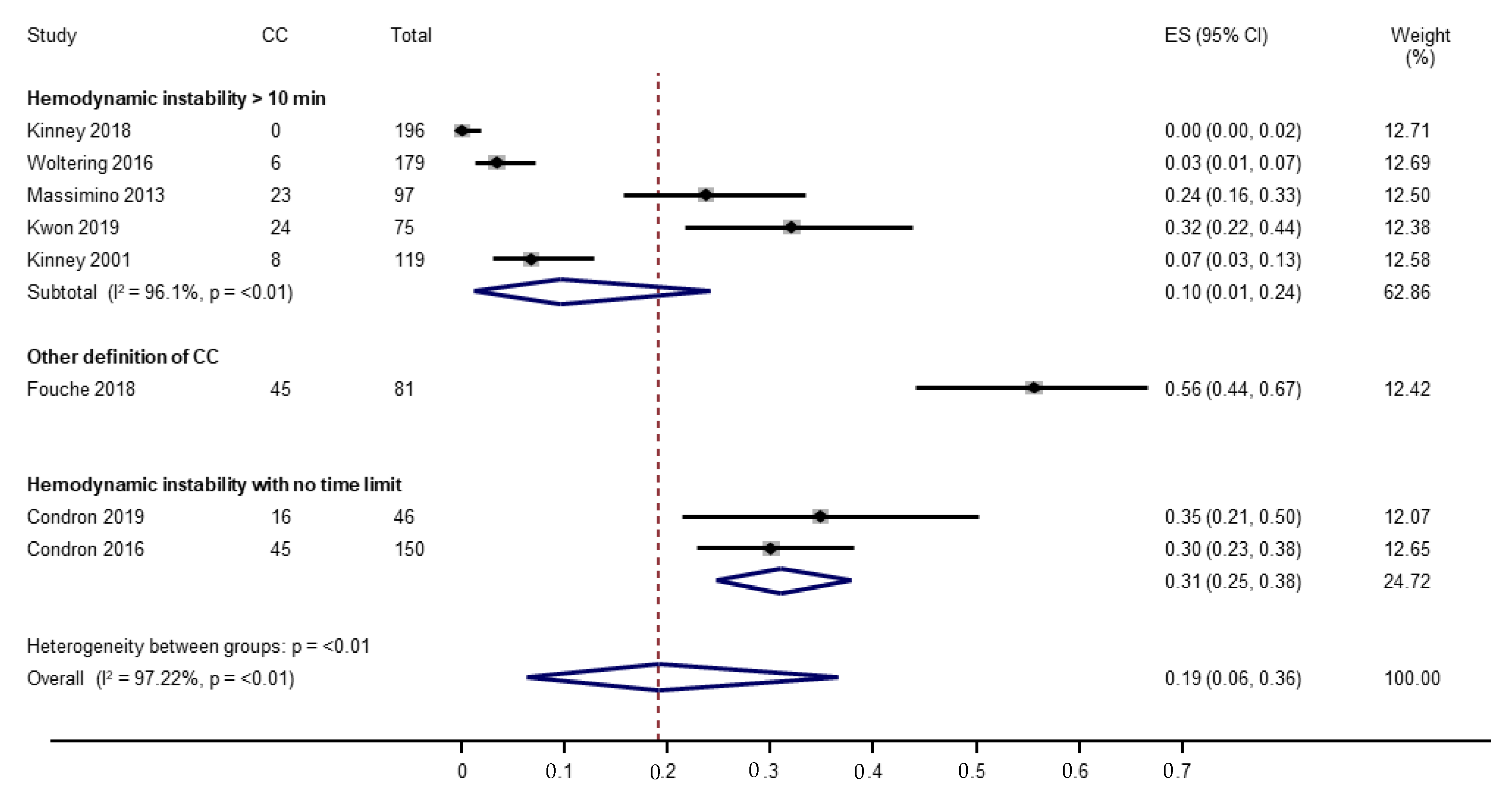
3.4.1. Incidence of Carcinoid Crisis
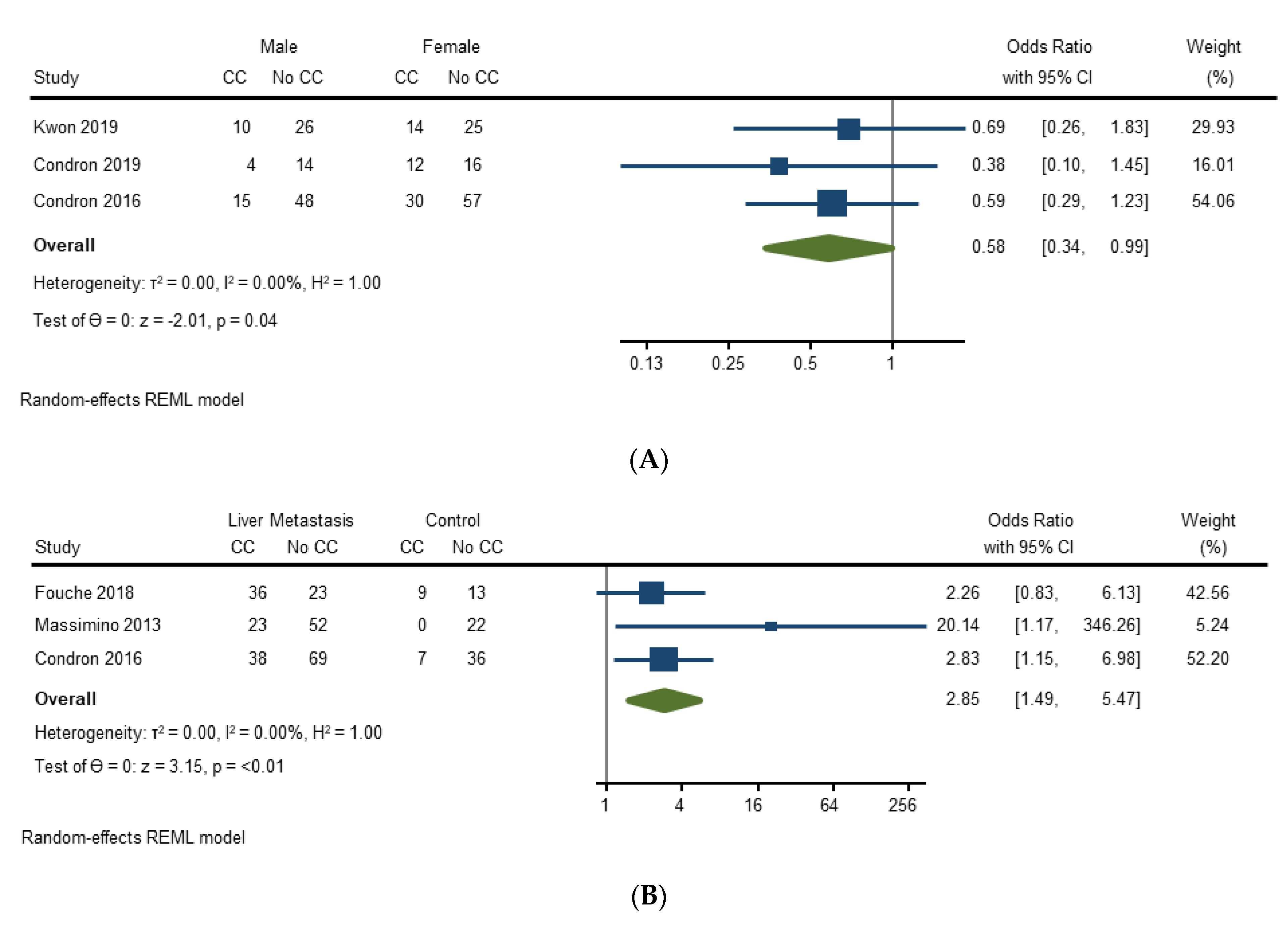
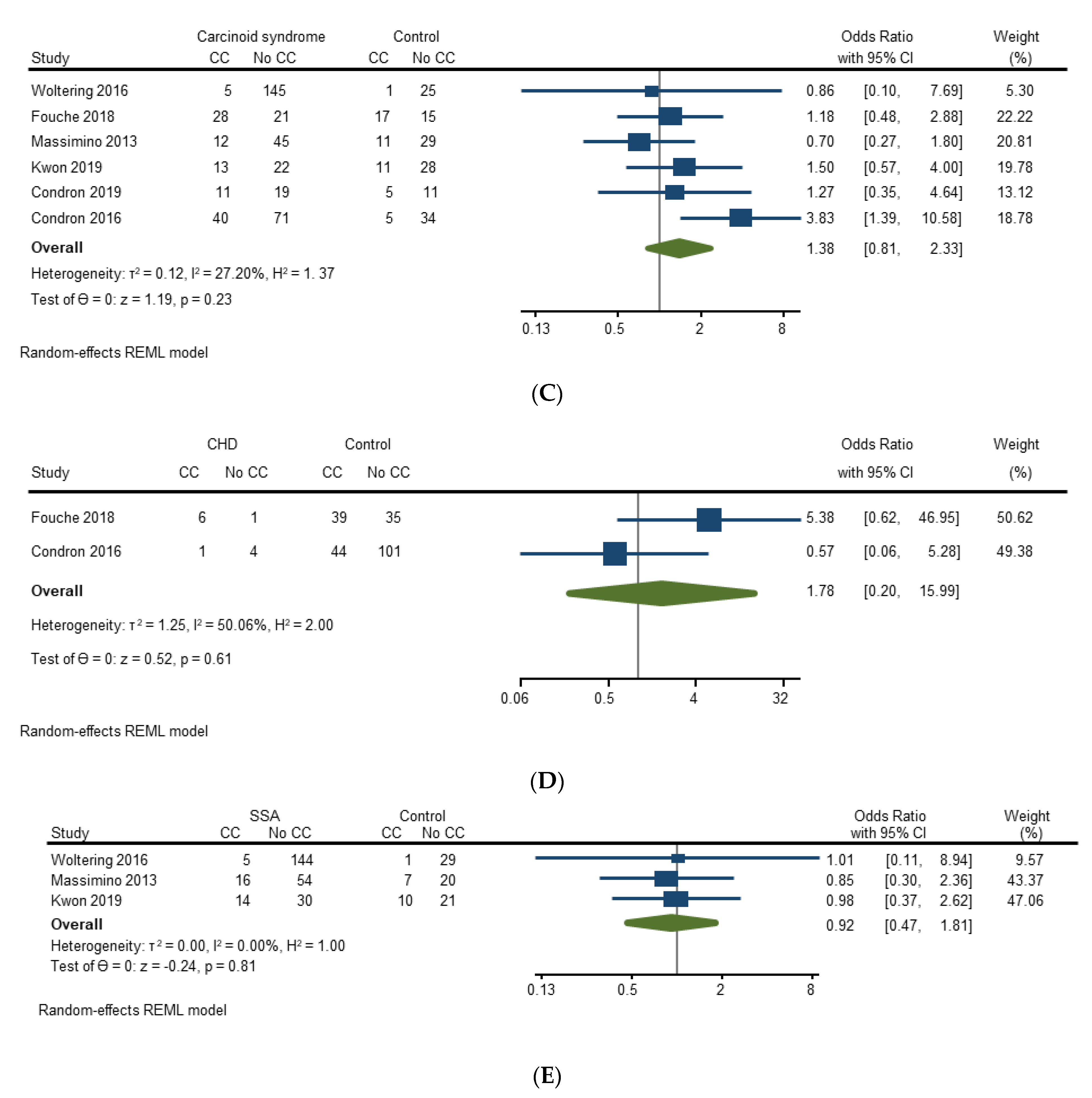
3.4.2. Risk Factors for Carcinoid Crisis
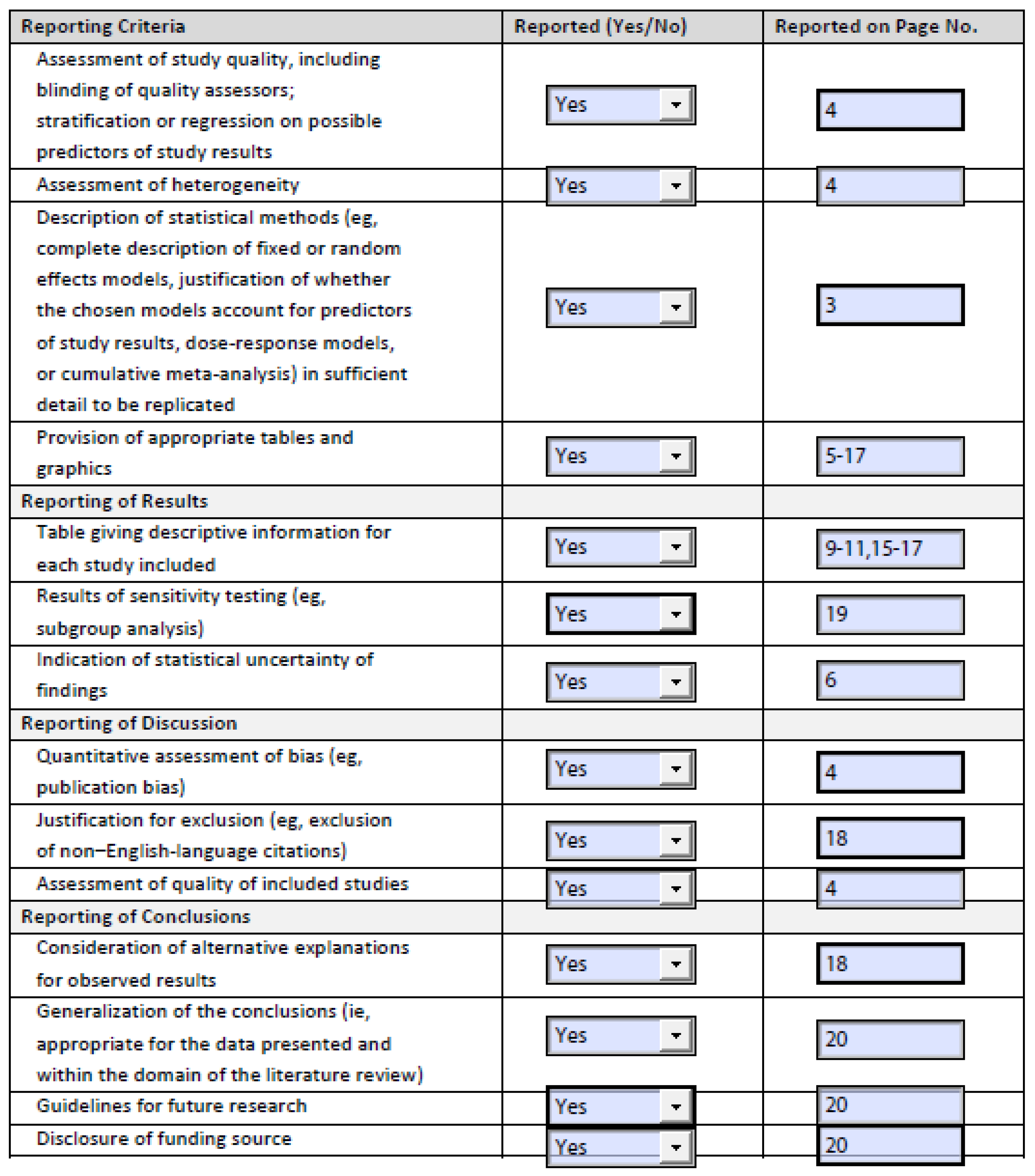
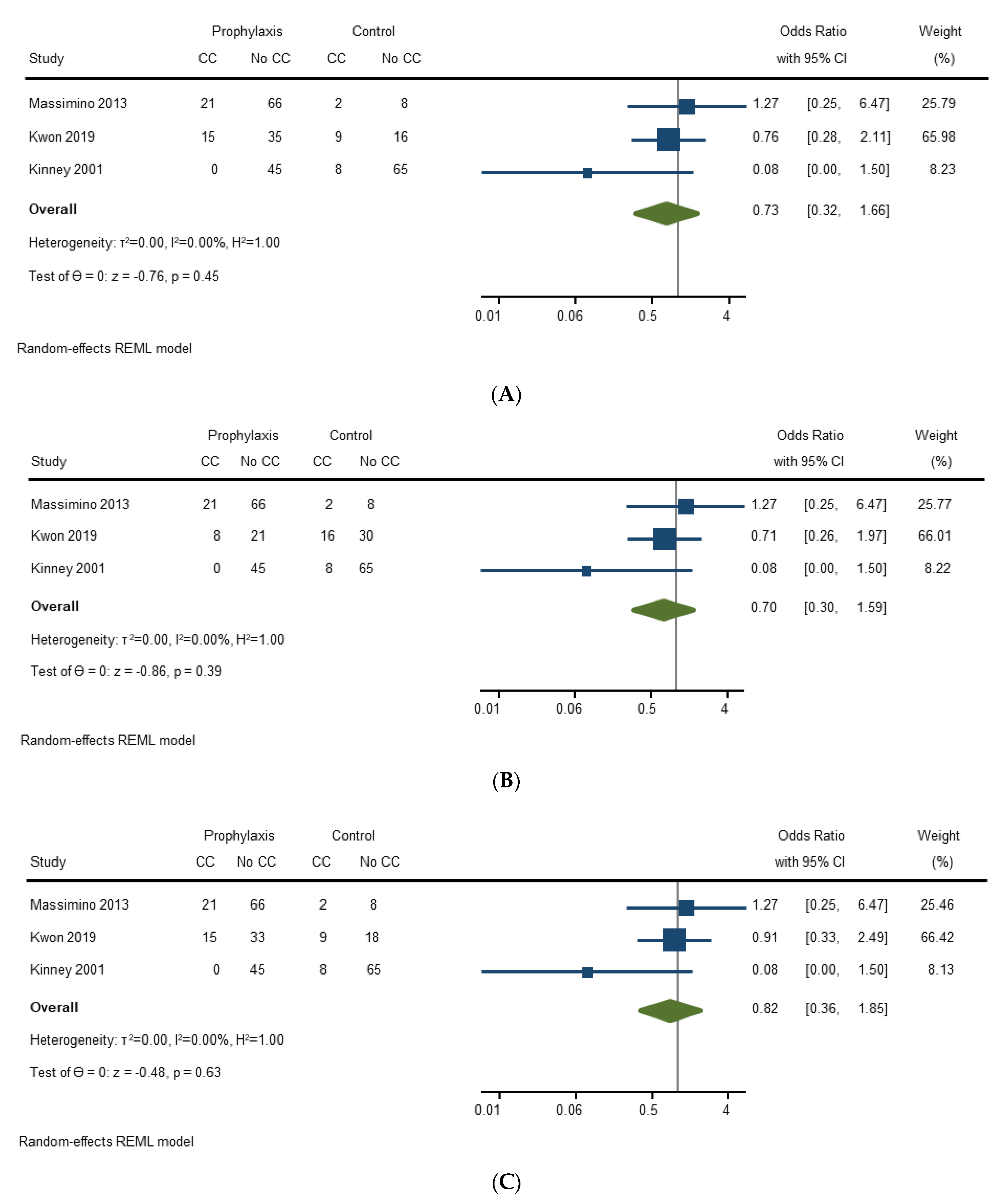
3.4.3. Prevention of Carcinoid Crisis with Octreotide
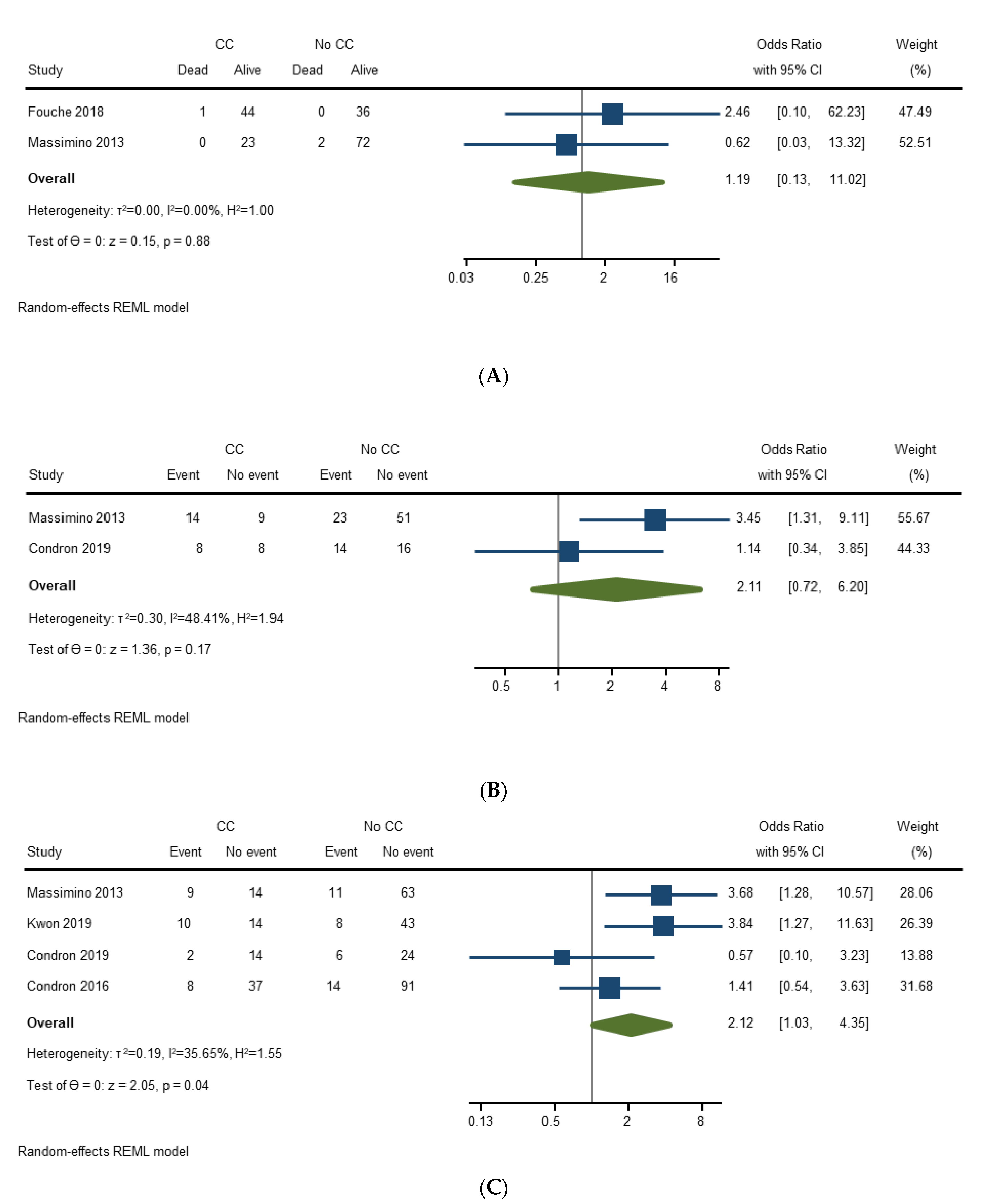
3.4.4. Prognosis of Carcinoid Crisis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
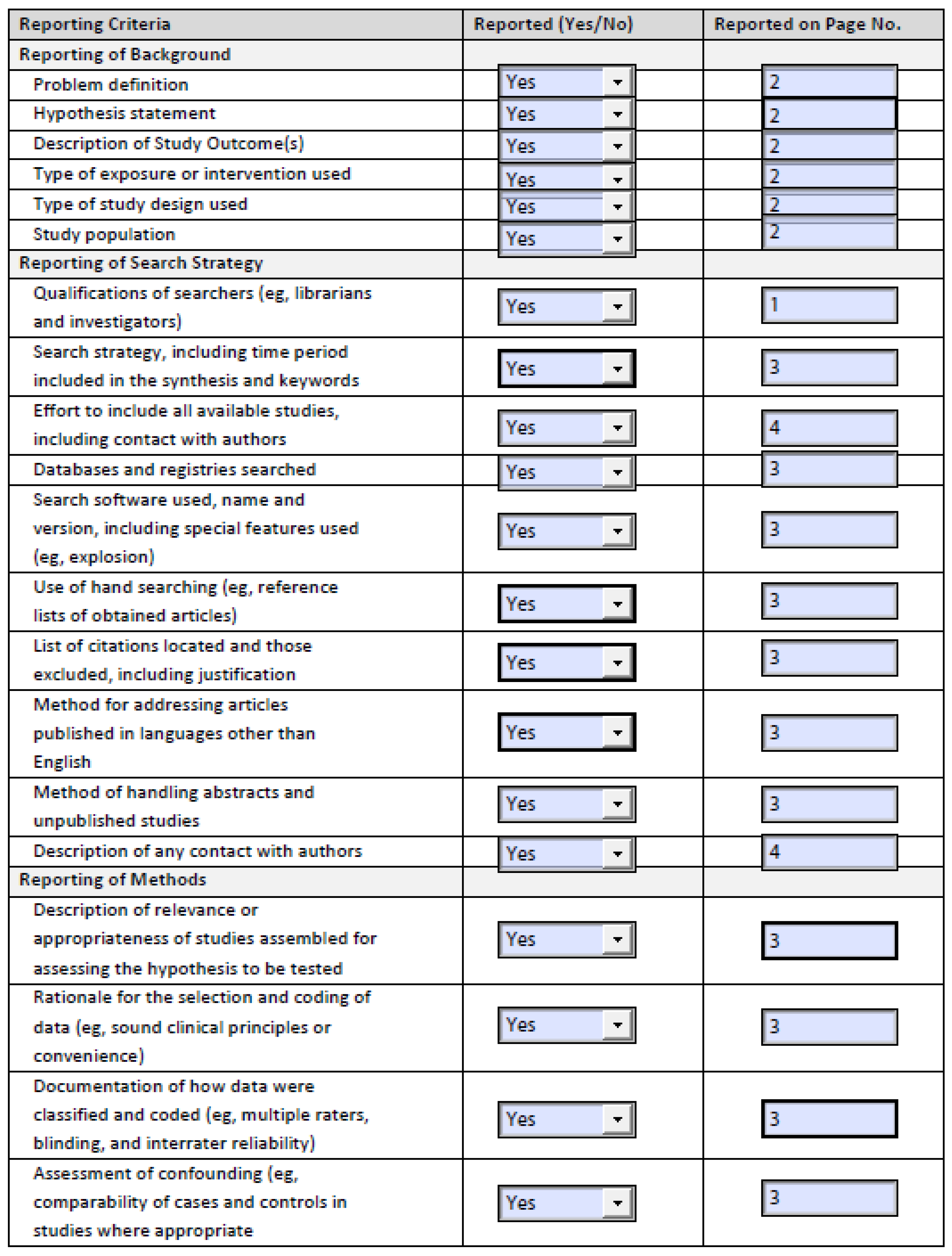
| Study | Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate |
|---|---|---|---|---|---|---|---|---|
| Kinney (2001) [20] | Low Risk | Low Risk | Low Risk | Unclear | Unclear/High Risk | Low Risk | Low Risk | Low Risk |
| Massimino (2013) [21] | Low Risk | Unclear/High Risk | Low Risk | Low Risk | Low risk | Low Risk | Low Risk | Low Risk |
| Condron (2016) [22] | Low Risk | Unclear/High Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Woltering (2016) [23] | Low Risk | Unclear/High Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Fouche (2018) [24] | Unclear/High Risk | Low Risk | Low Risk | Unclear/High Risk | Unclear/High Risk | Low Risk | Low Risk | Low Risk |
| Kinney (2018) [25] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Condron (2018) [26] | Low Risk | Unclear/High Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Kwon (2019) [27] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Study | Long-Acting Release (LAR) Octreotide | Preoperative Bolus of Octreotide | Preoperative Infusion of Octreotide | Intraoperative Bolus of Octreotide | Intraoperative Infusion of Octreotide | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (n/d) Patients | Dose | Duration | % (n/d) Patients | Dose | % (n/d) Patients | Infusion Rate | Duration | % (n/d) Patients | Dose | % (n/d) Patients | Infusion Rate | ||
| Kinney (2001) [20] | 26% (31/119) | Median 300 μg (range 50–1000 μg) | 38% (45/119) | Median 350 μg (range 30–4000 μg) | - | ||||||||
| Massimino (2013) [21] | 72% (70/97) | 90% (87/97) | Median 500 μg (range 100–1100 μg) | 52% (50/97) | Median 350 μg (range 100–5500 μg) | 8% (8/97) | Intraoperative bolus and intraoperative infusion frequencies and percentages are calculated, not directly stated in article. | ||||||
| Condron (2016) [22] | Yes (% not specified) | 100% (150/150) | 500 μg | Yes (% not specified) | 100% (150/150) | 500 μg/h | 86% (129/150) compliance of octreotide protocol. | ||||||
| Woltering (2016) [23] | 83% (149/179) | 100% (179/179) | 500 μg | 100% (179/179) | 500 μg/h | 100% (179/179) | 500 μg/h | - | |||||
| Fouché (2018) [24] | Yes (% not specified) | 40 μg/h 80 μg/h if patient had prior carcinoid syndrome, hepatic metastases, or carcinoid heart disease | 12–48 h | Yes (% not specified) | 0.5–2 μg/kg (if patient has ioCS) | Yes (% not specified) | 40 μg/h 80 μg/h if patient had prior carcinoid syndrome, hepatic metastases, or carcinoid heart disease | Difficult to understand how the hourly dose was given preoperatively. 79% (64/81) compliance of octreotide protocol. | |||||
| Kinney (2018) [25] | 28% (48/169) | 77% (130/169) | 500 μg | 23% (39/169) | Median 500 μg (IQR 250, 650) | Overlapping short- and long-acting; unclear % receiving prophylaxis. | |||||||
| Condron (2018) [26] | 100% (46/46) | 30 mg (3 patients received 20 mg, 1 patient received 10 mg) | At least 28 days | 100% (46/46) | 500 μg | 100% (46/46) | 500 μg/h | ||||||
| Kwon (2019) [27] | 59% (44/75) | 28% (21/75) | Median 150 μg (range 100–300 μg) | 36% (27/75) | Median 150 μg/h (range 50–300 μg/h) | 27% (20/75) | Median 150 μg (range 20–510 μg) | 64% (48/75) | Median 150 μg/h (range 50–300 μg/h) | Other strategies included H-blockers. | |||
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, J.R.; Cardona, K.; Fraker, D.L.; Kebebew, E.; Untch, B.R.; Wang, Y.Z.; Law, C.H.; Liu, E.H.; Kim, M.K.; Menda, Y.; et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017, 46, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Schurr, P.G.; Strate, T.; Rese, K.; Kaifi, J.T.; Reichelt, U.; Petri, S.; Kleinhans, H.; Yekebas, E.F.; Izbicki, J.R. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: An institutional experience. Ann. Surg. 2007, 245, 273–281. [Google Scholar] [CrossRef]
- Kulke, M.H.; Siu, L.L.; Tepper, J.E.; Fisher, G.; Jaffe, D.; Haller, D.G.; Ellis, L.M.; Benedetti, J.K.; Bergsland, E.K.; Hobday, T.J.; et al. Future Directions in the Treatment of Neuroendocrine Tumors: Consensus Report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting. J. Clin. Oncol. 2011, 29, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.; Raoof, M.; Ituarte, P.H.G.; Williams, J.; Melstrom, L.; Li, D.; Lee, B.; Singh, G. Resection of the Primary Gastrointestinal Neuroendocrine Tumor Improves Survival with or Without Liver Treatment. Ann. Surg. 2019, 270, 1131–1137. [Google Scholar] [CrossRef]
- Tierney, J.F.; Chivukula, S.V.; Wang, X.; Pappas, S.G.; Schadde, E.; Hertl, M.; Poirier, J.; Keutgen, X.M. Resection of primary tumor may prolong survival in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2018, 165, 644–651. [Google Scholar] [CrossRef]
- Basuroy, R.; Srirajaskanthan, R.; Ramage, J. A Multimodal Approach to the Management of Neuroendocrine Tumour Liver Metastases. Int. J. Hepatol. 2012, 2012, 819193. [Google Scholar] [CrossRef]
- Ejaz, A.; Reames, B.N.; Maithel, S.; Poultsides, G.A.; Bauer, T.W.; Fields, R.C.; Weiss, M.J.; Marques, H.P.; Aldrighetti, L.; Pawlik, T.M. Cytoreductive debulking surgery among patients with neuroendocrine liver metastasis: A multi-institutional analysis. HPB 2018, 20, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Janson, E.T.; Kianmanesh, R.; Kos-Kudła, B.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2017, 105, 245–254. [Google Scholar] [CrossRef]
- Wonn, S.M.; Pommier, R.F. Carcinoid Crisis: History, Dogmas, and Data. In Neuroendocrine Tumors; Cloyd, J.M., Pawlik, T.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 87–103. [Google Scholar]
- Kahil, M.E.; Brown, H.; Fred, H.L. The Carcinoid Crisis. Arch Intern. Med. 1964, 114, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, K.; Kaye, A.D.; Boudreaux, J.P.; Fox, C.J.; Lang, P.; Kalarickal, P.L.; Gomez, S.; Primeaux, P.J. Carcinoid syndrome and perioperative anesthetic considerations. J. Clin. Anesthesia 2011, 23, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Mayer, R.J. Carcinoid tumors. N. Engl. J. Med. 1999, 340, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.C.; Carter, R.F.; Wright, P.D. Somatostatin, anaesthesia, and the carcinoid syndrome. Peri-operative administration of a somatostatin analogue to suppress carcinoid tumour activity. Anaesthesia 1987, 42, 627–632. [Google Scholar] [CrossRef]
- Seymour, N.; Sawh, S.C. Mega-dose intravenous octreotide for the treatment of carcinoid crisis: A systematic review. Can. J. Anaesth. 2013, 60, 492–499. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for As-sessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://wwwohrica/programs/clinical_epidemiology/oxfordasp (accessed on 23 July 2021).
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Kinney, M.A.O.; Warner, M.E.; Nagorney, D.M.; Rubin, J.; Schroeder, D.R.; Maxson, P.M. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br. J. Anaesth. 2001, 87, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Massimino, K.; Harrskog, O.; Pommier, S.; Pommier, R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J. Surg. Oncol. 2013, 107, 842–846. [Google Scholar] [CrossRef]
- Condron, M.E.; Pommier, S.J.; Pommier, R.F. Continuous infusion of octreotide combined with perioperative octreotide bolus does not prevent intraoperative carcinoid crisis. Surgery 2016, 159, 358–367. [Google Scholar] [CrossRef]
- Woltering, E.A.; Wright, A.E.; Stevens, M.A.; Wang, Y.-Z.; Boudreaux, J.P.; Mamikunian, G.; Riopelle, J.M.; Kaye, A.D. Development of effective prophylaxis against intraoperative carcinoid crisis. J. Clin. Anesthesia 2016, 32, 189–193. [Google Scholar] [CrossRef]
- Fouché, M.; Bouffard, Y.; Le Goff, M.-C.; Prothet, J.; Malavieille, F.; Sagnard, P.; Christin, F.; Hayi-Slayman, D.; Pasquer, A.; Poncet, G.; et al. Intraoperative carcinoid syndrome during small-bowel neuroendocrine tumour surgery. Endocr. Connect. 2018, 7, 1245–1250. [Google Scholar] [CrossRef] [Green Version]
- Kinney, M.A.; Nagorney, D.M.; Clark, D.F.; O’Brien, T.D.; Turner, J.D.; Marienau, M.E.; Schroeder, D.R.; Martin, D.P. Partial hepatic resections for metastatic neuroendocrine tumors: Perioperative outcomes. J. Clin. Anesthesia 2018, 51, 93–96. [Google Scholar] [CrossRef]
- Condron, M.E.; Jameson, N.E.; Limbach, K.E.; Bingham, A.E.; Sera, V.A.; Anderson, R.B.; Schenning, K.; Yockelson, S.; Harukuni, I.; Kahl, E.A.; et al. A prospective study of the pathophysiology of carcinoid crisis. Surgery 2018, 165, 158–165. [Google Scholar] [CrossRef]
- Kwon, D.H.; Paciorek, A.; Mulvey, C.K.; Chan, H.; Fidelman, N.; Meng, L.; Nakakura, E.K.; Zhang, L.; Bergsland, E.K.; Van Loon, K. Periprocedural Management of Patients Undergoing Liver Resection or Embolotherapy for Neuroendocrine Tumor Metastases. Pancreas 2019, 48, 496–503. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Jones, W.P.G. Serotonin release from carcinoid tumours. Can. J. Anaesth. Soc. J. 1962, 9, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Bean, W.B.; Funk, D. The vasculo-cardiac syndrome of metastatic carcinoid. AMA Arch. Intern Med. 1959, 103, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.G.; Filsoufi, F.; Adams, D.H.; Raikhelkar, J.; Zaku, B.; Fischer, G.W. Management of patients undergoing mul-tivalvular surgery for carcinoid heart disease: The role of the anaesthetist. Br. J. Anaesth. 2008, 101, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Wonn, S.M.; Ratzlaff, A.N.; Pommier, S.J.; McCully, B.H.; Pommier, R.F. A prospective study of carcinoid crisis with no perioperative octreotide. Surgery 2021, 171, 88–93. [Google Scholar] [CrossRef]
- Naraev, B.G.; Halland, M.; Halperin, D.M.; Purvis, A.J.; O’Dorisio, T.M.; Halfdanarson, T. Management of Diarrhea in Patients with Carcinoid Syndrome. Pancreas 2019, 48, 961–972. [Google Scholar] [CrossRef]
- Giraldi, L.; Vecchioni, A.; Carioli, G.; Bilotta, M.; La Rosa, S.; Imperatori, A.; Volante, M.; Brizzi, M.P.; Inzani, F.; Petrone, G.; et al. Risk factors for pancreas and lung neuroendocrine neoplasms: A case–control study. Endocrine 2021, 71, 233–241. [Google Scholar] [CrossRef]
- Hackeng, W.M.; Dreijerink, K.M.; de Leng, W.W.; Morsink, F.H.; Valk, G.D.; Vriens, M.R.; Offerhaus, G.J.A.; Geisenberger, C.; Brosens, L.A. Genome Methylation Accurately Predicts Neuroendocrine Tumor Origin: An Online Tool. Clin. Cancer Res. 2021, 27, 1341–1350. [Google Scholar] [CrossRef]
| Study | Study Design | Study Population | N * | Definition of CC | Incidence of CC | Comments |
|---|---|---|---|---|---|---|
| Kinney (2001) [20] | Retrospective Single institution January 1983–December 1996 | Tumor Type: Metastatic carcinoid tumors Carcinoid Syndrome: Not specified, patients with preoperative carcinoid syndrome symptoms included Carcinoid Heart Disease: 20.2% (24/119) Surgical Procedures: Hepatic arterial ligation, resection or biopsy of hepatic metastases, hepatic carcinoid cryotherapy, and small or large bowel resection or diversion—alone or combined | 119 | Flushing, urticaria, ventricular fibrillation, SBP < 80 mmHg for >10 min, bronchospasm, acidosis (pH < 7.2), tachycardia (pulse > 120 bpm). | 6.7% (8/119) | 8 patients had intraoperative complication. 15 patients had perioperative complication or postoperative death. |
| Massimino (2013) [21] | Retrospective Single institution January 2007–January 2011 | Tumor Type: Gastrointestinal carcinoid tumors, metastases included Carcinoid Syndrome: 58.8% (57/97) Carcinoid Heart Disease: 2.1% (2/97) Surgical Procedures: Abdominal operations including hepatic resection, bowel resection, cholecystectomy, resection of mesenteric mass, and others | 97 | SBP ≤ 80 mmHg for ≥10 min, report of hemodynamic instability (hypotension, sustained hypertension, or tachycardia) not due to acute blood loss or other obvious causes, or if anesthesiologist or attending surgeon declared carcinoid crisis occurred in anesthesia record or operative report. | 24% (23/97) | 18 patients had prolonged hypotension. 5 patients had hemodynamic instability consistent with carcinoid crisis. |
| Condron (2016) [22] | Prospective Single institution January 2011–August 2014 | Tumor Type: Carcinoid tumors, metastases included Carcinoid Syndrome: 74% (111/150) Carcinoid Heart Disease: 3% (5/150) Surgical Procedures: Bowel resection, hepatic resection, resection of mesenteric nodal mass, and others | 150 (127 patients) | SBP < 80 or > 180 mmHg, heart rate > 120 beats per minute, or if patient displayed physiology that would be expected to cause end organ dysfunction if sustained. Not attributable to other causes. Consensus of the surgeon and attending anesthesiologist necessary to declare crisis. | 30% (45/150) | - |
| Woltering (2016) [23] | Retrospective Single institution May 2006–March 2012 | Tumor Type: Small bowel neuroendocrine tumors with distant metastases Carcinoid Syndrome: 85.2% (150/176) “potential” carcinoid syndrome Carcinoid Heart Disease: N/A Surgical Procedures: Not specified | 179 (150 patients) | SBP < 80 mmHg for >10 min that could not be explained by other factors. Anesthesia or surgical record noting intraoperative hemodynamic instability (hypertension, hypotension, or tachycardia) or containing the word “crisis” was further reviewed for possible carcinoid crisis. | 3.4% (6/179) | Operations described as “cytoreductive surgeries.” No additional details. |
| Fouché (2018) [24] | Retrospective Single institution January 2007–December 2015 | Tumor Type: Small bowel neuroendocrine tumors, metastases included Carcinoid Syndrome: 60.4% (49/81) Carcinoid Heart Disease: 8.6% (7/81) Surgical Procedures: Small bowel neuroendocrine tumor resections (operations for other neuroendocrine tumor location or for hepatic metastases only were excluded); 12/81 had liver resection | 81 | Highly probable intraoperative carcinoid syndrome (ioCS): Rapid (≤ 5 min) heart rate or arterial blood pressure changes ≥40%, not explained by surgical/anesthetic management and regressive ≥20% after octreotide bolus injection. Probable ioCS: Did not meet all criteria of highly probable ioCS. Suspected ioCS: Anesthesia record has octreotide injection due to manifestation that did not meet criteria for highly probable or probable ioCS. Carcinoid crisis: Life-threatening ioCS refractory to octreotide boluses. | ioCS: 55.6% (45/81) CC: 0% (0/81) | Main outcome is intraoperative carcinoid syndrome (ioCS). Multiple instances of ioCS recorded (139 instances for 45 patients). Authors note octreotide protocol was respected for 64 patients; 11 patients had lower doses. |
| Kinney (2018) [25] | Retrospective Single institution January 1997–June 2015 | Tumor Type: Neuroendocrine tumors with liver metastases Carcinoid Syndrome: Yes (no % given) Carcinoid Heart Disease: 8.3% (14/169) Surgical Procedures: Partial hepatectomy (major and minor)/ablation, +/−small bowel resection, +/−hepatic artery ligation | 196 (169 patients) | Sudden onset of at least 2: Flushing or urticaria not explained by an allergic reaction, bronchospasm or bronchodilator administration, SBP < 80 mmHg for >10 min not explained by volume status or hemorrhage and treated with pressors, dysrhythmia not explained by volume status or hemorrhage, or pulse > 120 bpm. | 0% (0/196) | 26 patients did not qualify as having a carcinoid crisis because they experienced only 1 of the criteria. Tachycardia was the most common criteria met. |
| Condron (2018) [26] | Prospective Single institution 2015–2017 | Tumor Type: Small bowel or lung carcinoid tumor with liver metastases Carcinoid Syndrome: 65.2% (30/46) Carcinoid Heart Disease: Excluded Surgical Procedures: Elective abdominal operations including hepatic debulking, prophylactic cholecystectomy, resection of primary tumor, resection of mesenteric nodal mass | 46 | SBP < 80 or > 180 mmHg, pulse > 120 bpm, or if patient displayed physiology expected to cause end organ dysfunction if sustained. Not attributable to other causes. Consensus of the surgeon and attending anesthesiologist necessary. | 35% (16/46) | - |
| Kwon (2019) [27] | Retrospective Single institution June 2012–December 2016 | Tumor Type: Neuroendocrine tumors with liver metastases Carcinoid Syndrome: 46.7% (35/75) Carcinoid Heart Disease: 10.7% (8/75) Surgical Procedures: Liver resection, other (thermal ablation, bland hepatic artery embolization, chemoembolization, Yttrium-90 radioembolization) | 75 | Carcinoid crisis (CC): Documentation of CC by any treating physician. Hemodynamic instability (HDI): >10 min of SBP < 80, > 180 mmHg, or pulse > 120 bpm not attributed to blood loss or other causes. | 32% (24/75) | 1 patient had CC alone. 21 patients had HDI alone. 2 patients had both CC and HDI. |
| Study | Risk Factors Evaluated | Risk Factors Associated |
|---|---|---|
| Massimino (2013) [21] | Unadjusted: Carcinoid syndrome, epidural catheter, epidural infusion, induction agents (propofol, etomidate, thiopental), hepatic metastases, hepatic resection, outpatient octreotide Adjusted: Age, gender, hepatic metastases, hepatic resection, epidural catheter | Unadjusted: Hepatic metastases (p ≤ 0.01), hepatic resection (p = 0.03), epidural catheter (p = 0.04) Adjusted: Hepatic metastases (p-value not specified) * |
| Condron (2016) [22] | Unadjusted: Age, estimated blood loss (EBL), duration of anesthesia, sex, carcinoid syndrome, carcinoid heart disease, location of primary tumor, hepatic metastases, mesenteric metastases, peritoneal metastases, ASA Adjusted: Age, ASA-3, ASA-4, carcinoid syndrome, carcinoid heart disease, duration of anesthesia, sex, hepatic metastases, mesenteric metastases, other metastases, primary tumor location, EBL | Unadjusted: EBL (p = 0.005), duration of anesthesia (p = 0.001), carcinoid syndrome (p = 0.006), hepatic metastases (p = 0.02) Adjusted: Age (p = 0.045), carcinoid syndrome (p = 0.014), duration of anesthesia (p = 0.022), hepatic metastases (p = 0.037) |
| Woltering (2016) [23] | Unadjusted: Hypertension, heart condition, potential carcinoid syndrome, preoperative SSA therapy | Unadjusted: Hypertension (CC 100% vs. no CC 55%) ** |
| Fouché (2018) [24] | Unadjusted: Carcinoid syndrome, preoperative diarrhea, preoperative cutaneous flush, carcinoid heart disease, hepatic metastases, elevated preoperative output of 5-hydroxylindoleacetic acid, premedication with antihistamine, intraoperative vasopressor use (ephedrine, phenylephrine, noradrenaline), hepatic resection Adjusted: N/A | Unadjusted: None significant |
| Condron (2018) [26] | Unadjusted: Age, sex, carcinoid syndrome, operative procedures, resection of mesenteric nodal mass, volume of hepatic metastases, estimated volume debulked, duration of anesthesia, estimated blood loss, preincision hemodynamics, preincision serotonin, preincision histamine, preincision kallikrein, preincision bradykinin, dose of octreotide LAR at time of operation, duration of long-acting SSA treatment prior to operation Adjusted: Age, anesthesia time, estimated blood loss, preincision serotonin, preincision histamine, preincision kallikrein, preincision bradykinin | Unadjusted: Preincision serotonin (p = 0.0064) Adjusted: Preincision serotonin (OR 1.1 [95% CI 1.01–1.19], p = 0.015) |
| Kwon (2019) [27] | Unadjusted and adjusted: Age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG), primary tumor location, tumor grade, extent of hepatic involvement by metastases, history of carcinoid syndrome, CGA greater than 2 x upper limit of normal and Urine 5-HIAA greater than 2 x upper limit of normal, long-acting SSA use in prior month | Unadjusted and adjusted: None significant *** |
| Study | Prophylactic Octreotide | Prophylactic Octreotide Strategy and % Patients | Other Octreotide Use | Risk Reduction (Y/N) and Strength of Association | Comments |
|---|---|---|---|---|---|
| Kinney (2001) [20] | Yes | Preoperative Bolus: 26% (31/119), median 300 μg, (range 50–1000 μg) | Intraoperative Bolus: 38% (45/119), median 350μg (range 30–4000 μg). Unclear if intraoperative bolus was administered as part of prevention technique or as treatment. | Intraoperative octreotide: Yes (p = 0.023) | FDA approved octreotide in 1988. Analysis on 1988–1996 data only (not all patients included), and intraoperative octreotide reduced risk (p = 0.010). No predictors of efficacy. |
| Massimino (2013) [21] | Yes | Preoperative Bolus: 90% (87/97), median 500 μg (range 100–1100 μg) Intraoperative Infusion: 8% (8/97), dose unspecified | Intraoperative Bolus: 52% (50/97), median 350 μg (range 100–5500 μg). Intraoperative bolus available as therapy. | Preoperative bolus: No (p = 0.77) | No predictors of efficacy. |
| Condron (2016) [22] | Yes | Preoperative Bolus: 100% (150/150), 500 μg Intraoperative Infusion: 100% (150/150), 500 μg/h 86% (129/150) compliance of octreotide protocol | Intraoperative Bolus: % not specified Intraoperative bolus described as part of crisis management. | 100% of patients received prophylactic octreotide—no comparison group. | |
| Woltering (2016) [23] | Yes | Preoperative Bolus: 100% (179/179), 500 μg Preoperative, intraoperative, and postoperative infusion: 100% (179/179), 500 μg/h | Intraoperative boluses are kept on hand and administered as necessary. Unclear what would trigger administration. | 100% of patients received prophylactic octreotide—no comparison group. | |
| Fouché (2018) [24] | Yes | 40 μg/h (80 μg/h if prior carcinoid syndrome, hepatic metastases, or carcinoid heart disease) infusion 12–48 h prior to operation. Same dose continued for intraoperative infusion. 79% (64/81) compliance of octreotide protocol | Intraoperative Bolus: 0.5–2 μg/kg (if patient has ioCS). Intraoperative boluses were administered to treat IoCS. | Octreotide protocol was respected in 64 patients and 11 patients had lower doses. No details about octreotide administration for remaining 6 patients. No clear control group. | |
| Kinney (2018) [25] | Yes | Preoperative Bolus: 77% (130/169), 500 μg | Intraoperative Bolus: 23% (39/169), median 500 μg (IQR 250, 650). Unclear if intraoperative bolus was administered as part of prevention technique or as treatment. | Did not evaluate efficacy of prophylactic octreotide | The clinical availability and use of SA and LAR octreotide changed over duration of study. |
| Condron (2018) [26] | Yes | Preoperative Bolus: 100% (46/46), 500 μg Intraoperative Infusion: 100% (46/46), 500 μg/h | None. | 100% of patients received prophylactic octreotide—no comparison group. No predictors of efficacy. | |
| Kwon (2019) [27] | Yes | Preoperative Bolus: 28% (21/75), median 150 μg (range 100–300 μg) Preoperative Infusion: 36% (27/75), median 150 μg/h (range 50–300 μg/h) Intraoperative Infusion: 64% (48/75), median 150 μg/h (range 50–300 μg/h) | Intraoperative Bolus: 27% (20/75), median 150 μg (range 20–510 μg). Unclear if intraoperative bolus was administered as part of prevention technique or as treatment. | Preoperative octreotide: No (p = 0.52) Intraoperative octreotide: No (p = 0.85) Preoperative or Intraoperative octreotide: No (p = 0.60) | No predictors of efficacy. |
| Study | Mortality Rate | Post-Operative Complication Rate | Incomplete Operation/Aborted Procedure Rate | Average Length of Stay | Comments |
|---|---|---|---|---|---|
| Massimino (2013) [21] | Unadjusted: CC 0/23 vs. no CC 2/74 | Unadjusted: Any complication: CC 60.9% vs. No CC 31.1% (p = 0.01) Major complication: CC 39.1% vs. No CC 14.9% | N/A | N/A | Postoperative period = 30 days Also significant different between minor and major complications for CC and no CC (p = 0.02) Major complication = Dindo Grade III+ |
| Condron (2016) [22] | N/A | Unadjusted: Major complication: p = 0.481 Adjusted: Major complication: OR 0.94 [95% CI 0.23–3.67] p = 0.93 | Unadjusted: CC 3/45 vs. no CC 0/105 | N/A | Postoperative period length not specified Major complication = Dindo Grade III+ |
| Fouché (2018) [24] | Unadjusted: CC 1/45 vs. no CC 0/36 Adjusted: N/A | N/A | N/A | N/A | Postoperative period length not specified No statistical analysis |
| Condron (2018) [26] | N/A | Unadjusted: Any: CC 50% vs. No CC 46.7% (p = 0.829) Clavien–Dindo I-II: CC 37.5% vs. No CC 26.7% (p = 0.447) Clavien–Dindo III-IV: CC 12.5% vs. No CC 20% (p = 0.523) | N/A | Unadjusted: Mean LOS: CC 11.6 vs. no CC 8.3 (p = 0.315) | Postoperative period length not specified |
| Kwon (2019) [27] | N/A | Unadjusted: Clavien–Dindo II-IV: CC 42% vs. No CC 16% (p = 0.01) Postoperative pulmonary embolism: CC 8% vs. No CC 0% (p = 0.04) Postoperative tachyarrhythmia requiring nodal blocker: CC 8% vs. No CC 0% (p = 0.04) | N/A | Unadjusted: Median LOS: CC 5.5 vs. no CC 4.0 (p = 0.13) | Postoperative period not specified Other outcomes evaluated that did not have association: hypotension requiring vasopressors, bleeding or coagulopathy requiring transfusions, hypoxemia requiring intensive care, infection, acute kidney injury, endoscopic or radiologic procedure, surgery, other |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Suz, P.; Reljic, T.; Are, A.C.; Kumar, A.; Powers, B.; Strosberg, J.; Denbo, J.W.; Fleming, J.B.; Anaya, D.A. Perioperative Carcinoid Crisis: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2966. https://doi.org/10.3390/cancers14122966
Xu A, Suz P, Reljic T, Are AC, Kumar A, Powers B, Strosberg J, Denbo JW, Fleming JB, Anaya DA. Perioperative Carcinoid Crisis: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(12):2966. https://doi.org/10.3390/cancers14122966
Chicago/Turabian StyleXu, Aileen, Pilar Suz, Tea Reljic, Abhirup C. Are, Ambuj Kumar, Benjamin Powers, Jonathan Strosberg, Jason W. Denbo, Jason B. Fleming, and Daniel A. Anaya. 2022. "Perioperative Carcinoid Crisis: A Systematic Review and Meta-Analysis" Cancers 14, no. 12: 2966. https://doi.org/10.3390/cancers14122966
APA StyleXu, A., Suz, P., Reljic, T., Are, A. C., Kumar, A., Powers, B., Strosberg, J., Denbo, J. W., Fleming, J. B., & Anaya, D. A. (2022). Perioperative Carcinoid Crisis: A Systematic Review and Meta-Analysis. Cancers, 14(12), 2966. https://doi.org/10.3390/cancers14122966