Long-Term Tubular Dysfunction in Childhood Cancer Survivors; DCCSS-LATER 2 Renal Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
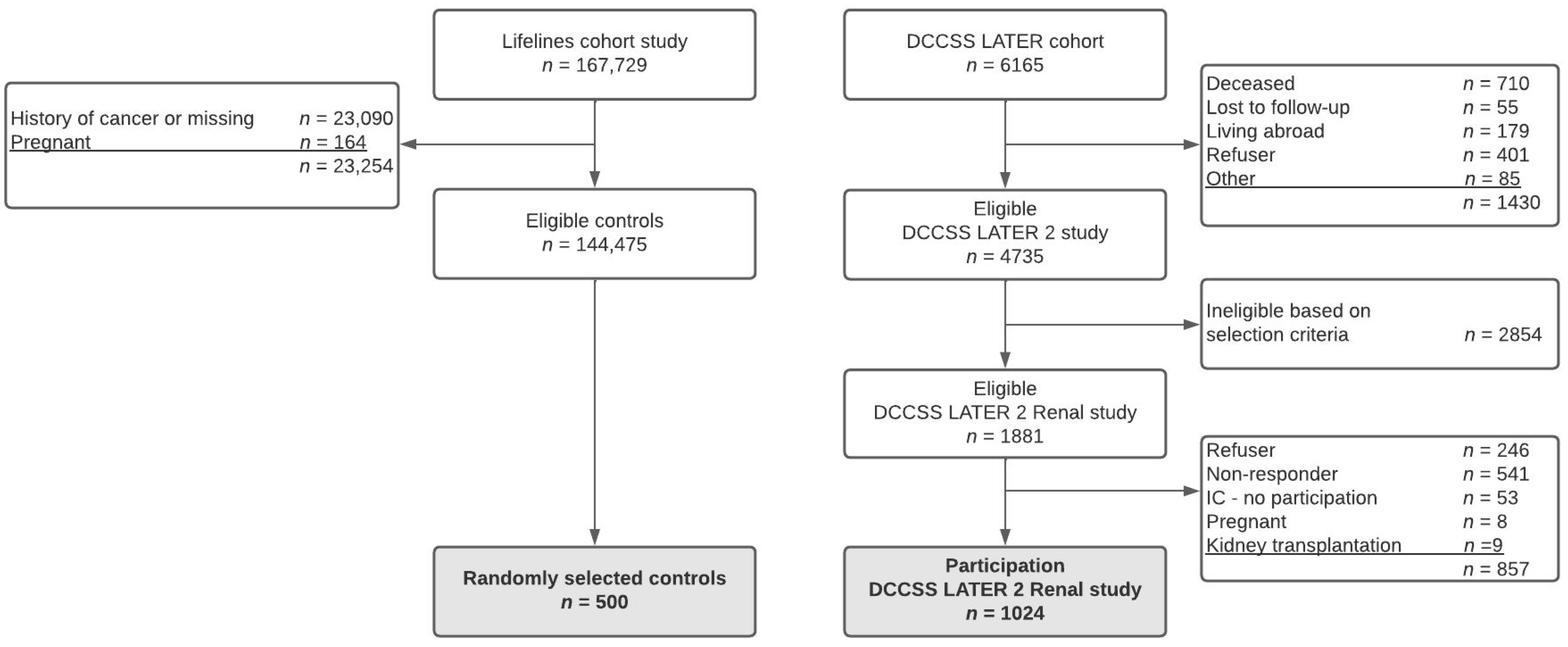
2.1. Study Population
2.2. Controls
2.3. Data Collection
2.4. Definition of Tubular Dysfunction
2.5. Definition of Glomerular Dysfunction
2.6. Statistical Analyses
3. Results
3.1. Study Population
3.2. Prevalence of Tubular Dysfunction
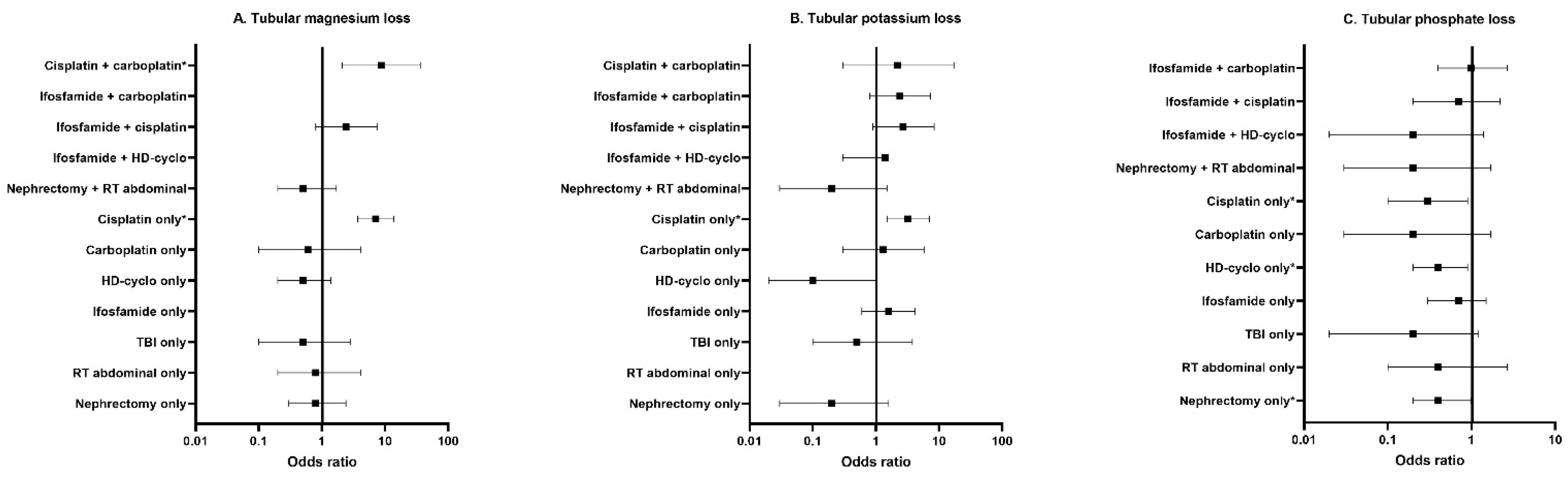
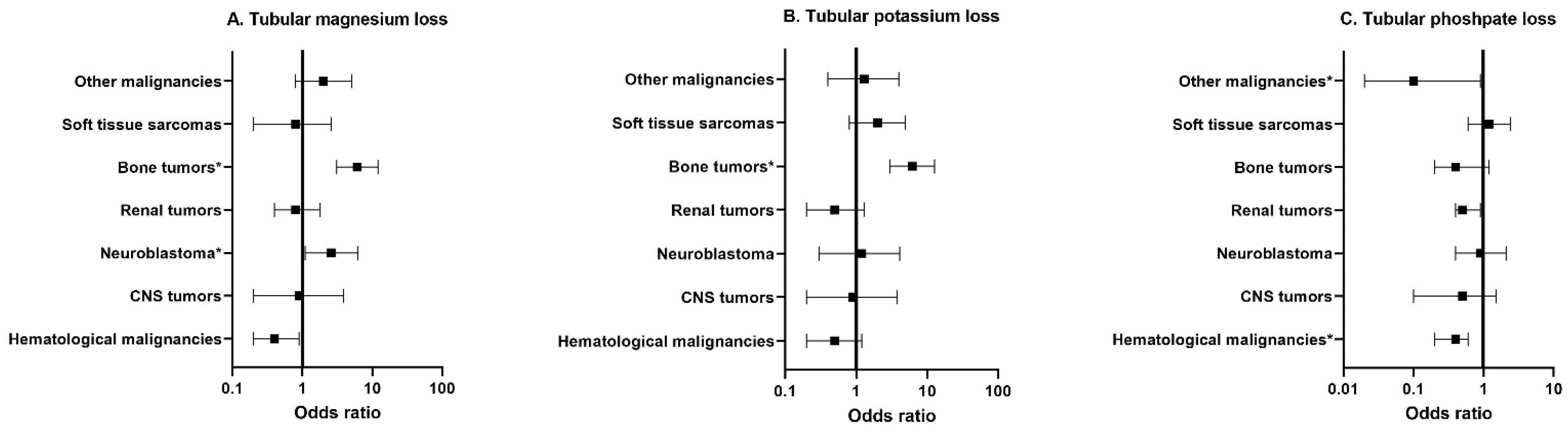
3.3. Risk Factors for Tubular Dysfunction
3.3.1. Risk Factors in CCS Compared to Controls
3.3.2. Tumor Type
3.3.3. Risk Factors among CCS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Kooijmans, E.C.; Bökenkamp, A.; Tjahjadi, N.S.; Tettero, J.M.; van Dulmen-den, E.B.; Van Der Pal, H.J.; Veening, M.A. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst. Rev. 2019, 3, Cd008944. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, M.J.; Skinner, R.; Castellino, S.M. Renal and Hepatic Health After Childhood Cancer. Pediatr. Clin. N. Am. 2020, 67, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Pearson, A.D.; English, M.W.; Price, L.; A Wyllie, R.; Coulthard, M.G.; Craft, A.W. Risk factors for ifosfamide nephrotoxicity in children. Lancet 1996, 348, 578–580. [Google Scholar] [CrossRef]
- Stöhr, W.; Patzer, L.; Paulides, M.; Kremers, A.; Beck, J.-D.; Langer, T.; Rossi, R. Growth impairment after ifosfamide-induced nephrotoxicity in children. Pediatr. Blood Cancer 2007, 48, 571–576. [Google Scholar] [CrossRef]
- Church, D.N.; Hassan, A.B.; Harper, S.J.; Wakeley, C.J.; Price, C.G.A. Osteomalacia as a late metabolic complication of Ifosfamide chemotherapy in young adults: Illustrative cases and review of the literature. Sarcoma 2007, 2007, 91586. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, W.; Paulides, M.; Bielack, S.; Jürgens, H.; Koscielniak, E.; Rossi, R.; Langer, T.; Beck, J. Nephrotoxicity of cisplatin and carboplatin in sarcoma patients: A report from the late effects surveillance system. Pediatr. Blood Cancer 2007, 48, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.; Parry, A.; Price, L.; Cole, M.; Craft, A.W.; Pearson, A.D. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: Relevance of age and dose as risk factors. Eur. J. Cancer 2009, 45, 3213–3219. [Google Scholar] [CrossRef]
- Skinner, R.; Parry, A.; Price, L.; Cole, M.; Craft, A.W.; Pearson, A.D. Glomerular toxicity persists 10 years after ifosfamide treatment in childhood and is not predictable by age or dose. Pediatr. Blood Cancer 2010, 54, 983–989. [Google Scholar] [CrossRef]
- Stöhr, W.; Paulides, M.; Bielack, S.; Jürgens, H.; Treuner, J.; Rossi, R.; Langer, T.; Beck, J. Ifosfamide-induced nephrotoxicity in 593 sarcoma patients: A report from the Late Effects Surveillance System. Pediatr. Blood Cancer 2007, 48, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, O.; Fawaz, O.; Rey, A.; Niaudet, P.; Ridola, V.; Orbach, D.; Bergeron, C.; Defachelles, A.S.; Gentet, J.-C.; Schmitt, C.; et al. Long-term evaluation of Ifosfamide-related nephrotoxicity in children. J. Clin. Oncol. 2009, 27, 5350–5355. [Google Scholar] [CrossRef] [PubMed]
- Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 5.0. Available online: http://www.survivorshipguidelines.org/ (accessed on 10 May 2022).
- Dutch Childhood Oncology Group. Richtlijn Follow-Up na Kinderkanker Meer dan 5 Jaar na Diagnose. 2010. Available online: www.skion.nl (accessed on 10 May 2022).
- Juergens, C.; Weston, C.; Lewis, I.; Whelan, J.; Paulussen, M.; Oberlin, O.; Michon, J.; Zoubek, A.; Juergens, H.; Craft, A. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr. Blood Cancer 2006, 47, 22–29. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; Mccarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticanc. Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, S.L.; Jaspers, M.W.; Van Der Pal, H.J.; Schouten-van Meeteren, A.Y.; Bouts, A.H.; Lieverst, J.A.; Bökenkamp, A.; Koning, C.C.E.; Oldenburger, F.; Wilde, J.C.H.; et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin. J. Am. Soc. Nephrol. 2012, 7, 1416–1427. [Google Scholar] [CrossRef]
- Mulder, R.L.; Knijnenburg, S.L.; Geskus, R.B.; Van Dalen, E.C.; Van Der Pal, H.J.H.; Koning, C.C.E.; Bouts, A.H.; Caron, H.N.; Kremer, L.C.M. Glomerular function time trends in long-term survivors of childhood cancer: A longitudinal study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1736–1746. [Google Scholar] [CrossRef]
- Kamps, W.A.; Bökkerink, J.P.; Hählen, K.; Hermans, J.; Riehm, H.; Gadner, H.; Schrappe, M.; Slater, R.; Ruiter, E.V.D.B.-D.; A Smets, L.; et al. Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: Results of Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988–1991). Blood 1999, 94, 1226–1236. [Google Scholar]
- Kamps, W.A.; Bökkerink, J.P.M.; Hakvoort-Cammel, F.G.A.J.; Veerman, A.J.P.; Weening, R.S.; Van Wering, E.R.; Van Weerden, J.F.; Hermans, J.; Slater, R.; Berg, E.V.D.; et al. BFM-oriented treatment for children with acute lymphoblastic leukemia without cranial irradiation and treatment reduction for standard risk patients: Results of DCLSG protocol ALL-8 (1991–1996). Leukemia 2002, 16, 1099–1111. [Google Scholar] [CrossRef][Green Version]
- Dekkers, I.A.; Blijdorp, K.; Cransberg, K.; Pluijm, S.M.; Pieters, R.; Neggers, S.J.; van den Heuvel-Eibrink, M.M. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin. J. Am. Soc. Nephrol. 2013, 8, 922–929. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.L.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; Van Zon, S.K.R.; Wijmenga, C.; Wolffenbuttel, B.H.R.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Klijs, B.; Scholtens, S.; Mandemakers, J.; Snieder, H.; Stolk, R.P.; Smidt, N. Representativeness of the LifeLines Cohort Study. PLoS ONE 2015, 10, e0137203. [Google Scholar] [CrossRef]
- den Elzen, W.P.J.; Brouwer, N.; Thelen, M.H.; Le Cessie, S.; Haagen, I.-A.; Cobbaert, C. NUMBER: Standardized reference intervals in the Netherlands using a ‘big data’ approach. Clin. Chem. Lab. Med. 2018, 57, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Topf, J.; Murray, P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 4, 195–206. [Google Scholar] [CrossRef]
- Elisaf, M.; Siamopoulos, K.C. Fractional excretion of potassium in normal subjects and in patients with hypokalaemia. Postgrad. Med. J. 1995, 71, 211–212. [Google Scholar] [CrossRef]
- Payne, R.B. Renal tubular reabsorption of phosphate (TmP/GFR): Indications and interpretation. Ann. Clin. Biochem. 1998, 35 Pt 2, 201–206. [Google Scholar] [CrossRef]
- Yu, H.; Yanagisawa, Y.; A Forbes, M.; Cooper, E.H.; A Crockson, R.; MacLennan, I.C. Alpha-1-microglobulin: An indicator protein for renal tubular function. J. Clin. Pathol. 1983, 36, 253–259. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Improving Global Outcomes and CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Guder, W.G.; Hofmann, W. Clinical role of urinary low molecular weight proteins: Their diagnostic and prognostic implications. Scand J. Clin. Lab. Investig. Suppl. 2008, 241, 95–98. [Google Scholar] [CrossRef]
- Patzer, L.; Ringelmann, F.; Kentouche, K.; Fuchs, D.; Zintl, F.; Brandis, M.; Zimmerhackl, L.; Misselwitz, J. Renal function in long-term survivors of stem cell transplantation in childhood. A prospective trial. Bone Marrow Transplant. 2001, 27, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Robles, N.R.; Gomez, J.L.; Pino, G.G.; Valladares, J.; Gallego, R.H.; Cerezo, I. Alpha-1-microglobulin: Prognostic value in chronic kidney disease. Med. Clin. 2021, 157, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Bökenkamp, A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020, 35, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- Matsui, M.; Saito, Y.; Yamaoka, S.; Yokokawa, Y.; Morikawa, Y.; Makimoto, A.; Yuza, Y. Kidney-protective Effect of Magnesium Supplementation in Cisplatin-containing Chemotherapy for Pediatric Cancer: A Retrospective Study. J. Pediatr. Hematol. Oncol. 2018, 40, 379–381. [Google Scholar] [CrossRef]
- Unwin, R.J.; Luft, F.C.; Shirley, D.G. Pathophysiology and management of hypokalemia: A clinical perspective. Nat. Rev. Nephrol. 2011, 7, 75–84. [Google Scholar] [CrossRef]
- Chang, A.R.; Grams, M.E. Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect modification by fasting. Am. J. Kidney Dis. 2014, 64, 567–573. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. Long Term Follow up of Survivors of Childhood Cancer: A National Clincial Guideline. 2013. Available online: http://www.sign.ac.uk/pdf/sign132.pdf (accessed on 22 March 2022).
- Loebstein, R.; Atanackovic, G.; Bishai, R.; Wolpin, J.; Khattak, S.; Hashemi, G.; Gobrial, M.; Baruchel, S.; Ito, S.; Koren, G. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J. Clin. Pharmacol. 1999, 39, 454–461. [Google Scholar]
- Kitterer, D.; Schwab, M.; Alscher, M.D.; Braun, N.; Latus, J. Drug-induced acid-base disorders. Pediatr. Nephrol. 2015, 30, 1407–1423. [Google Scholar] [CrossRef]
- Le Deley, M.C.; Paulussen, M.; Lewis, I.; Brennan, B.; Ranft, A.; Whelan, J.; Le Teuff, G.; Michon, J.; Ladenstein, R.; Marec-Bérard, P.; et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. 2014, 32, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.C.; Seidel, K.; Leisenring, W.M.; A Mulrooney, D.; Tersak, J.M.; Glick, R.D.; A Burnweit, C.; Green, D.M.; Diller, L.R.; A Smith, S.; et al. Solid organ transplantation after treatment for childhood cancer: A retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2019, 20, 1420–1431. [Google Scholar] [CrossRef]
- Park, P.G.; Hong, C.R.; Kang, E.; Park, M.; Lee, H.; Kang, H.J.; Shin, H.Y.; Ha, I.-S.; Cheong, H.I.; Yoon, H.J.; et al. Acute Kidney Injury in Pediatric Cancer Patients. J. Pediatr. 2019, 208, 243–250.e3. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Children’s Cancer Study Group Late Effects Group: Therapy Based Long Term Follow up Practice Statement. 2011. Available online: http://www.cclg.org.uk/ (accessed on 10 May 2022).
- Kremer, L.C.; Mulder, R.L.; Oeffinger, K.C.; Bhatia, S.; Landier, W.; Levitt, G.; Constine, L.S.; Wallace, W.H.; Caron, H.N.; Armenian, S.H.; et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr. Blood Cancer 2013, 60, 543–549. [Google Scholar] [CrossRef]
- Cobbaert, C.; Weykamp, C.; Franck, P.; De Jonge, R.; Kuypers, A.; Steigstra, H.; Gunnewiek, J.K.; Van Loon, D.; Jansen, R. Systematic monitoring of standardization and harmonization status with commutable EQA-samples—Five year experience from the Netherlands. Clin. Chim. Acta 2012, 414, 234–240. [Google Scholar] [CrossRef]
- McWilliam, S.J.; Antoine, D.J.; Smyth, R.L.; Pirmohamed, M. Aminoglycoside-induced nephrotoxicity in children. Pediatr. Nephrol. 2017, 32, 2015–2025. [Google Scholar] [CrossRef]
- Fanos, V.; Cataldi, L. Amphotericin B-induced nephrotoxicity: A review. J. Chemother. 2000, 12, 463–470. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
| Age | Male Range (mmol/L) | Female Range (mmol/L) |
|---|---|---|
| 25–35 years | 1.00–1.35 | 0.96–1.44 |
| 45–55 years | 0.90–1.35 | 0.88–1.42 |
| 65–75 years | 0.80–1.35 | 0.80–1.35 |
| Characteristics | Underlying Cohort | Invited Study Population | Non-Participants b | Participants | Controls |
|---|---|---|---|---|---|
| n = 6165 | n = 1881 | n = 787 | n = 1024 | n = 500 | |
| Sex, n (%) | |||||
| Male | 3433 (55.7) | 1009 (53.6) | 484 (61.5) | 505 (49.3) | 241 (48.2) |
| Female | 2731 (44.3) | 872 (46.4) | 303 (38.5) | 519 (50.7) | 259 (51.8) |
| Transgender | 1 (0.01) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Primary childhood cancer (ICCC), n (%) | |||||
| Leukemias, myeloproliferative diseases and myelodysplastic diseases | 2094 (34.0) | 569 (30.2) | 225 (28.6) | 317 (31.0) | − |
| Lymphomas and reticuloendothelial neoplasms | 1062 (17.2) | 150 (8.0) | 68 (8.6) | 79 (7.7) | − |
| CNS and miscellaneous intracranial and intraspinal neoplasms | 844 (13.7) | 121 (6.4) | 55 (7.0) | 62 (6.1) | − |
| Neuroblastoma and other peripheral nervous cell tumors | 324 (5.3) | 94 (5.0) | 28 (3.6) | 65 (6.3) | − |
| Retinoblastoma | 33 (0.5) | 2 (0.1) | 1 (0.1) | 1 (0.1) | − |
| Renal tumors | 596 (9.7) | 476 (25.3) | 200 (25.4) | 254 (24.8) | − |
| Hepatic tumors | 52 (0.8) | 34 (1.8) | 22 (2.8) | 12 (1.2) | − |
| Bone tumors | 370 (6.0) | 148 (7.9) | 67 (8.5) | 78 (7.6) | − |
| Soft tissue and other extraosseous sarcomas | 450 (7.3) | 168 (8.9) | 72 (9.1) | 92 (9.0) | − |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | 232 (3.8) | 99 (5.3) | 41 (5.2) | 52 (5.1) | − |
| Other malignant epithelial neoplasms and malignant melanomas | 102 (1.7) | 18 (1.0) | 8 (1.0) | 10 (1.0) | − |
| Other and unspecified malignant neoplasms | 6 (0.1) | 2 (0.1) | 0 (0) | 2 (0.2) | − |
| Age at diagnosis (yr), n (%) * | |||||
| 0–4 | 2727 (45.3) | 994 (52.9) | 417 (53.1) | 537 (52.4) | − |
| 5–9 | 1628 (27.1) | 476 (25.3) | 198 (25.2) | 265 (25.9) | − |
| 10–14 | 1285 (21.4) | 312 (16.6) | 128 (16.3) | 171 (16.7) | − |
| 15–17 | 376 (6.3) | 98 (5.2) | 43 (5.5) | 51 (5.0) | − |
| Treatment period, n (%) | |||||
| 1963–1969 | 119 (1.9) | 20 (1.1) | 6 (0.8) | 14 (1.4) | − |
| 1970–1979 | 978 (15.9) | 130 (6.9) | 54 (6.9) | 72 (7.0) | − |
| 1980–1989 | 1931 (31.3) | 477 (25.4) | 184 (23.4) | 272 (26.6) | − |
| 1990–1999 | 2541 (41.2) | 1093 (58.1) | 479 (60.9) | 576 (56.3) | − |
| 2000–2001 | 596 (9.7) | 161 (8.6) | 64 (8.1) | 90 (8.8) | − |
| Age at participation/invitation (yr), n (%) # | |||||
| <18 | 49 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 18–30 | 1313 (32.9) | 640 (39.1) | 205 (37.8) | 381 (37.2) | 182 (36.4) |
| 30–40 | 1511 (37.9) | 709 (43.3) | 244 (45.1) | 446 (43.6) | 216 (43.2) |
| >40 | 1118 (28.0) | 286 (17.5) | 92 (17.0) | 197 (19.2) | 102 (20.4) |
| Follow-up time since childhood cancer diagnosis (yr), n (%) | |||||
| 10–20 | 981 (15.9) | 328 (17.4) | 133 (16.9) | 186 (18.2) | − |
| 20–30 | 1931 (31.3) | 1078 (57.3) | 469 (59.6) | 569 (55.6) | − |
| 30–40 | 1393 (22.6) | 351 (18.7) | 136 (17.3) | 197 (19.2) | − |
| 40–50 | 460 (7.5) | 112 (6.0) | 48 (6.1) | 61 (6.0) | − |
| 50–60 | 46 (0.7) | 12 (0.6) | 1 (0.1) | 11 (1.1) | − |
| Surgery, n (%) a | |||||
| No | 2912 (47.2) | 694 (36.9) | 281 (35.7) | 385 (37.6) | − |
| Yes | 3185 (51.7) | 1182 (62.8) | 503 (63.9) | 637 (62.2) | − |
| Missing | 68 (1.1) | 5 (0.3) | 3 (0.4) | 2 (0.2) | − |
| Radiotherapy, n (%) a | |||||
| No | 3608 (58.5) | 1177 (62.6) | 533 (67.7) | 596 (58.2) | − |
| Yes | 2527 (41.0) | 703 (37.4) | 254 (32.3) | 427 (41.7) | − |
| Missing | 30 (0.5) | 1 (0.05) | 0 (0) | 1 (0.1) | − |
| Chemotherapy, n (%) a | |||||
| No | 1123 (18.2) | 35 (1.9) | 15 (1.9) | 20 (2.0) | − |
| Yes | 5005 (81.2) | 1845 (98.1) | 771 (98.0) | 1004 (98.0) | − |
| Missing | 37 (0.6) | 1 (0.05) | 1 (0.1) | 0 (0) | − |
| Stem cell transplantation/reinfusion, n (%) a,* | |||||
| No | 5532 (89.7) | 1624 (86.4) | 698 (88.8) | 863 (84.3) | − |
| Autologous transplant | 155 (2.5) | 90 (4.8) | 34 (4.3) | 56 (5.5) | − |
| Allogeneic HSCT | 231 (3.7) | 153 (8.1) | 51 (6.5) | 95 (9.3) | − |
| Missing | 98 (1.6) | 13 (0.7) | 3 (0.4) | 10 (1.0) | − |
| Therapy, n (%) | |||||
| No treatment | 61 (1.0) | 0 (0) | 0 (0) | 0 (0) | − |
| Surgery only | 575 (9.3) | 17 (0.9) | 8 (1.0) | 9 (0.9) | − |
| Chemotherapy only (±surgery) | 2967 (48.1) | 1160 (61.7) | 525 (66.7) | 587 (57.3) | − |
| Radiotherapy only (±surgery) | 484 (7.9) | 18 (1.0) | 7 (0.9) | 11 (1.1) | − |
| Chemotherapy and radiotherapy (±surgery) | 2030 (32.9) | 684 (36.4) | 246 (31.3) | 416 (40.6) | − |
| Missing | 48 (0.8) | 2 (0.1) | 1 (0.1) | 1 (0.1) | − |
| Potentially nephrotoxic cancer treatment, n (%) a | |||||
| Nephrectomy | 622 (10.1) | 492 (26.2) | 207 (26.3) | 264 (25.8) | − |
| Unilateral | 605 (97.3) | 478 (97.2) | 204 (98.6) | 255 (96.6) | − |
| Bilateral partial | 17 (2.7) | 14 (2.9) | 3 (1.5) | 9 (3.4) | − |
| Radiotherapy renal area | 467 (7.6) | 273 (14.5) | 90 (11.4) | 175 (17.1) | − |
| Total body irradiation | 221 (3.6) | 143 (7.6) | 52 (6.6) | 85 (8.4) | − |
| Ifosfamide | 714 (11.6) | 524 (27.9) | 206 (26.2) | 300 (29.3) | − |
| HD-cyclophosphamide | 833 (13.5) | 504 (26.8) | 208 (26.4) | 278 (27.2) | − |
| Cisplatin | 457 (7.4) | 328 (17.4) | 142 (18.0) | 175 (17.1) | − |
| Carboplatin | 422 (6.9) | 284 (15.1) | 125 (15.9) | 151 (14.7) | − |
| Allogeneic HSCT | 231 (3.8) | 153 (8.1) | 51 (6.5) | 95 (9.3) | − |
| Tubular Function Parameter | CCS (n) | Prevalence a | Controls (n) | Prevalence a | p-Value |
|---|---|---|---|---|---|
| Tubular magnesium loss | 999 | 56/999 (5.6) | 500 | 25/500 (5.0) | 0.63 |
| Magnesium supplementation | 1024 | 20/1024 (2.0) | 500 | 0/500 (0) | <0.001 |
| Tubular potassium loss | 1003 | 45/1003 (4.5) | 500 | 20/500 (4.0) | 0.66 |
| Potassium supplementation | 1024 | 9/1024 (0.9%) | 500 | 0/500 (0) | 0.04 |
| Tubular phosphate loss | 997 | 55/997 (5.5) | 500 | 54/500 (10.8) | <0.001 |
| Phosphate supplementation | 1024 | 3/1024 (0.3%) | 500 | 0/500 (0) | 0.55 |
| Low molecular weight proteinuria | 931 | 187/931 (20.1) | 498 | 2/498 (0.4) | <0.001 |
| Metabolic acidosis | 1002 | 26/1002 (2.5) | - | - | - |
| Total Number of Tubular Outcomes | CCS (n) | Controls (n) |
|---|---|---|
| 0 | 658 (71.1) | 401 (80.5) |
| 1 | 213 (23.0) | 93 (18.7) |
| 2 | 45 (4.9) | 4 (0.8) |
| 3 | 9 (1.0) | 0 (0) |
| 4 | 1 (0.1) | 0 (0) |
| LMWP | Renal Magnesium Loss | Renal Potassium Loss | Renal Phosphate Loss | |||||
|---|---|---|---|---|---|---|---|---|
| CCS | Controls | CCS | Controls | CCS | Controls | CCS | Controls | |
| G1 (eGFR ≥ 90) | 111/692 (16.0) | 2/427 (0.5) | 34/715 (4.8) | 25/429 (5.8) | 26/717 (3.6) | 19/429 (4.6) | 36/713 (5.0) | 48/429 (11.2) |
| G2 (eGFR 60–89) | 51/185 (27.6) | 0/71 (0.0) | 18/189 (9.5) | 0/71 (0.0) | 15/191 (7.9) | 1/71 (1.4) | 17/190 (8.9) | 6/71 (8.5) |
| G3 (eGFR 45–59) | 14/21 (66.7) | 0/0 (0.0) | 2/21 (9.5) | 0/0 (0.0) | 2/21 (9.5) | 0/0 (0.0) | 1/21 (4.8) | 0/0 (0.0) |
| G4 (eGFR 15–44) | 3/3 (100) | 0/0 (0.0) | 2/4 (50.0) | 0/0 (0.0) | 0/4 (0.0) | 0/0 (0.0) | 1/4 (25.0) | 0/0 (0.0) |
| G5 (eGFR < 15) | 1/1 (100) | 0.0 (0.0) | 0/1 (0.0) | 0.0 (0.0) | 0/1 (0.0) | 0.0 (0.0) | 0/1 (0.0) | 0.0 (0.0) |
| No albuminuria | 112/756 (14.8) | 2/492 (0.4) | 37/775 (4.8) | 25/494 (5.1) | 32/776 (4.1) | 20/494 (4.0) | 41/775 (5.3) | 53/494 (10.7) |
| Microalbuminuria | 60/138 (43.5) | 0/5 (0.0) | 13/140 (9.3) | 0/5 (0.0) | 12/142 (8.5) | 0/5 (0.0) | 13/142 (9.2) | 1/5 (20.0) |
| Macroalbuminuria | 6/10 (60.0) | 0/1 (0.0) | 4/10 (40.0) | 0/1 (0.0) | 1/10 (10.0) | 0/1 (0.0) | 0/10 (0.0) | 0/1 (0.0) |
| Tubular Magnesium Loss n= 56/999 | Tubular Potassium Loss n= 43/1003 | Tubular Phosphate Loss n= 55/997 | Low Molecular Weight Proteinuria n= 187/931 | |||||||||
| Model 1 | Prevalence a | OR (95% CI) Multivariable | Prevalence a | OR (95% CI) Multivariable | Prevalence a | OR (95% CI) Multivariable | Prevalence a | OR (95% CI) Multivariable | ||||
| Nephrectomy | ||||||||||||
| No | 46/738 (6.2) | 1.0 (ref) | 40/741 (5.4) | 1.0 (ref) | 42/736 (5.7) | 1.0 (ref) | 149/687 (21.7) | 1.0 (ref) | ||||
| Yes | 10/261 (3.8) | 0.9 (0.3–2.7) | 5/262 (1.9) | 0.6 (0.2–2.1) | 13/261 (5.0) | 1.2 (0.5–2.9) | 38/244 (15.6) | 0.5 (0.3–0.8) | ||||
| Abdominal RT | ||||||||||||
| No | 45/811 (5.5) | 1.0 (ref) | 35/814 (4.3) | 1.0 (ref) | 42/808 (5.2) | 1.0 (ref) | 152/754 (20.2) | 1.0 (ref) | ||||
| Yes | 9/173 (5.2) | 1.1 (0.4–2.8) | 7/174 (4.0) | 1.9 (0.7–5.2) | 10/174 (5.7) | 1.3 (0.5–3.0) | 32/162 (19.8) | 1.1 (0.6–2.0) | ||||
| TBI | ||||||||||||
| No | 52/902 (5.8) | 1.0 (ref) | 40/904 (4.4) | 1.0 (ref) | 48/898 (5.3) | 1.0 (ref) | 166/837 (19.8) | 1.0 (ref) | ||||
| Yes | 2/82 (2.4) | 0.8 (01.1–4.2) | 2/84 (2.4) | 0.8 (0.2–3.8) | 4/84 (4.8) | 1.0 (0.3–3.0) | 18/79 (22.8) | 1.1 (0.6–2.1) | ||||
| Ifosfamide | ||||||||||||
| No | 49/703 (7.0) | 1.0 (ref) | 21/706 (3.0) | 1.0 (ref) | 28/702 (4.0) | 1.0 (ref) | 92/652 (14.1) | 1.0 (ref) | ||||
| Yes | 7/296 (2.4) | 0.2 (0.1–0.6) | 24/297 (8.1) | 2.4 (1.2–4.7) | 27/295 (9.2) | 2.3 (1.2–4.3) | 95/279 (34.1) | 2.2 (1.5–3.3) | ||||
| HD-cyclo | ||||||||||||
| No | 52/731 (7.1) | 1.0 (ref) | 41/734 (5.6) | 1.0 (ref) | 44/731 (6.0) | 1.0 (ref) | 148/685 (21.6) | 1.0 (ref) | ||||
| Yes | 4/266 (1.5) | 0.5 (0.1–1.7) | 4/267 (1.5) | 0.5 (0.1–1.5) | 11/264 (4.2) | 0.8 (0.4–1.9) | 39/244 (16.0) | 0.7 (0.4–1.2) | ||||
| Cisplatin | ||||||||||||
| No | 20/829 (2.4) | 1.0 (ref) | 26/832 (3.1) | 1.0 (ref) | 45/826 (5.4) | 1.0 (ref) | 156/771 (20.2) | 1.0 (ref) | ||||
| Yes | 36/170 (21.2) | 10.5 (4.1–27.2) | 19/171 (11.1) | 3.5 (1.6–7.5) | 10/171 (5.8) | 1.2 (0.5–2.8) | 31/160 (19.4) | 0.8 (0.5–1.3) | ||||
| Carboplatin | ||||||||||||
| No | 49/852 (5.8) | 1.0 (ref) | 33/855 (3.9) | 1.0 (ref) | 42/849 (4.9) | 1.0 (ref) | 149/790 (18.9) | 1.0 (ref) | ||||
| Yes | 7/147 (4.8) | 1.1 (0.4–3.3) | 12/148 (8.1) | 1.6 (0.7–3.8) | 13/148 (8.8) | 1.5 (0.7–3.3) | 38/141 (27.0) | 1.3 (0.8–2.1) | ||||
| HSCT | ||||||||||||
| No | 51/899 (5.7) | NA | 42/903 (4.7) | NA | 50/897 (5.6) | NA | 164/838 (19.6) | NA | ||||
| Yes | 2/91 (2.2) | 2/91 (2.2) | 4/91 (4.4) | 19/84 (22.6) | ||||||||
| Gender | ||||||||||||
| Male | 21/492 (4.3) | NA | 19/496 (3.8) | NA | 29/492 (5.9) | NA | 91/461 (19.7) | NA | ||||
| Female | 35/507 (6.9) | 26/507 (5.1) | 26/505 (5.1) | 96/470 (20.4) | ||||||||
| Age at diagnosis | - | 1.0 (0.96–1.1) | - | 1.1 (0.99–1.1) | NA | - | NA | |||||
| FU duration (yr) | ||||||||||||
| 10–19 | 11/181 (6.1) | 1.0 (ref) | 12/181 (6.6) | 1.0 (ref) | 4/180 (2.2) | 1.0 (ref) | 37/162 (22.8) | 1.0 (ref) | ||||
| 20–29 | 20/554 (3.6) | 0.9 (0.4–2.2) | 23/555 (4.1) | 0.9 (0.4–1.9) | 38/550 (6.9) | 4.7 (1.4–15.5) | 96/513 (18.7) | 0.9 (0.6–1.6) | ||||
| ≥30 | 25/264 (9.5) | 1.3 (0.5–3.4) | 10/267 (3.7) | 0.8 (0.3–2.0) | 13/267 (4.9) | 3.3 (0.9–12.5) | 54/256 (21.1) | 0.9 (0.5–1.5) | ||||
| eGFR (per 1 mL/min/1.73 m2) | 0.98 (0.96–0.99) | 0.98 (0.96–0.99) | ||||||||||
| ACR (per 1 mg/mmol) | 1.0 (0.9–1.02) | 1.06 (1.02–1.09) | ||||||||||
| Model 2 | Prevalence a | OR (95% CI) Multivariable | Ptrend * | Prevalencea | OR (95% CI) Multivariable | Ptrend * | Prevalence a | OR (95% CI) Multivariable | Ptrend * | Prevalence a | OR (95% CI) Multivariable | Ptrend * |
| Abdominal RT dose, Gy | ||||||||||||
| None | 42/808 (5.2) | 1.0 (ref) | 152/754 (20.2) | 1.0 (ref) | ||||||||
| <20 | 3/47 (6.4) | 1.6 (0.4–6.4) | 8/43 (18.6) | 1.6 (0.6–3.9) | ||||||||
| 20–30 | 2/54 (3.7) | 0.9 (0.2–4.5) | 9/50 (18.0) | 1.3 (0.5–3.2) | ||||||||
| >30 | 5/71 (7.0) | 1.4 (0.5–3.9) | 0.66 | 15/67 (22.4) | 1.0 (0.5–2.1) | 0.95 | ||||||
| Ifosfamide dose, mg/m2 | ||||||||||||
| None | 21/706 (3.0) | 1.0 (ref) | 28/702 (4.0) | 1.0 (ref) | 92/652 (14.1) | 1.0 (ref) | ||||||
| ≤12,000 | 5/99 (5.1) | 3.7 (1.2–11.7) | 6/99 (6.1) | 1.6 (0.6–4.5) | 17/91 (18.7) | 1.1 (0.6–2.2) | ||||||
| 12,001–42,000 | 9/97 (9.3) | 2.4 (0.9–6.4) | 9/97 (9.3) | 2.4 (1.0–5.9) | 27/92 (29.3) | 2.0 (1.1–3.6) | ||||||
| >42,000 | 9/99 (9.1) | 3.7 (1.3–10.7) | 0.56 | 12/97 (12.4) | 4.1 (1.6–10.4) | 0.39 | 50/94 (53.2) | 6.2 (3.4–11.5) | 0.16 | |||
| Cisplatin dose mg/m2 | ||||||||||||
| None | 20/829 (2.4) | 1.0 (ref) | 26/832 (3.1) | 1.0 (ref) | 45/826 (5.4) | 1.0 (ref) | 156/771 (20.2) | 1.0 (ref) | ||||
| ≤300 | 6/57 (10.5) | 5.8 (1.7–19.9) | 2/58 (3.4) | 1.0 (0.2–5.3) | 2/58 (3.4) | 0.8 (0.2–3.9) | 12/55 (21.8) | 1.1 (0.5–2.5) | ||||
| 301–500 | 10/57 (17.5) | 9.2 (2.9–29.2) | 3/57 (5.3) | 1.8 (0.4–7.5) | 2/57 (3.5) | 0.5 (0.1–3.6) | 9/54 (16.7) | 1.0 (0.4–2.3) | ||||
| >500 | 20/55 (36.4) | 22.0 (7.2–67.3) | 0.72 | 14/55 (25.5) | 17.7 (6.2–50.4) | 0.84 | 6/55 (10.9) | 3.6 (1.2–10.9) | 0.85 | 10/50 (20.0) | 1.1 (0.5–2.6) | 0.36 |
| Carboplatin dose, mg/m2 | ||||||||||||
| None | 33/855 (3.9) | 1.0 (ref) | 42/849 (4.9) | 1.0 (ref) | 149/790 (18.9) | 1.0 (ref) | ||||||
| ≤1500 | 5/51 (9.8) | 1.1 (0.2–5.7) | 5/51 (9.8) | 1.6 (0.5–5.4) | 17/49 (34.7) | 1.2 (0.5–2.6) | ||||||
| 1501–2800 | 1/49 (2.0) | 0.6 (0.1–5.2) | 6/49 (12.2) | 2.8 (1.0–7.9) | 12/47 (25.5) | 2.5 (1.1–5.5) | ||||||
| >2800 | 6/46 (13.0) | 5.1 (1.7–15.8) | 0.04 | 2/46 (4.3) | 0.7 (0.2–3.5) | 0.74 | 9/43 (20.9) | 0.9 (0.3–2.1) | 0.02 | |||
| Cisplatin dose mg/m2 | ||||||||||||
| None | 20/829 (2.4) | 1.0 (ref) | 26/832 (3.1) | 1.0 (ref) | 45/826 (5.4) | 1.0 (ref) | 156/771 (20.2) | 1.0 (ref) | ||||
| ≤300 | 6/57 (10.5) | 5.8 (1.7–19.9) | 2/58 (3.4) | 1.0 (0.2–5.3) | 2/58 (3.4) | 0.8 (0.2–3.9) | 12/55 (21.8) | 1.1 (0.5–2.5) | ||||
| 301–500 | 10/57 (17.5) | 9.2 (2.9–29.2) | 3/57 (5.3) | 1.8 (0.4–7.5) | 2/57 (3.5) | 0.5 (0.1–3.6) | 9/54 (16.7) | 1.0 (0.4–2.3) | ||||
| >500 | 20/55 (36.4) | 22.0 (7.2–67.3) | 0.72 | 14/55 (25.5) | 17.7 (6.2–50.4) | 0.84 | 6/55 (10.9) | 3.6 (1.2–10.9) | 0.85 | 10/50 (20.0) | 1.1 (0.5–2.6) | 0.36 |
| Carboplatin dose, mg/m2 | ||||||||||||
| None | 33/855 (3.9) | 1.0 (ref) | 42/849 (4.9) | 1.0 (ref) | 149/790 (18.9) | 1.0 (ref) | ||||||
| ≤1500 | 5/51 (9.8) | 1.1 (0.2–5.7) | 5/51 (9.8) | 1.6 (0.5–5.4) | 17/49 (34.7) | 1.2 (0.5–2.6) | ||||||
| 1501–2800 | 1/49 (2.0) | 0.6 (0.1–5.2) | 6/49 (12.2) | 2.8 (1.0–7.9) | 12/47 (25.5) | 2.5 (1.1–5.5) | ||||||
| >2800 | 6/46 (13.0) | 5.1 (1.7–15.8) | 0.04 | 2/46 (4.3) | 0.7 (0.2–3.5) | 0.74 | 9/43 (20.9) | 0.9 (0.3–2.1) | 0.02 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooijmans, E.C.M.; van der Pal, H.J.H.; Pluijm, S.M.F.; van der Heiden-van der Loo, M.; Kremer, L.C.M.; Bresters, D.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; Loonen, J.J.; Louwerens, M.; et al. Long-Term Tubular Dysfunction in Childhood Cancer Survivors; DCCSS-LATER 2 Renal Study. Cancers 2022, 14, 2754. https://doi.org/10.3390/cancers14112754
Kooijmans ECM, van der Pal HJH, Pluijm SMF, van der Heiden-van der Loo M, Kremer LCM, Bresters D, van Dulmen-den Broeder E, van den Heuvel-Eibrink MM, Loonen JJ, Louwerens M, et al. Long-Term Tubular Dysfunction in Childhood Cancer Survivors; DCCSS-LATER 2 Renal Study. Cancers. 2022; 14(11):2754. https://doi.org/10.3390/cancers14112754
Chicago/Turabian StyleKooijmans, Esmee C. M., Helena J. H. van der Pal, Saskia M. F. Pluijm, Margriet van der Heiden-van der Loo, Leontien C. M. Kremer, Dorine Bresters, Eline van Dulmen-den Broeder, Marry M. van den Heuvel-Eibrink, Jacqueline J. Loonen, Marloes Louwerens, and et al. 2022. "Long-Term Tubular Dysfunction in Childhood Cancer Survivors; DCCSS-LATER 2 Renal Study" Cancers 14, no. 11: 2754. https://doi.org/10.3390/cancers14112754
APA StyleKooijmans, E. C. M., van der Pal, H. J. H., Pluijm, S. M. F., van der Heiden-van der Loo, M., Kremer, L. C. M., Bresters, D., van Dulmen-den Broeder, E., van den Heuvel-Eibrink, M. M., Loonen, J. J., Louwerens, M., Neggers, S. J. C., Ronckers, C., Tissing, W. J. E., de Vries, A. C. H., Kaspers, G. J. L., Bökenkamp, A., Veening, M. A., & on behalf of the Dutch LATER Study Group. (2022). Long-Term Tubular Dysfunction in Childhood Cancer Survivors; DCCSS-LATER 2 Renal Study. Cancers, 14(11), 2754. https://doi.org/10.3390/cancers14112754








