Using a Clinicopathologic and Gene Expression (CP-GEP) Model to Identify Stage I–II Melanoma Patients at Risk of Disease Relapse
Abstract
:Simple Summary
Abstract
1. Introduction
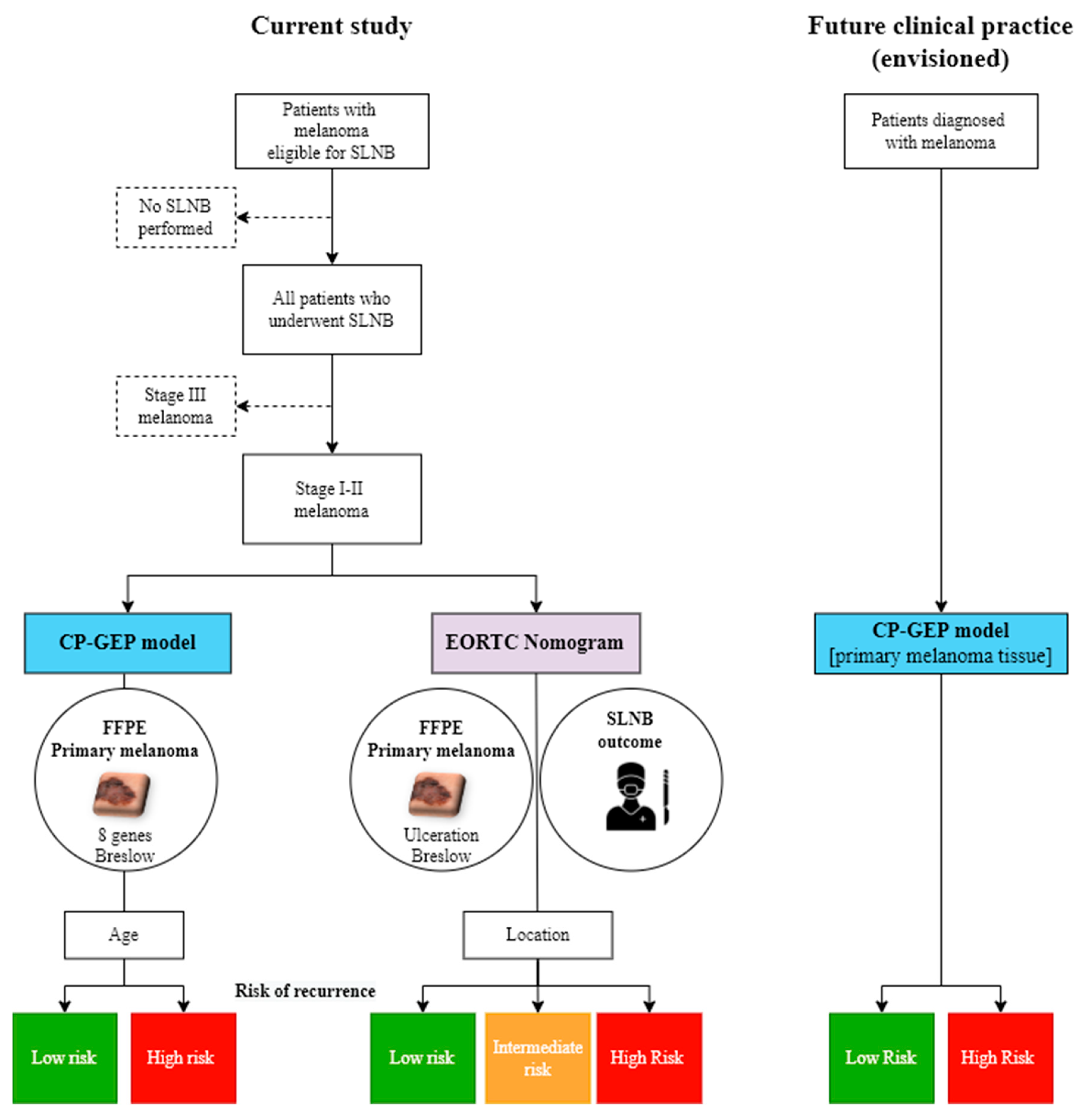
2. Patients and Methods
2.1. Study Population
2.2. CP-GEP Model
2.3. Outcomes
2.4. Statistical Analysis
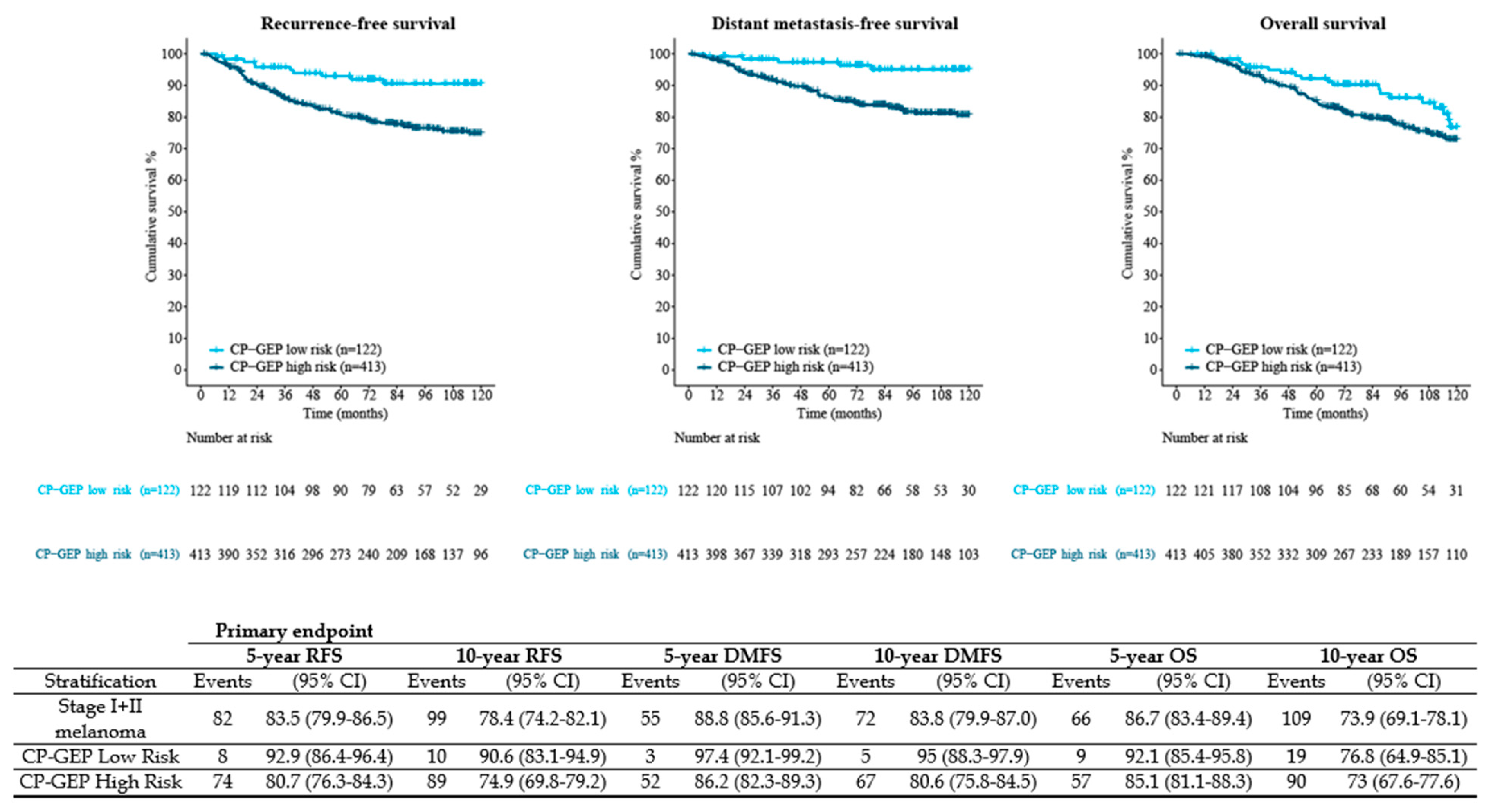
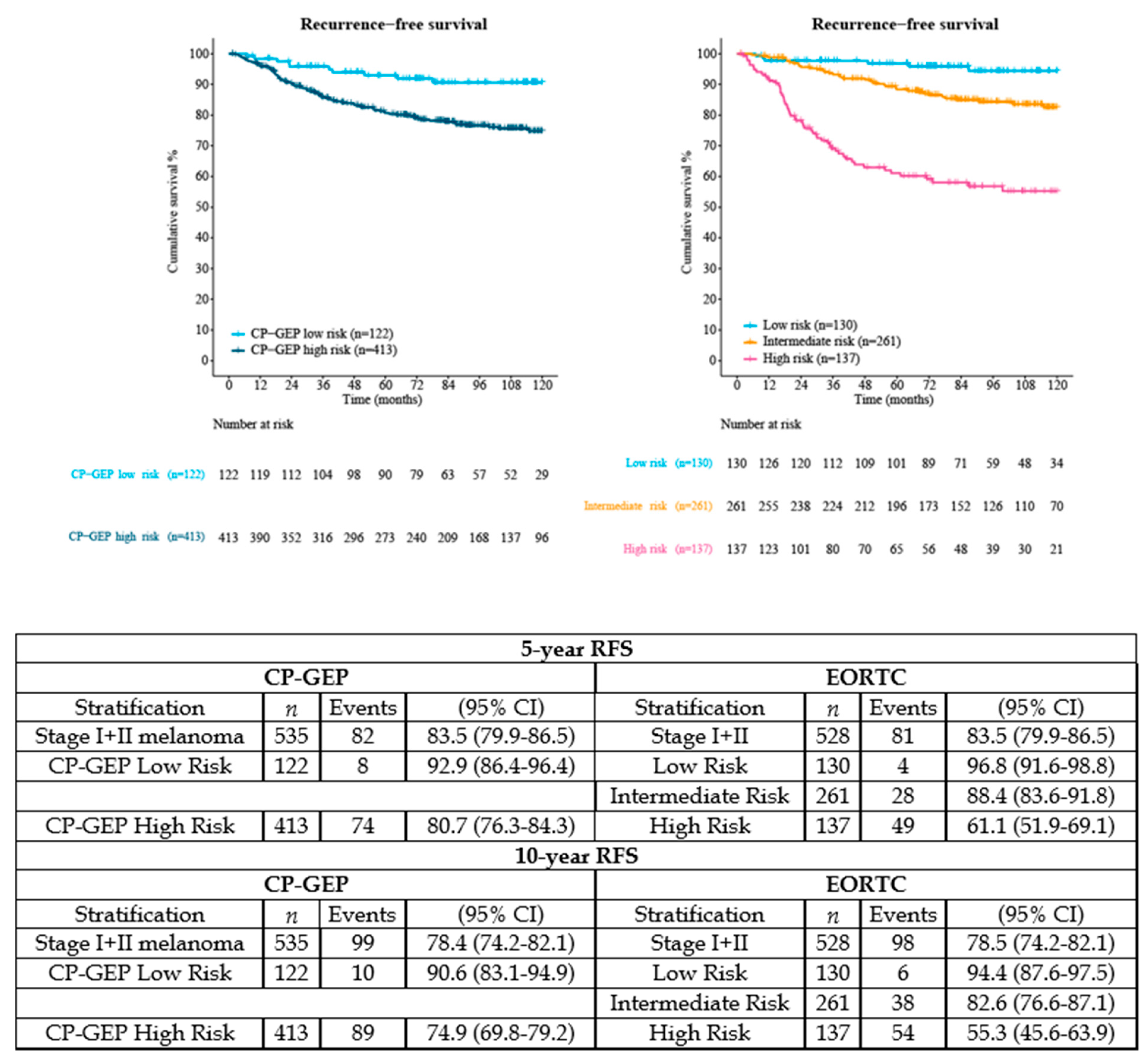
3. Results
3.1. Study Population
3.2. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whiteman, D.C.; Baade, P.D.; Olsen, C.M. More people die from thin melanomas (≤1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J. Investig. Dermatol. 2015, 135, 1190–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landow, S.M.; Gjelsvik, A.; Weinstock, M.A. Mortality burden and prognosis of thin melanomas overall and by subcategory of thickness, SEER registry data, 1992–2013. J. Am. Acad. Dermatol. 2017, 76, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. LBA3_PR—Pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the Keynote 716 double-blinded phase III trial. Ann. Oncol. 2021, 32 (Suppl. 5), S1283–S1346. [Google Scholar]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, V.G.; Hauschild, A.; Santinami, M.; Mandala, M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.S.; Dutriaux, C.; Haydon, A.; Mortier, L.; et al. Adverse events (AEs) over time in patients (pts) treated with adjuvant dabrafenib plus trametinib (D + T) or placebo (Pbo) in the COMBI-AD trial. Ann. Oncol. 2018, 29, viii446. [Google Scholar] [CrossRef]
- Gorry, C.; McCullagh, L.; Barry, M. Economic Evaluation of Systemic Treatments for Advanced Melanoma: A Systematic Review. Value Health 2020, 23, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Mulder, E.E.A.P.; Smit, L.; Grunhagen, D.J.; Verhoef, C.; Sleijfer, S.; van der Veldt, A.A.M.; Uyl-de Groot, C.A. Cost-effectiveness of adjuvant systemic therapies for patients with high-risk melanoma in Europe: A model-based economic evaluation. ESMO Open 2021, 6, 100303. [Google Scholar] [CrossRef]
- Verver, D.; van Klaveren, D.; Franke, V.; van Akkooi, A.C.J.; Rutkowski, P.; Keilholz, U.; Eggermont, A.M.M.; Nijsten, T.; Grünhagen, D.J.; Verhoef, C. Development and validation of a nomogram to predict recurrence and melanoma-specific mortality in patients with negative sentinel lymph nodes. Br. J. Surg. 2019, 106, 217–225. [Google Scholar] [CrossRef]
- Verver, D.; van Klaveren, D.; van Akkooi, A.C.J.; Rutkowski, P.; Powell, B.; Robert, C.; Testori, A.; van Leeuwen, B.L.; van der Veldt, A.A.M.; Keilholz, U.; et al. Risk stratification of sentinel node-positive melanoma patients defines surgical management and adjuvant therapy treatment considerations. Eur. J. Cancer 2018, 96, 25–33. [Google Scholar] [CrossRef]
- El Sharouni, M.-A.; Ahmed, T.; Varey, A.H.R.; Elias, S.G.; Witkamp, A.J.; Sigurdsson, V.; Suijkerbuijk, K.P.M.; van Diest, P.J.; Scolyer, R.A.; van Gils, C.H.; et al. Development and Validation of Nomograms to Predict Local, Regional, and Distant Recurrence in Patients With Thin (T1) Melanomas. J. Clin. Oncol. 2021, 39, 1243–1252. [Google Scholar] [CrossRef]
- El Sharouni, M.A.; Ahmed, T.; Witkamp, A.J.; Sigurdsson, V.; van Gils, C.H.; Nieweg, O.E.; Scolyer, R.A.; Thompson, J.F.; van Diest, P.J.; Lo, S.N. Predicting recurrence in patients with sentinel node-negative melanoma: Validation of the EORTC nomogram using population-based data. Br. J. Surg. 2021, 108, 550–553. [Google Scholar] [CrossRef]
- Amaral, T.M.S.; Hoffmann, M.-C.; Sinnberg, T.; Niessner, H.; Sülberg, H.; Eigentler, T.K.; Garbe, C. Clinical validation of a prognostic 11-gene expression profiling score in prospectively collected FFPE tissue of patients with AJCC v8 stage II cutaneous melanoma. Eur. J. Cancer 2020, 125, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Gerami, P.; Cook, R.W.; Wilkinson, J.; Russell, M.C.; Dhillon, N.; Amaria, R.N.; Gonzalez, R.; Lyle, S.; Johnson, C.E.; Oelschlager, K.M.; et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin. Cancer Res. 2015, 21, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Garg, M.; Couturier, D.-L.; Nsengimana, J.; Fonseca, N.A.; Wongchenko, M.; Yan, Y.; Lauss, M.; Jönsson, G.B.; Newton-Bishop, J.; Parkinson, C.; et al. Tumour gene expression signature in primary melanoma predicts long-term outcomes. Nat. Commun. 2021, 12, 1137. [Google Scholar] [CrossRef]
- Bellomo, D.; Arias-Mejias, S.M.; Ramana, C.; Heim, J.B.; Quattrocchi, E.; Sominidi-Damodaran, S.; Bridges, A.G.; Lehman, J.S.; Hieken, T.J.; Jakub, J.W.; et al. Model Combining Tumor Molecular and Clinicopathologic Risk Factors Predicts Sentinel Lymph Node Metastasis in Primary Cutaneous Melanoma. JCO Precis. Oncol. 2020, 4, 319–334. [Google Scholar] [CrossRef]
- Johansson, I.; Tempel, D.; Dwarkasing, J.T.; Rentroia-Pacheco, B.; Mattsson, J.; Ny, L.; Olofsson Bagge, R. Validation of a clinicopathological and gene expression profile model to identify patients with cutaneous melanoma where sentinel lymph node biopsy is unnecessary. Eur. J. Surg. Oncol. 2021, 48, 320–325. [Google Scholar] [CrossRef]
- Meves, A.; Nikolova, E.; Heim, J.B.; Squirewell, E.J.; Cappel, M.A.; Pittelkow, M.R.; Otley, C.C.; Behrendt, N.; Saunte, D.M.; Lock-Andersen, J.; et al. Tumor Cell Adhesion As a Risk Factor for Sentinel Lymph Node Metastasis in Primary Cutaneous Melanoma. J. Clin. Oncol. 2015, 33, 2509–2515. [Google Scholar] [CrossRef]
- Mulder, E.E.A.P.; Dwarkasing, J.T.; Tempel, D.; van der Spek, A.; Bosman, L.; Verver, D.; Mooyaart, A.L.; van der Veldt, A.A.M.; Verhoef, C.; Nijsten, T.E.C.; et al. Validation of a clinicopathological and gene expression profile model for sentinel lymph node metastasis in primary cutaneous melanoma. Br. J. Dermatol. 2021, 184, 944–951. [Google Scholar] [CrossRef]
- Verver, D.; Rekkas, A.; Garbe, C.; van Klaveren, D.; van Akkooi, A.C.J.; Rutkowski, P.; Powell, B.; Robert, C.; Testori, A.; van Leeuwen, B.L.; et al. The EORTC-DeCOG nomogram adequately predicts outcomes of patients with sentinel node-positive melanoma without the need for completion lymph node dissection. Eur. J. Cancer 2020, 134, 9–18. [Google Scholar] [CrossRef]
- Lyth, J.; Mikiver, R.; Nielsen, K.; Isaksson, K.; Ingvar, C. Prognostic instrument for survival outcome in melanoma patients: Based on data from the population-based Swedish Melanoma Register. Eur. J. Cancer 2016, 59, 171–178. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; et al. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Oude Ophuis, C.M.; van Akkooi, A.C.; Rutkowski, P.; Voit, C.A.; Stepniak, J.; Erler, N.S.; Eggermont, A.M.; Wouters, M.W.; Grunhagen, D.J.; Verhoef, C.K. Effects of time interval between primary melanoma excision and sentinel node biopsy on positivity rate and survival. Eur. J. Cancer 2016, 67, 164–173. [Google Scholar] [CrossRef]
- Parrett, B.M.; Accortt, N.A.; Li, R.; Dosanjh, A.S.; Thummala, S.; Kullar, R.; Cleaver, J.E.; Kashani-Sabet, M.; Leong, S.P. The effect of delay time between primary melanoma biopsy and sentinel lymph node dissection on sentinel node status, recurrence, and survival. Melanoma Res. 2012, 22, 386–391. [Google Scholar] [CrossRef]
- Van Akkooi, A.C.; de Wilt, J.H.; Verhoef, C.; Graveland, W.J.; van Geel, A.N.; Kliffen, M.; Eggermont, A.M. High positive sentinel node identification rate by EORTC melanoma group protocol. Prognostic indicators of metastatic patterns after sentinel node biopsy in melanoma. Eur. J. Cancer 2006, 42, 372–380. [Google Scholar] [CrossRef]
- Thakur, R.; Laye, J.P.; Lauss, M.; Diaz, J.M.S.; O’Shea, S.J.; Poźniak, J.; Filia, A.; Harland, M.; Gascoyne, J.; Randerson-Moor, J.A.; et al. Transcriptomic Analysis Reveals Prognostic Molecular Signatures of Stage I Melanoma. Clin. Cancer Res. 2019, 25, 7424–7435. [Google Scholar] [CrossRef] [Green Version]
- Zager, J.S.; Gastman, B.R.; Leachman, S.; Gonzalez, R.C.; Fleming, M.D.; Ferris, L.K.; Ho, J.; Miller, A.R.; Cook, R.W.; Covington, K.R.; et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer 2018, 18, 130. [Google Scholar] [CrossRef]
- Cook, R.W.; Middlebrook, B.; Wilkinson, J.; Covington, K.R.; Oelschlager, K.; Monzon, F.A.; Stone, J.F. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn. Pathol. 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhaw, B.N.; Zitelli, J.A.; Brodland, D.G. Estimation of Prognosis in Invasive Cutaneous Melanoma: An Independent Study of the Accuracy of a Gene Expression Profile Test. Dermatol. Surg. 2018, 44, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, E.C.; DeBloom, J.R.; Lee, J.H.; Sussman, J.J.; Covington, K.R.; Caruso, H.G.; Quick, A.P.; Cook, R.W.; Slingluff, C.L.; McMasters, K.M. Long-Term Outcomes in a Multicenter, Prospective Cohort Evaluating the Prognostic 31-Gene Expression Profile for Cutaneous Melanoma. JCO Precis. Oncol. 2021, 5, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Gastman, B.R.; Gerami, P.; Kurley, S.J.; Cook, R.W.; Leachman, S.; Vetto, J.T. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J. Am. Acad. Dermatol. 2019, 80, 149–157.e144. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.A.; Bartlett, E.K.; Dusza, S.W.; Bichakjian, C.K. Use of a prognostic gene expression profile test for T1 cutaneous melanoma: Will it help or harm patients? J. Am. Acad. Dermatol. 2019, 80, e161–e162. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.A.; Coit, D.G.; Dusza, S.W.; Yu, A.; McLean, L.; Hu, Y.; Nanda, J.K.; Matsoukas, K.; Mancebo, S.E.; Bartlett, E.K. Performance of Gene Expression Profile Tests for Prognosis in Patients With Localized Cutaneous Melanoma: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 953–962. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Fort Washington, U. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Cutaneous Melanoma, Version 2. 2021. Available online: NCCN.org (accessed on 1 June 2021).
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
| Variable | Sahlgrenska University Hospital n = 367 | Erasmus MC Cancer Institute n = 168 | Combined n = 535 | |
|---|---|---|---|---|
| Gender | Female | 181 (49.3%) | 80 (47.6%) | 261 (48.8%) |
| Male | 186 (50.7%) | 87 (51.8%) | 273 (51.0%) | |
| Unknown | 0 (0%) | 1 (0.6%) | 1 (0.2%) | |
| Age (years) | 61 (50–72) | 57 (46–67) | 60 (48–70) | |
| Breslow depth (mm) | 1.80 (1.30, 3.00) | 1.90 (1.30, 3.20) | 1.80 (1.30, 3.00) | |
| Ulceration | Absent | 261 (71.1%) | 122 (72.6%) | 383 (71.6%) |
| Present | 106 (28.9%) | 39 (23.2%) | 145 (27.1%) | |
| Unknown | 0 (0%) | 7 (4.2%) | 7 (1.3%) | |
| Disease stages * | IA | 29 (7.9%) | 10 (6.0%) | 39 (7.3%) |
| IB | 156 (42.5%) | 70 (41.7%) | 226 (42.2%) | |
| IIA | 108 (29.4%) | 40 (23.8%) | 148 (27.7%) | |
| IIB | 44 (12.0%) | 28 (16.7%) | 72 (13.5%) | |
| IIC | 30 (8.2%) | 13 (7.7%) | 43 (8.0%) | |
| Unknown | 0 (0%) | 7 (4.2%) | 7 (1.3%) | |
| Histologic type | SSM | 180 (49.0%) | 101 (60.1%) | 281 (52.5%) |
| NM | 138 (37.6%) | 50 (29.8%) | 188 (35.1%) | |
| LMM | 5 (1.4%) | 1 (0.6%) | 6 (1.1%) | |
| ALM | 4 (1.1%) | 3 (1.8%) | 7 (1.3%) | |
| Other | 40 (10.9%) | 9 (5.4%) | 49 (9.2%) | |
| Unknown | 0 (0%) | 4 (2.4%) | 4 (0.7%) | |
| Biopsy location | Head/Neck | 0 (0%) | 1 (0.6%) | 1 (0.2%) |
| Trunk | 176 (48.0%) | 80 (47.6%) | 256 (47.9%) | |
| Upper extremities | 80 (21.8%) | 30 (17.9%) | 110 (20.6%) | |
| Lower extremities | 111 (30.2%) | 56 (33.3%) | 167 (31.2%) | |
| Unknown | 0 (0%) | 1 (0.6%) | 1 (0.2%) | |
| Clark level | II | 17 (4.6%) | 9 (5.4%) | 26 (4.9%) |
| III | 169 (46.0%) | 46 (27.4%) | 215 (40.2%) | |
| IV | 156 (42.5%) | 58 (34.5%) | 214 (40%) | |
| V | 12 (3.3%) | 6 (3.6%) | 18 (3.4%) | |
| Unknown | 13 (3.5%) | 49 (29.2%) | 62 (11.6%) | |
| Angiolymphatic Invasion | Absent | 0 (0%) | 58 (34.5%) | 58 (10.8%) |
| Present | 0 (0%) | 6 (3.6%) | 6 (1.1%) | |
| Unknown | 367 (100%) | 104 (61.9%) | 471 (88.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulder, E.E.A.P.; Johansson, I.; Grünhagen, D.J.; Tempel, D.; Rentroia-Pacheco, B.; Dwarkasing, J.T.; Verver, D.; Mooyaart, A.L.; van der Veldt, A.A.M.; Wakkee, M.; et al. Using a Clinicopathologic and Gene Expression (CP-GEP) Model to Identify Stage I–II Melanoma Patients at Risk of Disease Relapse. Cancers 2022, 14, 2854. https://doi.org/10.3390/cancers14122854
Mulder EEAP, Johansson I, Grünhagen DJ, Tempel D, Rentroia-Pacheco B, Dwarkasing JT, Verver D, Mooyaart AL, van der Veldt AAM, Wakkee M, et al. Using a Clinicopathologic and Gene Expression (CP-GEP) Model to Identify Stage I–II Melanoma Patients at Risk of Disease Relapse. Cancers. 2022; 14(12):2854. https://doi.org/10.3390/cancers14122854
Chicago/Turabian StyleMulder, Evalyn E. A. P., Iva Johansson, Dirk J. Grünhagen, Dennie Tempel, Barbara Rentroia-Pacheco, Jvalini T. Dwarkasing, Daniëlle Verver, Antien L. Mooyaart, Astrid A. M. van der Veldt, Marlies Wakkee, and et al. 2022. "Using a Clinicopathologic and Gene Expression (CP-GEP) Model to Identify Stage I–II Melanoma Patients at Risk of Disease Relapse" Cancers 14, no. 12: 2854. https://doi.org/10.3390/cancers14122854
APA StyleMulder, E. E. A. P., Johansson, I., Grünhagen, D. J., Tempel, D., Rentroia-Pacheco, B., Dwarkasing, J. T., Verver, D., Mooyaart, A. L., van der Veldt, A. A. M., Wakkee, M., Nijsten, T. E. C., Verhoef, C., Mattsson, J., Ny, L., Hollestein, L. M., & Olofsson Bagge, R. (2022). Using a Clinicopathologic and Gene Expression (CP-GEP) Model to Identify Stage I–II Melanoma Patients at Risk of Disease Relapse. Cancers, 14(12), 2854. https://doi.org/10.3390/cancers14122854










