Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Cases
Inclusion Criteria
2.2. Statistical Analysis
3. Results
3.1. Patient Data and Overall Incidence and Survival Trends
3.1.1. GI adenocarcinomas Trends across Populations
3.1.2. Survival by Time Trends and Site in Indigenous Compared to Non-Indigenous Peoples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreto, S.G.; Pandol, S.J. Young-Onset Carcinogenesis—The Potential Impact of Perinatal and Early Life Metabolic Influences on the Epigenome. Front. Oncol. 2021, 11, 653289. [Google Scholar] [CrossRef] [PubMed]
- Done, J.Z.; Fang, S.H. Young-Onset Colorectal Cancer: A Review. World J. Gastrointest. Oncol. 2021, 13, 856–866. [Google Scholar] [CrossRef] [PubMed]
- El Din, K.S.; Loree, J.; Sayre, E.C.; Gill, S.; Brown, C.; Dau, H.; De Vera, M.A. Trends in the epidemiology of young-onset colorectal cancer: A worldwide systematic review. BMC Cancer 2020, 20, 288. [Google Scholar] [CrossRef] [Green Version]
- di Martino, E.; Smith, L.; Bradley, S.H.; Hemphill, S.; Wright, J.; Renzi, C.; Bergin, R.; Emery, J.; Neal, R.D. Incidence trends for twelve cancers in younger adults—A rapid review. Br. J. Cancer 2022, 126, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Schell, D.; Ullah, S.; Brooke-Smith, M.E.; Hollington, P.; Yeow, M.; Karapetis, C.S.; Watson, D.I.; Pandol, S.; Roberts, C.T.; Barreto, S.G. Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017. Cancers 2022, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Singal, A.G.; Baron, J.A.; Sandler, R.S. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology 2018, 155, 1716–1719.e4. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Coppola, G.; Palma, G.; Barbieri, A.; Luciano, A.; Del Prete, P.; Rossetti, S.; Berretta, M.; Facchini, G.; Perdonà, S.; et al. Microbiota effects on cancer: From risks to therapies. Oncotarget 2018, 9, 17915–17927. [Google Scholar] [CrossRef] [Green Version]
- Barreto, S.G. We Asked the Experts: Providing the Road Map to Uncovering the Pathophysiology of Young-Onset Cancer to Guide Treatment and Preventive Strategies. World J. Surg. 2020, 44, 3212–3213. [Google Scholar] [CrossRef] [PubMed]
- Arık, A.; Dodd, E.; Cairns, A.; Streftaris, G. Socioeconomic disparities in cancer incidence and mortality in England and the impact of age-at-diagnosis on cancer mortality. PLoS ONE 2021, 16, e0253854. [Google Scholar] [CrossRef]
- Hoebel, J.; Kroll, L.E.; Fiebig, J.; Lampert, T.; Katalinic, A.; Barnes, B.; Kraywinkel, K. Socioeconomic Inequalities in Total and Site-Specific Cancer Incidence in Germany: A Population-Based Registry Study. Front. Oncol. 2018, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. The Health and Welfare of Australia’s Aboriginal and Torres Strait Islander People, an Overview 2011; Contract No Cat. No Ihw 42; AIHW: Canberra, Australia, 2011.
- Ryder, C.; Mackean, T.; Hunter, K.; Towers, K.; Rogers, K.; Holland, A.J.A.; Ivers, R. Factors contributing to longer length of stay in Aboriginal and Torres Strait Islander children hospitalised for burn injury. Inj. Epidemiol. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Devi, S. Native American health left out in the cold. Lancet 2011, 377, 1481–1482. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Mortality and Life Expectancy of Indigenous Australian’s 2008 to 2012; Contract No Cat. No Ihw 140; AIHW: Canberra, Australia, 2014.
- Hill, K.; Barker, B.; Vos, T. Excess Indigenous Mortality: Are Indigenous Australians More Severely Disadvantaged Than Other Indigenous Populations? Int. J. Epidemiol. 2007, 36, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Chong, A.; Roder, D. Exploring differences in survival from cancer among Indigenous and non-Indigenous Australians: Implications for health service delivery and research. Asian Pac. J. Cancer Prev. 2010, 11, 953–961. [Google Scholar] [PubMed]
- Condon, J.R.; Barnes, T.; Armstrong, B.K.; Selva-Nayagam, S.; Elwood, J.M. Stage at diagnosis and cancer survival for Indigenous Australians in the Northern Territory. Med. J. Aust. 2005, 182, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.P.; O’Rourke, P.K.; Mallitt, K.-A.; Garvey, G.; Green, A.C.; Coory, M.D.; Valery, P.C. Cancer incidence and mortality in Indigenous Australians in Queensland, 1997-2006. Med. J. Aust. 2010, 193, 590–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valery, P.C.; Coory, M.; Stirling, J.; Green, A. Cancer diagnosis, treatment, and survival in Indigenous and non-Indigenous Australians: A matched cohort study. Lancet 2006, 367, 1842–1848. [Google Scholar] [CrossRef]
- Zhang, X.; Condon, J.R.; Rumbold, A.R.; Cunningham, J.; Roder, D.M. Estimating cancer incidence in Indigenous Australians. Aust. N. Z. J. Public Health 2011, 35, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Brands, J.; Garvey, G.; Anderson, K.; Cunningham, J.; Chynoweth, J.; Wallington, I.; Morris, B.; Knott, V.; Webster, S.; Kinsella, L.; et al. Development of a National Aboriginal and Torres Strait Islander Cancer Framework: A Shared Process to Guide Effective Policy and Practice. Int. J. Environ. Res. Public Health 2018, 15, 942. [Google Scholar] [CrossRef] [Green Version]
- Condon, J.R.; Zhang, X.; Baade, P.; Griffiths, K.; Cunningham, J.; Roder, D.M.; Coory, M.; Jelfs, P.L.; Threlfall, T. Cancer survival for Aboriginal and Torres Strait Islander Australians: A national study of survival rates and excess mortality. Popul. Health Metrics 2014, 12, 1. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Australia’s Health 2016; Australia’s Health Series No. 15. Cat. No. AUS 199; AIHW: Canberra, Australia, 2016.
- Australian Institute of Health and Welfare. Profile of Indigenous Australians; AIHW: Canberra, Australia, 2020.
- Northern Territory Legislation. Cancer (Registration) Act. Available online: https://legislation.nt.gov.au/legislation/cancer-registration-act-2009 (accessed on 5 June 2022).
- Breau, G.; Ellis, U. Risk Factors Associated with Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control 2020, 27, 1073274820976670. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, Y.S.; Yang, H.-J.; Park, S.-K.; Park, J.H.; Park, D.I.; Sohn, C.I. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clin. Gastroenterol. Hepatol. 2018, 17, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.-H.; Wu, K.; Ng, K.; Zauber, A.G.; Nguyen, L.; Song, M.; He, X.; Fuchs, C.S.; Ogino, S.; Willett, W.C.; et al. Association of Obesity with Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019, 5, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine Tay, E.E.; Guthridge, S.; Zhang, X. Preventable Cancers in the Northern Territory—A Snapshot. Chronicle 2010, 17, 2–3. [Google Scholar]
- Australian Bureau of Statistics. Overweight and Obesity, Australia. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/overweight-and-obesity/latest-release (accessed on 5 June 2022).
- Chakrabarti, S.; Peterson, C.Y.; Sriram, D.; Mahipal, A. Early Stage Colon Cancer: Current Treatment Standards, Evolving Paradigms, and Future Directions. World J. Gastrointest. Oncol. 2020, 12, 808–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, I.-H.; Kim, J.S.; Kim, S.W.; Kim, J.G.; Oh, S.T.; Kang, W.K.; Lee, M.A. Different clinical characteristics in sporadic young-age onset colorectal cancer. Medicine 2016, 95, e4840. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.; Leiting, J.L.; Habermann, E.B.; Cleary, S.; Kendrick, M.L.; Smoot, R.L.; Nagorney, D.M.; Truty, M.J.; Grotz, T.E. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery 2019, 166, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; La Torre, M.; et al. Early Onset Pancreatic Cancer: Risk Factors, Presentation and Outcome. Pancreatology 2015, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ntala, C.; Debernardi, S.; Feakins, R.M.; Crnogorac-Jurcevic, T. Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC Gastroenterol. 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, J.-B.; Feletto, E.; Worthington, J.; Roder, D.; Canuto, K.; Miller, C.; D’Onise, K.; Canfell, K. The potential for tailored screening to reduce bowel cancer mortality for Aboriginal and Torres Strait Islander peoples in Australia: Modelling study. J. Cancer Policy 2022, 32, 100325. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.C.; Wirtalla, C.; Armstrong, K.; Mahmoud, N.N. Gender Influences Treatment and Survival in Colorectal Cancer Surgery. Dis. Colon Rectum 2009, 52, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H.; Gekid Cancer Survival Working Group. Sex Differences in Colorectal Cancer Survival: Population-Based Analysis of 164,996 Colorectal Cancer Patients in Germany. PLoS ONE 2013, 8, e68077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Bureau of Statistics. Smoking. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/smoking/latest-release#data-download (accessed on 5 June 2022).
- Alcohol. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/alcohol-consumption/latest-release (accessed on 5 June 2022).
- National Health Survey: First Results: Overweight and Obesity. 4364.0.55.001. 2019. Available online: https://iepcp.org.au/wp-content/uploads/2019/01/4364.0.55.001-national-health-survey-first-results-2017-18.pdf (accessed on 5 June 2022).
- Ward, M.C.; Agarwal, A.; Bish, M.; James, R.; Faulks, F.; Pitson, J.; Yuen, N.; Mnatzaganian, G. Trends in obesity and impact on obstetric outcomes in a regional hospital in Victoria, Australia. Aust. N. Z. J. Obstet. Gynaecol. 2019, 60, 204–211. [Google Scholar] [CrossRef] [Green Version]
- McCall, S.J.; Li, Z.; Kurinczuk, J.J.; Sullivan, E.; Knight, M. Binational Cohort Study Comparing the Management and Outcomes of Pregnant Women with a Bmi > 50–59.9 Kg/M(2) and Those with a Bmi ≥ 60 Kg/M(2). BMJ Open 2018, 8, e021055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Q.D.; Hsieh, M.-C.; Gibbs, J.F.; Wu, X.-C. Social determinants of health associated with poor outcome for rural patients following resected pancreatic cancer. J. Gastrointest. Oncol. 2021, 12, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Dhahri, A.; Kaplan, J.; Naqvi, S.M.H.; Brownstein, N.C.; Ntiri, S.O.; Imanirad, I.; Felder, S.I.; Dineen, S.P.; Sanchez, J.; Dessureault, S.; et al. The impact of socioeconomic status on survival in stage III colon cancer patients: A retrospective cohort study using the SEER census-tract dataset. Cancer Med. 2021, 10, 5643–5652. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.; Crengle, S.; Kamaka, M.L.; Chen, T.H.; Palafox, N.; Jackson-Pulver, L. Indigenous Health in Australia, New Zealand, and the Pacific. Lancet 2006, 367, 1775–1785. [Google Scholar] [CrossRef]
- Australian Government. Australian Instititute of Health and Welfare. Indigenous Life Expectancy and Deaths. Available online: https://www.aihw.gov.au/reports/australias-health/indigenous-life-expectancy-and-deaths (accessed on 9 April 2022).
- Moore, S.P.; Antoni, S.; Colquhoun, A.; Healy, B.; Ellison-Loschmann, L.; Potter, J.D.; Garvey, G.; Bray, F. Cancer Incidence in Indigenous People in Australia, New Zealand, Canada, and the USA: A Comparative Population-Based Study. Lancet Oncol. 2015, 16, 1483–1492. [Google Scholar] [CrossRef]
- Banham, D.; Roder, D.; Eckert, M.; Howard, N.J.; Canuto, K.; Brown, A. Cancer treatment and the risk of cancer death among Aboriginal and non-Aboriginal South Australians: Analysis of a matched cohort study. BMC Health Serv. Res. 2019, 19, 771. [Google Scholar] [CrossRef]
- Diaz, A.; Whop, L.J.; Valery, P.C.; Moore, S.P.; Cunningham, J.; Garvey, G.; Condon, J.R. Cancer outcomes for Aboriginal and Torres Strait Islander Australians in rural and remote areas. Aust. J. Rural Health 2015, 23, 4–18. [Google Scholar] [CrossRef]
- Cunningham, J.; Rumbold, A.R.; Zhang, X.; Condon, J. Incidence, aetiology, and outcomes of cancer in Indigenous peoples in Australia. Lancet Oncol. 2008, 9, 585–595. [Google Scholar] [CrossRef]
- Siegel, J.; Engelhardt, K.E.; Hornor, M.A.; Morgan, K.A.; Lancaster, W.P. Travel distance and its interaction with patient and hospital factors in pancreas cancer care. Am. J. Surg. 2020, 221, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.P.; Green, A.C.; Bray, F.; Garvey, G.; Coory, M.; Martin, J.; Valery, P.C. Survival disparities in Australia: An analysis of patterns of care and comorbidities among indigenous and non-indigenous cancer patients. BMC Cancer 2014, 14, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, M. The Voice of Indigenous Data: Beyond the Markers of Disadvantage. Griffith Rev. 2018, 60, 256. [Google Scholar]
- Walter, M.; Suina, M. Indigenous data, indigenous methodologies and indigenous data sovereignty. Int. J. Soc. Res. Methodol. 2018, 22, 233–243. [Google Scholar] [CrossRef]
- Salmon, M.; Skelton, F.; Thurber, K.A.; Bennetts Kneebone, L.; Gosling, J.; Lovett, R.; Walter, M. Intergenerational and Early Life Influences on the Well-Being of Australian Aboriginal and Torres Strait Islander Children: Overview and Selected Findings from Footprints in Time, the Longitudinal Study of Indigenous Children. J. Dev. Orig. Health Dis. 2019, 10, 17–23. [Google Scholar] [CrossRef]
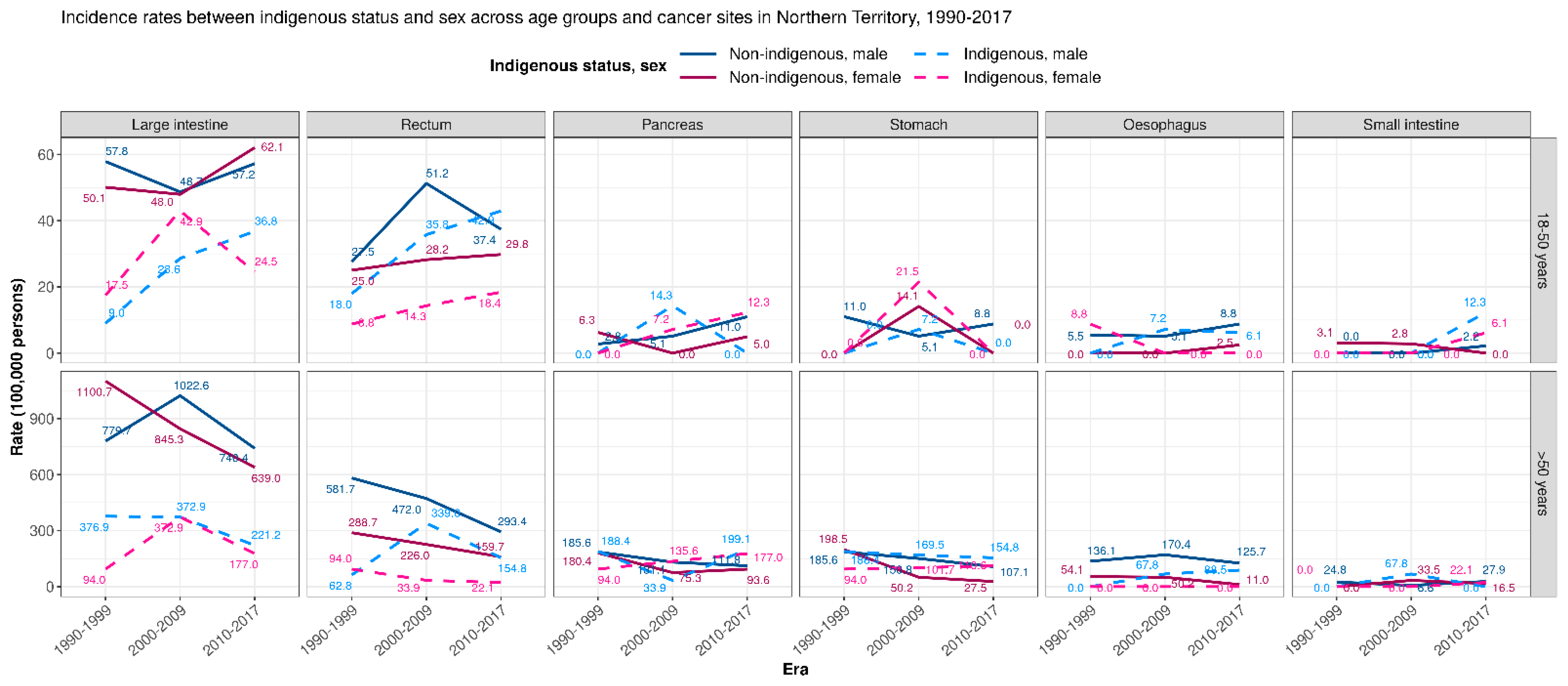
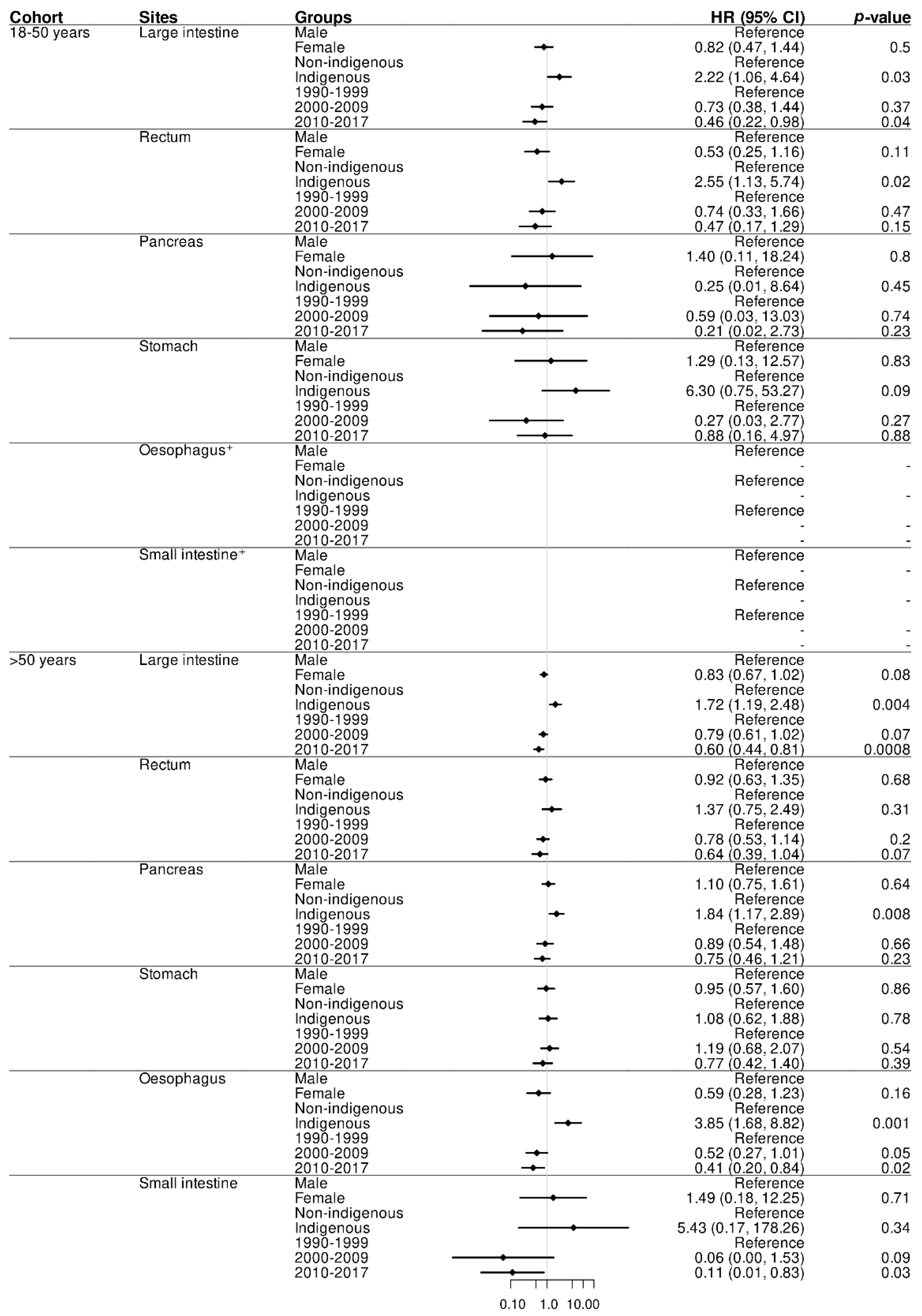
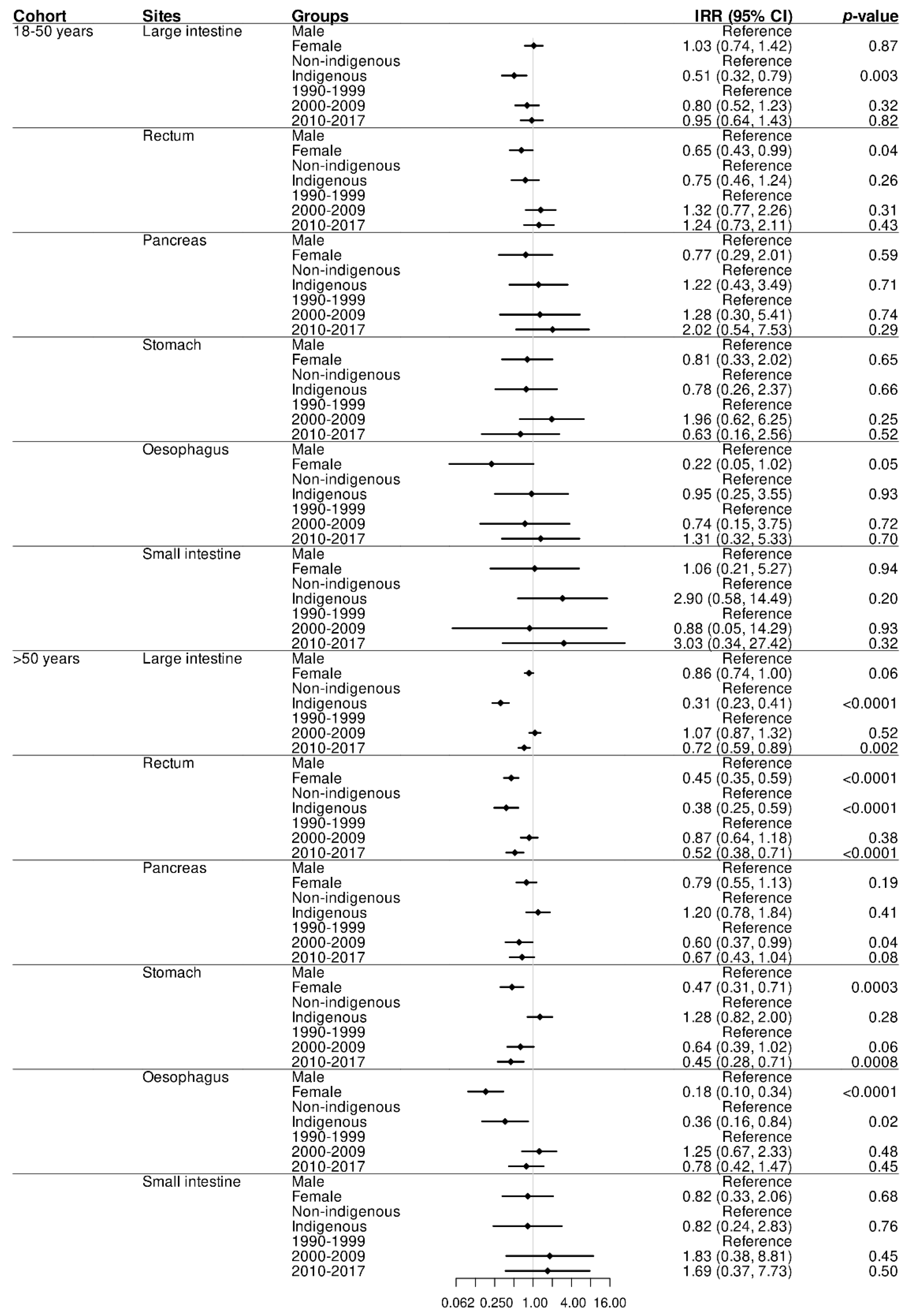
| (18–50 Years) | (>50 Years) | ||||
|---|---|---|---|---|---|
| n = 298 (18.5%) | n = 1310 (81.5%) | ||||
| n | % | n | % | p-Value | |
| Age (years): median (IQR) | 45 | (39–49) | 64 | (58–72) | <0.0001 |
| Sex | 0.07 | ||||
| Male | 172 | 57.7 | 834 | 63.7 | |
| Female | 126 | 42.3 | 476 | 36.3 | |
| Indigenous | <0.0001 | ||||
| Non-Indigenous | 240 | 80.5 | 1179 | 90.0 | |
| Indigenous | 58 | 19.5 | 131 | 10.0 | |
| Era | 0.4 | ||||
| 1990–1999 | 72 | 24.2 | 275 | 21.0 | |
| 2000–2009 | 103 | 34.6 | 501 | 38.2 | |
| 2010–2017 | 123 | 41.3 | 534 | 40.8 | |
| Primary site | 0.0006 | ||||
| Large intestine (excl. Appendix) | 147 | 49.3 | 704 | 53.7 | |
| Rectum | 97 | 32.6 | 276 | 21.1 | |
| Pancreas | 17 | 5.7 | 122 | 9.3 | |
| Stomach | 19 | 6.4 | 108 | 8.2 | |
| Oesophagus | 12 | 4.0 | 81 | 6.2 | |
| Small intestine | 6 | 2.0 | 19 | 1.5 | |
| (18–50 Years) | (>50 Years) | |||||
|---|---|---|---|---|---|---|
| n = 298 | n = 1310 | |||||
| * IR (95% CI) | IRR (95% CI) | p-Value | * IR (95% CI) | IRR (95% CI) | p-Value | |
| Overall | 95.64 (85.09, 107.14) | 1321.70 (1251.09, 1395.26) | ||||
| Age (years) | - | 1.15 (1.13, 1.17) | <0.0001 | - | 1.06 (1.06, 1.07) | <0.0001 |
| Sex | ||||||
| Male | 5.39 (4.09, 7.10) | Reference | - | 196.86 (172.34, 224.87) | Reference | - |
| Female | 4.41 (3.31, 5.87) | 0.82 (0.65, 1.03) | 0.09 | 131.05 (113.74, 151.01) | 0.67 (0.59, 0.75) | <0.0001 |
| Indigenous | ||||||
| Non-Indigenous | 5.91 (4.61, 7.56) | Reference | - | 232.99 (209.68, 258.88) | Reference | - |
| Indigenous | 4.02 (2.87, 5.62) | 0.68 (0.51, 0.91) | 0.009 | 110.73 (91.44, 134.09) | 0.48 (0.40, 0.57) | <0.0001 |
| Era | ||||||
| 1990–1999 | 4.66 (3.40, 6.38) | Reference | - | 189.49 (161.17, 222.79) | Reference | - |
| 2000–2009 | 4.83 (3.56, 6.57) | 1.04 (0.77, 1.41) | 0.81 | 177.91 (154.23, 205.23) | 0.94 (0.81, 1.09) | 0.40 |
| 2010–2017 | 5.14 (3.82, 6.90) | 1.10 (0.82, 1.48) | 0.51 | 122.92 (106.80, 141.48) | 0.65 (0.56, 0.75) | <0.0001 |
| Cancer site | ||||||
| Large intestine | 27.22 (21.68, 34.18) | - | - | 900.31 (807.56, 1003.71) | - | - |
| Rectum | 17.96 (13.91, 23.19) | 351.68 (304.95, 405.58) | ||||
| Pancreas | 3.15 (1.91, 5.20) | - | - | 156.02 (128.44, 189.53) | - | - |
| Stomach | 3.52 (2.18, 5.67) | - | - | 138.12 (112.54, 169.50) | - | - |
| Oesophagus | 2.22 (1.23, 4.00) | - | - | 103.59 (82.15, 130.63) | - | - |
| Small intestine | 1.11 (0.49, 2.51) | - | - | 24.30 (15.39, 38.36) | - | - |
| Cancer Sites | (18–50 Years) | (>50 Years) |
|---|---|---|
| n = 298 (18.5%) | n = 1310 (81.5%) | |
| Median (95% CI) | Median (95% CI) | |
| Overall | 3.37 (1.59–8.66) | 2.91 (1.24–7.46) |
| Large intestine | 3.75 (1.79–9.97) | 4.17 (1.75–8.45) |
| Rectum | 3.98 (1.90–9.62) | 4.06 (1.67–9.03) |
| Pancreas | 1.59 (0.97–5.59) | 0.98 (0.56–1.49) |
| Stomach | 1.51 (1.12–2.79) | 1.55 (0.85–2.81) |
| Oesophagus | 2.19 (1.56–3.56) | 1.64 (1.06–2.57) |
| Small intestine | 1.73 (0.80–4.19) | 1.86 (0.82–4.98) |
| (18–50 Years) | (>50 Years) | |||
|---|---|---|---|---|
| n = 298 | n = 1310 | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years) | 1.02 (1.00, 1.05) | 0.07 | 1.04 (1.03, 1.05) | <0.0001 |
| Sex | ||||
| Male | Reference | - | Reference | - |
| Female | 0.88 (0.62, 1.27) | 0.5 | 0.84 (0.72, 0.98) | 0.03 |
| Indigenous | ||||
| Non-Indigenous | Reference | - | Reference | - |
| Indigenous | 2.06 (1.36, 3.11) | 0.0007 | 1.66 (1.32, 2.08) | <0.0001 |
| Era | ||||
| 1990–1999 | Reference | - | Reference | - |
| 2000–2009 | 0.63 (0.42, 0.97) | 0.03 | 0.81 (0.68, 0.97) | 0.02 |
| 2010–2017 | 0.45 (0.29, 0.72) | 0.0007 | 0.64 (0.52, 0.78) | <0.0001 |
| Cancer site | ||||
| Large intestine | Reference | Reference | ||
| Rectum | 0.94 (0.63, 1.42) | 0.78 | 1.01 (0.82, 1.23) | 0.95 |
| Pancreas | 2.30 (1.19, 4.46) | 0.01 | 5.76 (4.58, 7.24) | <0.0001 |
| Stomach | 2.73 (1.49, 5.02) | 0.001 | 2.91 (2.28, 3.70) | <0.0001 |
| Oesophagus | 1.87 (0.88, 3.98) | 0.1 | 3.29 (2.51, 4.30) | <0.0001 |
| Small intestine | 1.88 (0.59, 6.02) | 0.29 | 2.01 (1.10, 3.68) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherdson, M.; Leemaqz, S.; Singh, G.; Ryder, C.; Ullah, S.; Canuto, K.; Young, J.P.; Price, T.J.; McKinnon, R.A.; Pandol, S.J.; et al. Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples. Cancers 2022, 14, 2870. https://doi.org/10.3390/cancers14122870
Shepherdson M, Leemaqz S, Singh G, Ryder C, Ullah S, Canuto K, Young JP, Price TJ, McKinnon RA, Pandol SJ, et al. Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples. Cancers. 2022; 14(12):2870. https://doi.org/10.3390/cancers14122870
Chicago/Turabian StyleShepherdson, Mia, Shalem Leemaqz, Gurmeet Singh, Courtney Ryder, Shahid Ullah, Karla Canuto, Joanne P. Young, Timothy J. Price, Ross A. McKinnon, Stephen J. Pandol, and et al. 2022. "Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples" Cancers 14, no. 12: 2870. https://doi.org/10.3390/cancers14122870
APA StyleShepherdson, M., Leemaqz, S., Singh, G., Ryder, C., Ullah, S., Canuto, K., Young, J. P., Price, T. J., McKinnon, R. A., Pandol, S. J., Roberts, C. T., & Barreto, S. G. (2022). Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples. Cancers, 14(12), 2870. https://doi.org/10.3390/cancers14122870







