Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Recruitment, Data Collection, and Measures
2.3. Statistical Analyses
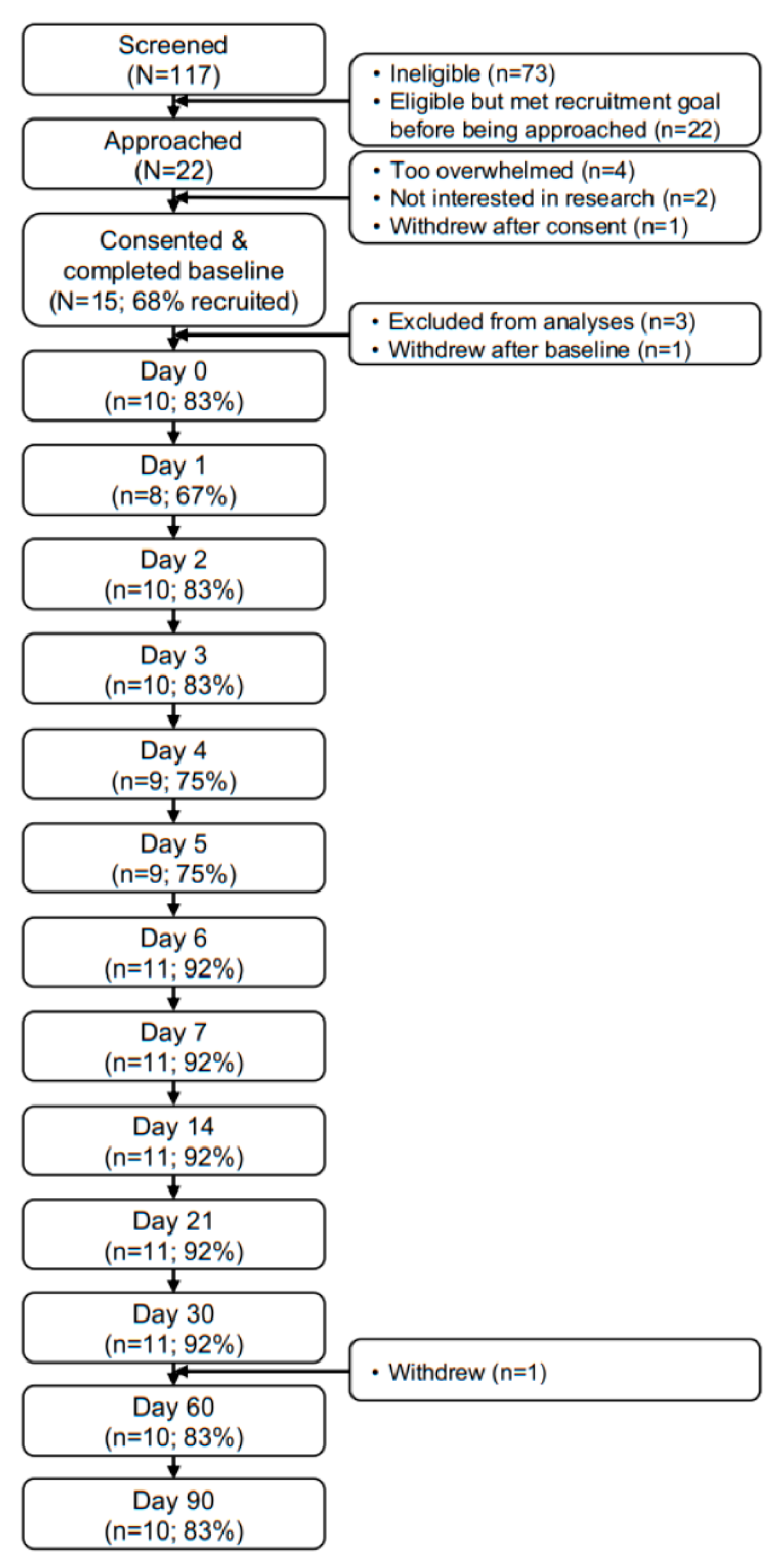
3. Results
3.1. Sample Characteristics
3.2. Feasibility and Acceptability
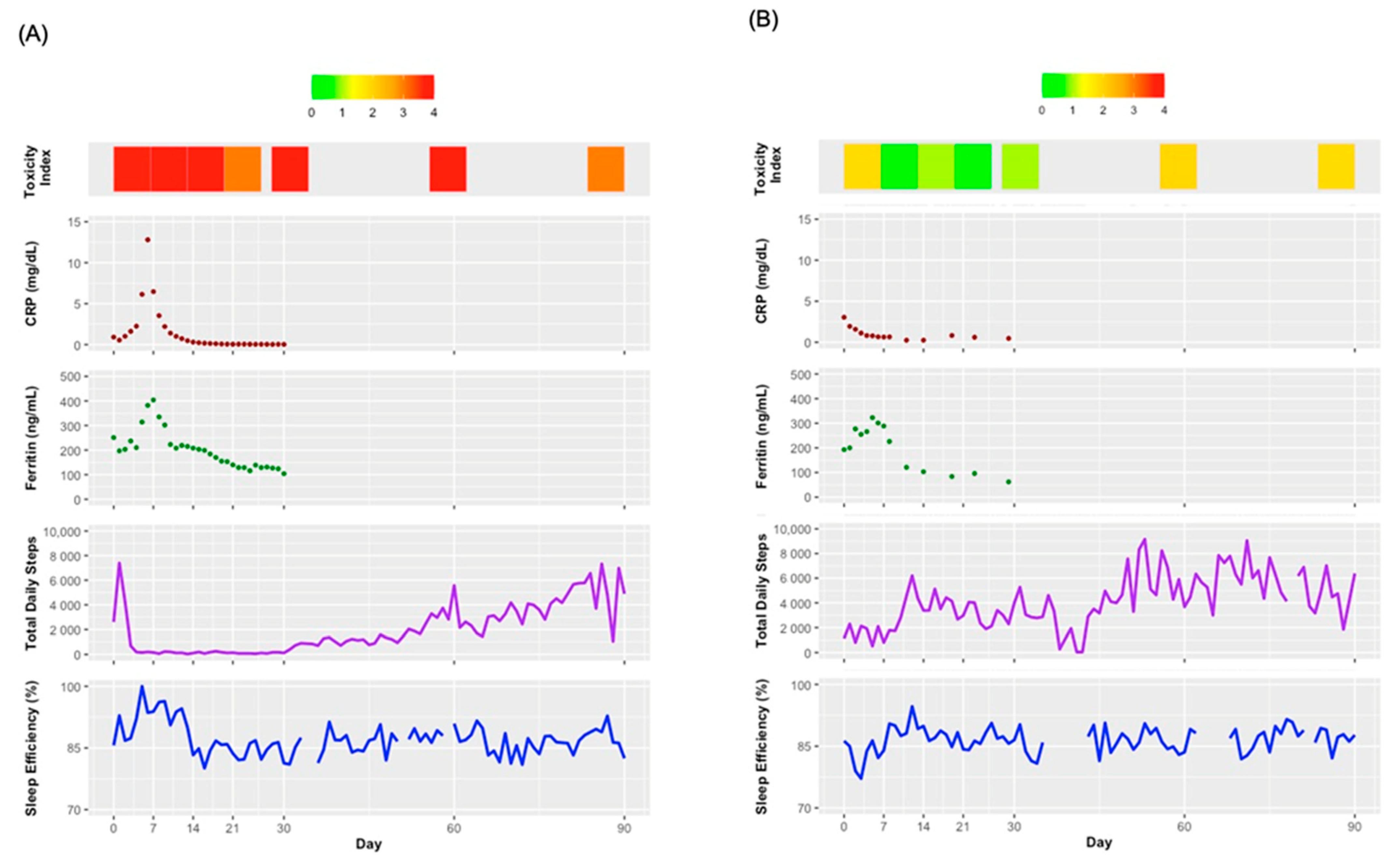
3.3. Preliminary Patterns of PROs and Activity Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.A.; Ruella, M.; Schuster, S.J. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N. Engl. J. Med. 2021, 384, 673–674. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Grigor, E.J.M.; Fergusson, D.; Kekre, N.; Montroy, J.; Atkins, H.; Seftel, M.D.; Daugaard, M.; Presseau, J.; Thavorn, K.; Hutton, B.; et al. Risks and Benefits of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Cancer: A Systematic Review and Meta-Analysis. Transfus. Med. Rev. 2019, 33, 98–110. [Google Scholar] [CrossRef]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood J. Am. Soc. Hematol. 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Tr. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, T.M.; Ryan, S.J.; Bennett, A.V.; Stover, A.M.; Saracino, R.M.; Rogak, L.J.; Jewell, S.T.; Matsoukas, K.; Li, Y.; Basch, E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): A systematic review. Support Care Cancer 2016, 24, 3669–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; De Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [Green Version]
- Lizee, T.; Basch, E.; Tremolieres, P.; Voog, E.; Domont, J.; Peyraga, G.; Urban, T.; Bennouna, J.; Septans, A.L.; Balavoine, M.; et al. Cost-Effectiveness of Web-Based Patient-Reported Outcome Surveillance in Patients With Lung Cancer. J. Thorac. Oncol. 2019, 14, 1012–1020. [Google Scholar] [CrossRef]
- Cox, S.M.; Lane, A.; Volchenboum, S.L. Use of Wearable, Mobile, and Sensor Technology in Cancer Clinical Trials. JCO Clin. Cancer Inform. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Smuck, M.; Odonkor, C.A.; Wilt, J.K.; Schmidt, N.; Swiernik, M.A. The emerging clinical role of wearables: Factors for successful implementation in healthcare. NPJ Digit. Med. 2021, 4, 45. [Google Scholar] [CrossRef]
- Wright, A.A.; Raman, N.; Staples, P.; Schonholz, S.; Cronin, A.; Carlson, K.; Keating, N.L.; Onnela, J.P. The HOPE Pilot Study: Harnessing Patient-Reported Outcomes and Biometric Data to Enhance Cancer Care. JCO Clin. Cancer Inform. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.F.; Bardyn, T.P. Research electronic data capture (REDCap). J. Med. Libr. Assoc. JMLA 2018, 106, 142. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, R.; Sidana, S.; Shah, G.L.; Scordo, M.; Hamilton, B.K.; Majhail, N.S. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol. Blood Marrow Transpl. 2019, 25, e155–e162. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Tedesco, V.E.T.; Mohan, C. Biomarkers for Predicting Cytokine Release Syndrome following CD19-Targeted CAR T Cell Therapy. J. Immunol. 2021, 206, 1561–1568. [Google Scholar] [CrossRef]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How We Design Feasibility Studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Pavic, M.; Klaas, V.; Theile, G.; Kraft, J.; Troster, G.; Guckenberger, M. Feasibility and Usability Aspects of Continuous Remote Monitoring of Health Status in Palliative Cancer Patients Using Wearables. Oncology 2019, 98, 386–395. [Google Scholar] [CrossRef]
- Bouchard, L.C.; Yanez, B.; Dahn, J.R.; Flury, S.C.; Perry, K.T.; Mohr, D.C.; Penedo, F.J. Brief report of a tablet-delivered psychosocial intervention for men with advanced prostate cancer: Acceptability and efficacy by race. Transl. Behav. Med. 2018, 9, 8. [Google Scholar] [CrossRef]
- Yanez, B.; Oswald, L.B.; Baik, S.H.; Buitrago, D.; Iacobelli, F.; Perez-Tamayo, A.; Guitelman, J.; Penedo, F.J.; Buscemi, J. Brief culturally informed smartphone interventions decrease breast cancer symptom burden among Latina breast cancer survivors. Psychooncology 2020, 29, 195–203. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Stein, D.M.; Victorson, D.E.; Choy, J.T.; Waimey, K.E.; Pearman, T.P.; Smith, K.; Dreyfuss, J.; Kinahan, K.E.; Sadhwani, D.; Woodruff, T.K.; et al. Fertility Preservation Preferences and Perspectives Among Adult Male Survivors of Pediatric Cancer and Their Parents. J. Adolesc. Young Adul. 2014, 3, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med. Care 2007, 45 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, R.D.; Spritzer, K.L.; Schalet, B.D.; Cella, D. PROMISA (R)-29 v2.0 profile physical and mental health summary scores. Qual. Life Res. 2018, 27, 1885–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez, B.; Pearman, T.; Lis, C.G.; Beaumont, J.L.; Cella, D. The FACT-G7: A rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M.; et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Basch, E.; Becker, C.; Rogak, L.J.; Schrag, D.; Reeve, B.B.; Spears, P.; Smith, M.L.; Gounder, M.M.; Mahoney, M.R.; Schwartz, G.K.; et al. Composite grading algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Clin. Trials 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Gresham, G.; Diniz, M.A.; Razaee, Z.S.; Luu, M.; Kim, S.; Hays, R.D.; Piantadosi, S.; Tighiouart, M.; Yothers, G.; Ganz, P.A.; et al. Evaluating Treatment Tolerability in Cancer Clinical Trials Using the Toxicity Index. J. Natl. Cancer Inst. 2020, 112, 1266–1274. [Google Scholar] [CrossRef]
- Rogatko, A.; Babb, J.S.; Wang, H.; Slifker, M.J.; Hudes, G.R. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin. Cancer Res. 2004, 10, 4645–4651. [Google Scholar] [CrossRef] [Green Version]
- Lichstein, K.L.; Durrence, H.H.; Taylor, D.J.; Bush, A.J.; Riedel, B.W. Quantitative criteria for insomnia. Behav. Res. Ther. 2003, 41, 427–445. [Google Scholar] [CrossRef]
- Banerjee, R.; Shah, N.; Dicker, A.P. Next-Generation Implementation of Chimeric Antigen Receptor T-Cell Therapy Using Digital Health. JCO Clin. Cancer Inform. 2021, 5, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.D.; Verneris, M.R.; Goy, A.; Maziarz, R.T. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J. Immunother. Cancer 2021, 9, e002056. [Google Scholar] [CrossRef] [PubMed]
- Penedo, F.J.; Oswald, L.B.; Kronenfeld, J.P.; Garcia, S.F.; Cella, D.; Yanez, B. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020, 21, e240–e251. [Google Scholar] [CrossRef]
- Holstein, S.A.; Lunning, M.A. CAR T-Cell Therapy in Hematologic Malignancies: A Voyage in Progress. Clin. Pharmacol. Ther. 2020, 107, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Jutagir, D.R.; Espinosa, A.; Lopez, M.; Rasool, B.; Gomez, T.; Brown, A.; Frankel, C.A.; Loo, K.; Goldberg, J.; Wolchok, J.D.; et al. Disparities in the use of checkpoint inhibitors and CAR T-cell therapy: A systematic review. J. Clin. Oncol. 2021, 39 (Suppl. S15), e18541. [Google Scholar] [CrossRef]
- Varon, M.L.; Baker, E.; Byers, E.; Cirolia, L.; Bogler, O.; Bouchonville, M.; Schmeler, K.; Hariprasad, R.; Pramesh, C.S.; Arora, S. Project ECHO Cancer Initiative: A Tool to Improve Care and Increase Capacity along the Continuum of Cancer Care. J. Cancer Educ. 2021, 36 (Suppl. S1), 25–38. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Hawk, E. Ensuring Equitable Cancer Care for All Patients; The ASCO Post; 2017; Available online: https://ascopost.com/issues/may-10-2017/ensuring-equitable-cancer-care-for-all-patients/ (accessed on 10 May 2022).
- Brammer, J.E.; Braunstein, Z.; Katapadi, A.; Porter, K.; Biersmith, M.; Guha, A.; Vasu, S.; Yildiz, V.O.; Smith, S.A.; Buck, B.; et al. Early toxicity and clinical outcomes after chimeric antigen receptor T-cell (CAR-T) therapy for lymphoma. J. Immunother. Cancer 2021, 9, e002303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Front. Oncol. 2019, 9, 1237. [Google Scholar] [CrossRef] [Green Version]

| Measure | Construct | Baseline | Day 0 | Days 1–6 | Day 7 | Day 14 | Day 21 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics survey | Demographics | X | ||||||||
| CCI | Comorbidities | X | ||||||||
| FACT-G | HRQOL | X | X | X | X | X | X | X | X | |
| PROMIS-29 + 2 Profile v2.1 | HRQOL | X | X | X | X | X | X | X | X | |
| FACT-G7 | HRQOL | X | ||||||||
| PRO-CTCAE items | Toxicity burden | X | X | X | X | X | X | X | X | |
| Study-specific survey | Acceptability | X |
| Variable | Statistic |
|---|---|
| Age, years; M (SD) range | 66 (7) 53–77 |
| Male gender; n (%) | 6 (50) |
| Race; n (%) | |
| White | 10 (83) |
| Black/African American | 2 (17) |
| Non-Hispanic; n (%) | 11 (92) |
| Highest education completed; n (%) | |
| Partial college or specialized training | 7 (58) |
| College or university | 4 (33) |
| Graduate degree | 1 (8) |
| Annual household income; n (%) | |
| USD20,000–USD39,999 | 2 (17) |
| USD40,000–USD59,999 | 1 (8) |
| USD60,000–USD100,000 | 3 (25) |
| >USD100,000 | 3 (25) |
| Prefer not to report | 3 (25) |
| Cancer diagnosis; n (%) | |
| Multiple myeloma | 7 (58) |
| Mantle cell lymphoma | 3 (25) |
| Chronic lymphoid leukemia | 1 (8) |
| Diffuse large B cell lymphoma | 1 (8) |
| Hospital length of stay, days; M (SD) range | 10 (7) 6–32 |
| Charlson Comorbidity Index; M (SD) range | 3 (1) 2–6 |
| Measure/Scale | Baseline | Day 0 | Day 7 | Day 14 | Day 21 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|---|---|---|
| FACT-G; M (SD) | ||||||||
| Total HRQOL | 81 (17) | 77 (19) | 75 (18) | 73 (18) | 75 (21) | 79 (19) | 85 (19) | 84 (19) |
| Physical well-being | 21 (5) | 20 (6) | 18 (7) | 19 (8) | 19 (7) | 21 (5) | 23 (5) | 23 (5) |
| Social well-being | 23 (5) | 23 (5) | 23 (5) | 22 (5) | 22 (5) | 23 (5) | 23 (5) | 23 (6) |
| Emotional well-being | 19 (4) | 20 (3) | 21 (2) | 20 (5) | 20 (4) | 20 (3) | 21 (3) | 20 (4) |
| Functional well-being | 17 (7) | 15 (7) | 13 (7) | 13 (5) | 14 (7) | 14 (7) | 18 (7) | 18 (8) |
| PROMIS-29 + 2 Profile v2.1; M (SD) | ||||||||
| Depression | 48 (7) | 47 (7) | 46 (6) | 48 (9) | 48 (9) | 47 (8) | 47 (7) | 45 (7) |
| Anxiety | 46 (7) | 46 (7) | 45 (6) | 47 (10) | 47 (8) | 48 (9) | 46 (8) | 45 (7) |
| Fatigue | 52 (11) | 56 (12) | 57 (13) | 58 (13) | 56 (11) | 55 (12) | 50 (10) | 47 (10) |
| Pain interference | 51 (9) | 52 (12) | 54 (10) | 55 (8) | 53 (11) | 52 (12) | 50 (9) | 49 (9) |
| Physical function | 43 (8) | 42 (7) | 38 (9) | 37 (11) | 39 (10) | 40 (9) | 44 (8) | 46 (8) |
| Sleep disturbance | 52 (10) | 54 (9) | 53 (9) | 50 (11) | 48 (8) | 48 (11) | 48 (9) | 47 (9) |
| Social roles and activities | 51 (10) | 48 (11) | 45 (12) | 44 (10) | 45 (10) | 44 (9) | 52 (10) | 52 (10) |
| Cognitive function | 55 (7) | 53 (9) | 52 (9) | 50 (7) | 50 (7) | 53 (7) | 55 (8) | 56 (6) |
| Global pain | 3 (3) | 3 (3) | 4 (3) | 3 (3) | 3 (3) | 3 (3) | 2 (3) | 3 (2) |
| Toxicity index; M (SD) | 2.9 (1.1) | 3.2 (0.8) | 3.4 (1.2) | 3.1 (0.9) | 2.6 (1.3) | 2.6 (1.1) | 2.8 (0.9) | 2.6 (0.8) |
| Daily steps; M (SD) a | - | 2155 (1425) | 1923 (947) | 2585 (1817) | 3171 (2273) | 3236 (2030) | 4575 (2083) | 5312 (1834) |
| Sleep efficiency; M (SD) a | - | 86% (3) | 86% (4) | 88% (4) | 86% (2) | 86% (3) | 87% (1) | 87% (1) |
| Measure | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | ||
| FACT-G7 total HRQOL; M (SD) | 17 (8) | 16 (5) | 17 (4) | 15 (5) | 18 (5) | 17 (6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oswald, L.B.; Li, X.; Carvajal, R.; Hoogland, A.I.; Gudenkauf, L.M.; Hansen, D.K.; Alsina, M.; Locke, F.L.; Rodriguez, Y.; Irizarry-Arroyo, N.; et al. Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization. Cancers 2022, 14, 2742. https://doi.org/10.3390/cancers14112742
Oswald LB, Li X, Carvajal R, Hoogland AI, Gudenkauf LM, Hansen DK, Alsina M, Locke FL, Rodriguez Y, Irizarry-Arroyo N, et al. Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization. Cancers. 2022; 14(11):2742. https://doi.org/10.3390/cancers14112742
Chicago/Turabian StyleOswald, Laura B., Xiaoyin Li, Rodrigo Carvajal, Aasha I. Hoogland, Lisa M. Gudenkauf, Doris K. Hansen, Melissa Alsina, Frederick L. Locke, Yvelise Rodriguez, Nathaly Irizarry-Arroyo, and et al. 2022. "Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization" Cancers 14, no. 11: 2742. https://doi.org/10.3390/cancers14112742
APA StyleOswald, L. B., Li, X., Carvajal, R., Hoogland, A. I., Gudenkauf, L. M., Hansen, D. K., Alsina, M., Locke, F. L., Rodriguez, Y., Irizarry-Arroyo, N., Robinson, E. J., Jim, H. S. L., Gonzalez, B. D., & Kirtane, K. (2022). Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization. Cancers, 14(11), 2742. https://doi.org/10.3390/cancers14112742






