Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System
Abstract
Simple Summary
Abstract
1. Introduction
2. Impact of Hyperoxia on Different Cancer Therapies
2.1. Conventional Therapies and Hyperoxia
2.1.1. Chemotherapy
2.1.2. Radiotherapy
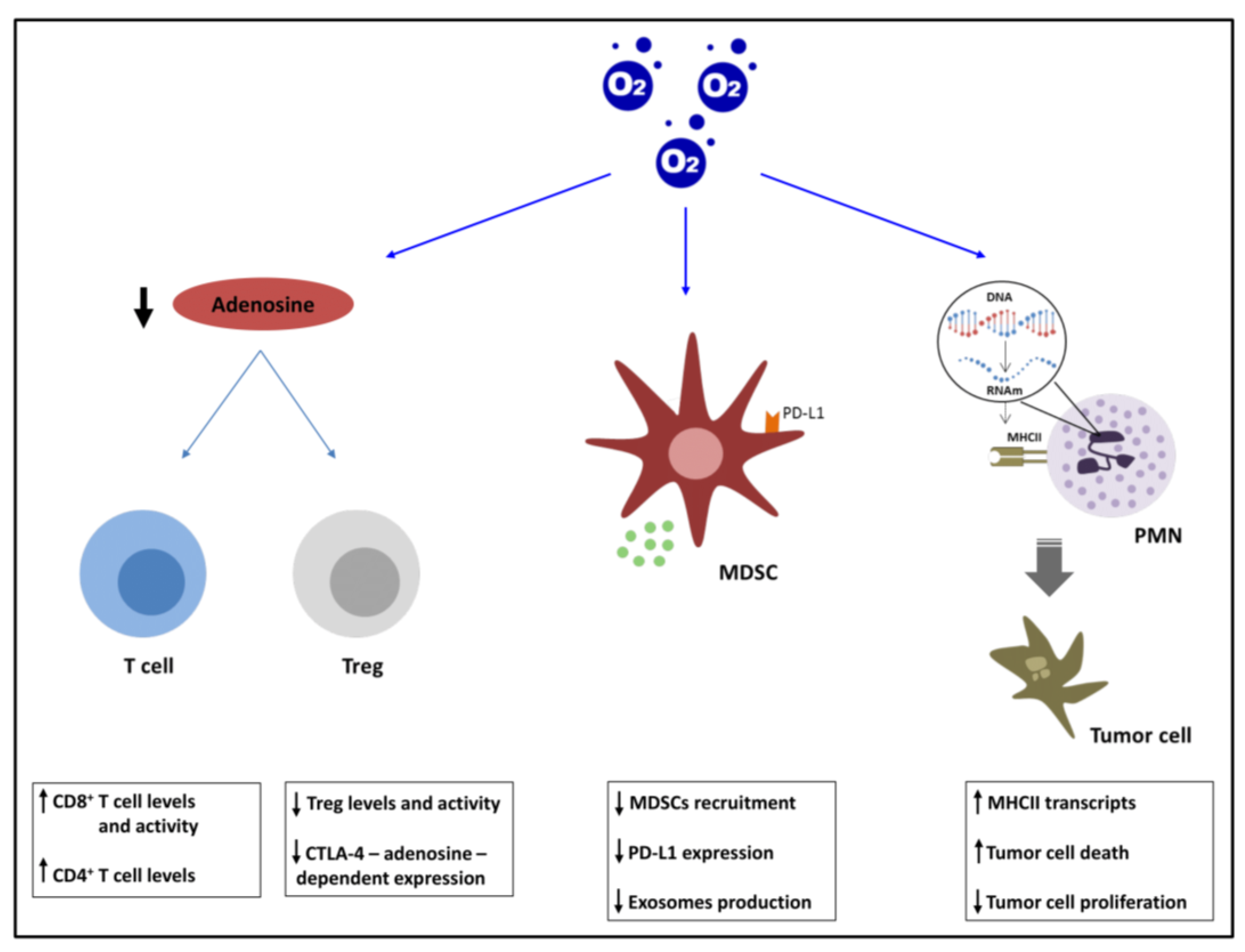
2.2. Cancer Immunotherapy and Hyperoxia
2.2.1. Adenosinergic Pathway
2.2.2. MDSCs and PDL1
2.2.3. PMNs
3. Adverse Effects Derived from the Use of Hyperoxia
3.1. Hyperoxia-Induced Acute Lung Injury
3.2. Protumor Inflammation
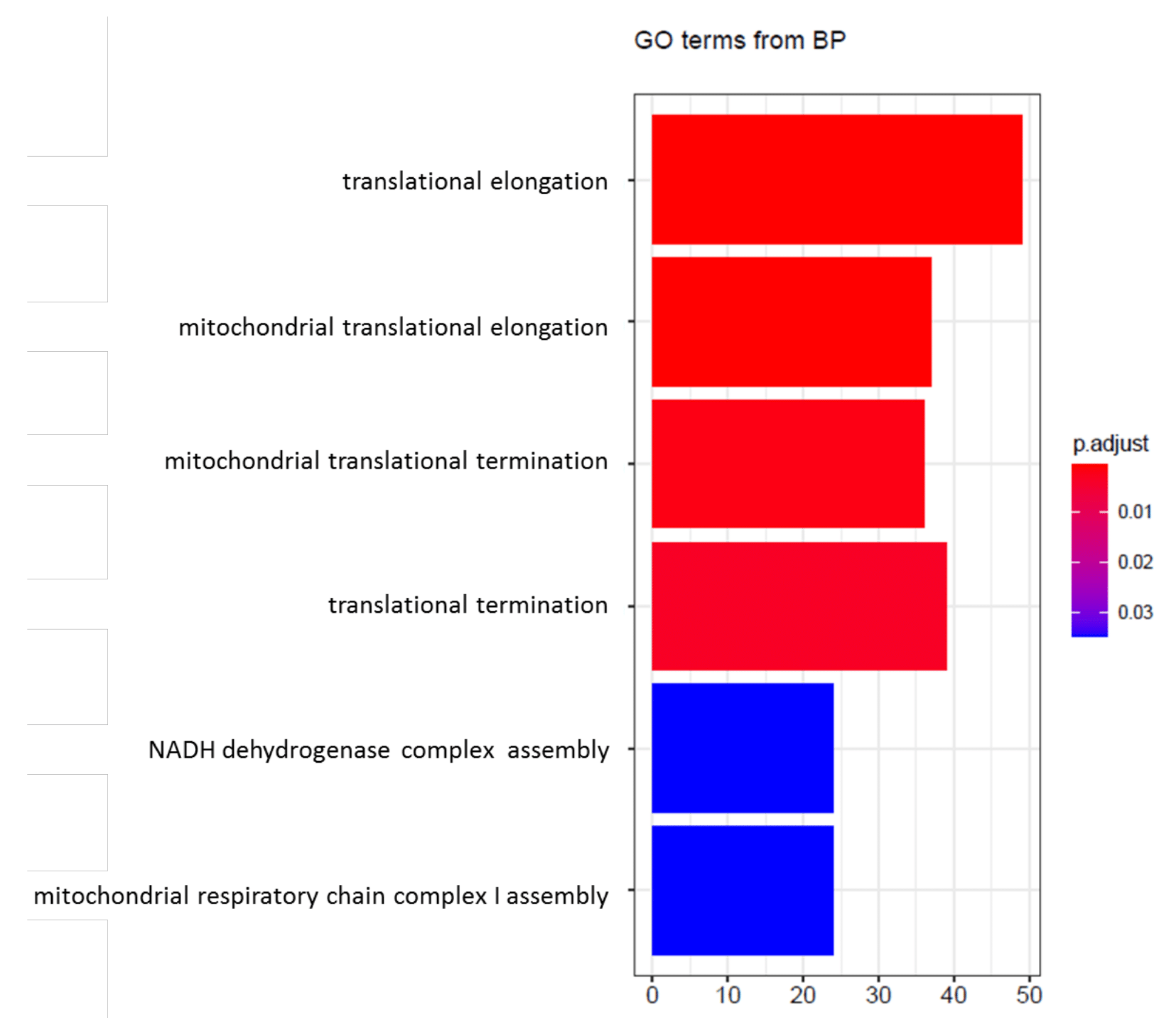
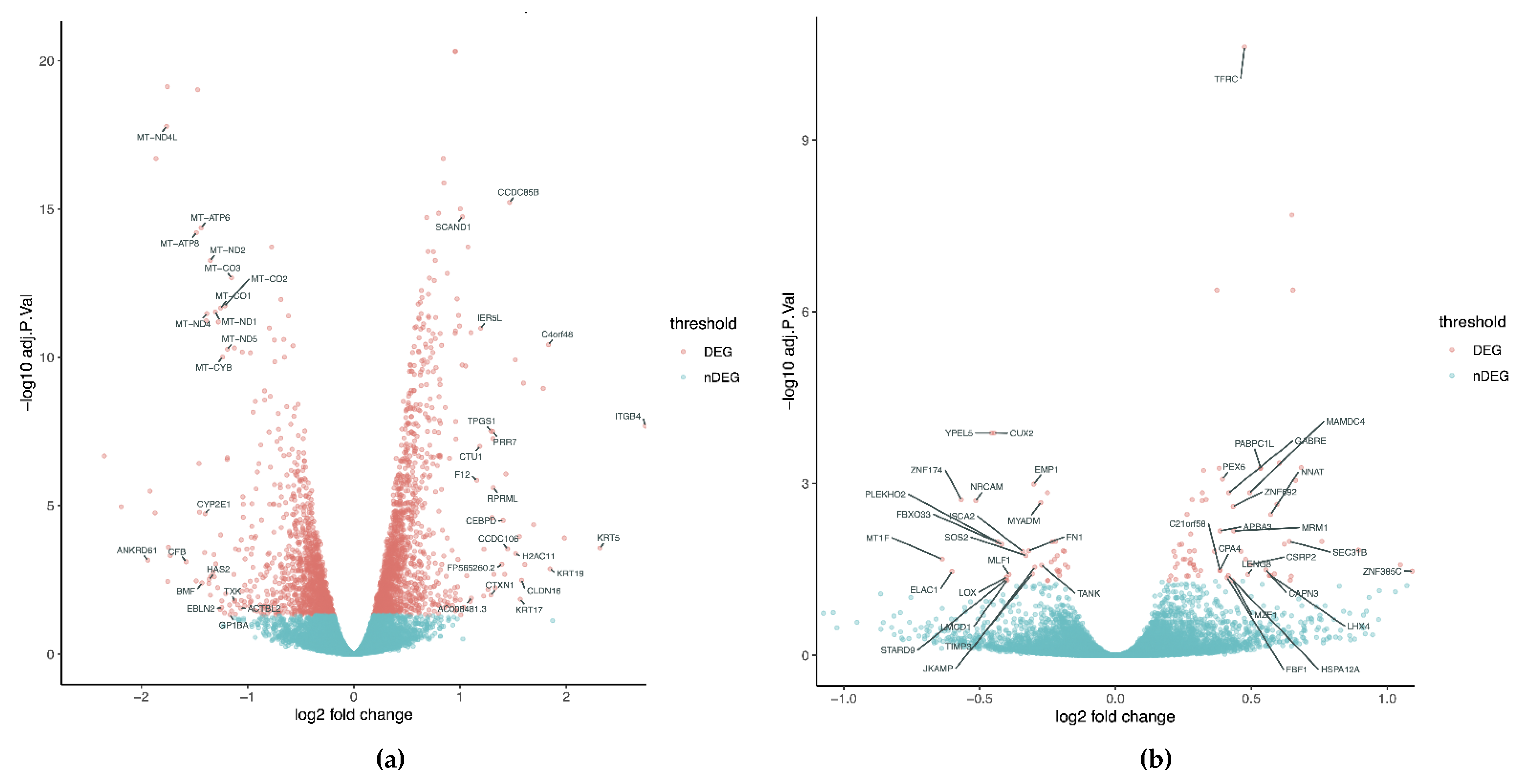
4. Transcriptional Profile
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michiels, C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Pringle, K.; Kind, K.; Thompson, J.; Roberts, C. Complex Interactions Between Hypoxia Inducible Factors, Insulin-Like Growth Factor-II and Oxygen in Early Murine Trophoblasts. Placenta 2007, 28, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and Developmental Control of O2 Homeostasis by Hypoxia-Inducible Factor 1. 1998. Available online: www.genesdev.org (accessed on 15 September 2021).
- Guimbellot, J.S.; Erickson, S.W.; Mehta, T.; Wen, H.; Page, G.P.; Sorscher, E.J.; Hong, J.S. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med. Genom. 2009, 2, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Benita, Y.; Kikuchi, H.; Smith, A.; Zhang, M.Q.; Chung, D.C.; Xavier, R.J. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009, 37, 4587–4602. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Chen, D.; Jiang, X.; Wang, W.; Li, D.; Shan, H. Hypoxic Hepatocellular Carcinoma Cells Acquire Arsenic Trioxide Resistance by Upregulating HIF-1α Expression. Am. J. Dig. Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Forsythe, A.J.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, X.; Li, J.; Cui, T.; Wei, Y.; Xu, J.; Zhu, Y.; Zhu, X. Mild Hypoxia Enhances the Expression of HIF and VEGF and Triggers the Response to Injury in Rat Kidneys. Front. Physiol. 2021, 12, 986. [Google Scholar] [CrossRef]
- Chen, J.; Cui, B.; Fan, Y.; Li, X.; Li, Q.; Du, Y.; Feng, Y.; Zhang, P. Protein kinase D1 regulates hypoxic metabolism through HIF-1α and glycolytic enzymes incancer cells. Oncol. Rep. 2018, 40, 1073–1082. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Rempel, A.; Pedersen, P.L. Glucose Catabolism in Cancer Cells: Identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J. Biol. Chem. 2001, 276, 43407–43412. [Google Scholar] [CrossRef]
- Hoefflin, R.; Harlander, S.; Schäfer, S.; Metzger, P.; Kuo, F.; Schönenberger, D.; Adlesic, M.; Peighambari, A.; Seidel, P.; Chen, C.-Y.; et al. HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun. 2020, 11, 4111. [Google Scholar] [CrossRef]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-inducible Factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cao, X.; Zhang, W.; Pan, G.; Yi, Q.; Zhong, W.; Yan, D. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J. 2018, 33, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kim, D.-Y.; Kim, W. Cultivation of human skin cells under physiological oxygen concentration modulates expression of skin significant genes and response to hydroxy acids. Biochem. Biophys. Res. Commun. 2021, 551, 161–167. [Google Scholar] [CrossRef]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Vélez, J.; Silva, L.M.R.; Gärtner, U.; Daugschies, A.; Mazurek, S.; Hermosilla, C.; Taubert, A. First Metabolic Insights into Ex Vivo Cryptosporidium parvum-Infected Bovine Small Intestinal Explants Studied under Physioxic Conditions. Biology 2021, 10, 963. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Q.; Zhang, Y.; Yu, M.; Jing, W.; Tian, W. Physioxia: A more effective approach for culturing human adipose-derived stem cells for cell transplantation. Stem Cell Res. Ther. 2018, 9, 148. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Salceda, S.; Caro, J. Hypoxia-inducible Factor 1α (HIF-1α) Protein Is Rapidly Degraded by the Ubiquitin-Proteasome System under Normoxic Conditions. J. Biol. Chem. 1997, 272, 22642–22647. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Thomlinson, R.H. Hypoxia and tumours. J. Clin. Pathol. 1977, 11, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Thomlinson, R.H.; Gray, L.H. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lee, D.; Lee, N.P.; Pu, J.K.S.; Wong, T.S.; Lui, W.M.; Fung, C.F.; Leung, G.K.K. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J. Neuro-Oncol. 2012, 109, 467–475. [Google Scholar] [CrossRef]
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy—PubMed. Cancer Res. 1998, 7, 1408–1416. [Google Scholar]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef]
- Daruwalla, J.; Christophi, C. Hyperbaric Oxygen Therapy for Malignancy: A Review. World J. Surg. 2006, 30, 2112–2131. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Y.; Huang, W.; Li, J.; Zhang, B.; Li, Z.; Yang, X. Hyperbaric Oxygen Potentiates Doxil Antitumor Efficacy by Promoting Tumor Penetration and Sensitizing Cancer Cells. Adv. Sci. 2018, 5, 1700859. [Google Scholar] [CrossRef]
- Raa, A.; Stansberg, C.; Steen, V.M.; Bjerkvig, R.; Reed, R.K.; Stuhr, L.E.B. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer 2007, 7, 23. [Google Scholar] [CrossRef][Green Version]
- Moen, I.; Tronstad, K.J.; Kolmannskog, O.; Salvesen, G.S.; Reed, R.K.; Stuhr, L.E.B. Hyperoxia increases the uptake of 5-fluorouracil in mammary tumors independently of changes in interstitial fluid pressure and tumor stroma. BMC Cancer 2009, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Minami, A.; Uchida, J.; Nakatani, T. Potential of hyperbaric oxygen in urological diseases. Int. J. Urol. 2019, 26, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Aphale, R.; Shah, S.M. A Randomised Clinical Trial to Compare the Efficacy of Hyperbaric Oxygen Therapy with Neoadjuvant Chemotherapy with Neoadjuvant Chemotherapy Alone for Carcinoma Breast: A Pilot Study. Indian J. Surg. 2020, 83, 511–515. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, Y.; Ji, X.; Meng, R. Advances in Normobaric Hyperoxia Brain Protection in Experimental Stroke. Front. Neurol. 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Thews, O.; Vaupel, P. Spatial oxygenation profiles in tumors during normo- and hyperbaric hyperoxia. Strahlenther. Onkol. 2015, 191, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, I.K.; Lee, S.H. Role of hyperoxic treatment in cancer. Exp. Biol. Med. 2020, 245, 851–860. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, I.K.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Kim, J.W.; Lee, S.H. Normobaric hyperoxia inhibits the progression of lung cancer by inducing apoptosis. Exp. Biol. Med. 2018, 243, 739–748. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, I.K.; Lee, H.I.; Lee, H.Y.; Kang, H.S.; Yeo, C.D.; Kang, H.H.; Moon, H.S.; Lee, S.H. Combination of carboplatin and intermittent normobaric hyperoxia synergistically suppresses benzo[a]pyrene-induced lung cancer. Korean J. Intern. Med. 2018, 33, 541–551. [Google Scholar] [CrossRef]
- Jiang, M.-S.; Yin, X.-Y.; Qin, B.; Xuan, S.-Y.; Yuan, X.-L.; Yin, H.; Zhu, C.; Li, X.; Yang, J.; Du, Y.-Z.; et al. Inhibiting Hypoxia and Chemotherapy-Induced Cancer Cell Metastasis under a Valid Therapeutic Effect by an Assistance of Biomimetic Oxygen Delivery. Mol. Pharm. 2019, 16, 4530–4541. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Sun, Y.; Liu, X.; Chen, Y.; Chen, C.; Yan, R.; Fan, T.; Yang, T.; Lu, Y.; et al. A smart O2-generating nanocarrier optimizes drug transportation comprehensively for chemotherapy improving. Acta Pharm. Sin. B 2021, 11, 3608–3621. [Google Scholar] [CrossRef]
- Rojas, A.; Carl, U.; Reghebi, K. Effect of normobaric oxygen on tumor radiosensitivity: Fractionated studies. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 547–553. [Google Scholar] [CrossRef]
- Rijpkema, M.; Kaanders, J.H.; Joosten, F.B.; van der Kogel, A.J.; Heerschap, A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int. J. Radiat. Oncol. 2002, 53, 1185–1191. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Bae, H.C.; Noh, K.H.; Song, K.-H.; Ye, S.-K.; Mao, C.-P.; Lee, K.-M.; Wu, T.-C.; Kim, T.W. Gain of HIF-1α under Normoxia in Cancer Mediates Immune Adaptation through the AKT/ERK and VEGFA Axes. Clin. Cancer Res. 2015, 21, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fu, Y.; Chen, F.; Zhang, J.; Jia, H.; Li, J.; Wang, H.; Wen, J. Hyperoxia sensitizes hypoxic HeLa cells to ionizing radiation by downregulating HIF-1α and VEGF expression. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Co, J.; De Moraes, M.V.; Katznelson, R.; Evans, A.W.; Shultz, D.; Laperriere, N.; Millar, B.-A.; Berlin, A.; Kongkham, P.; Tsang, D.S. Hyperbaric Oxygen for Radiation Necrosis of the Brain. Can. J. Neurol. Sci. 2020, 47, 92–99. [Google Scholar] [CrossRef]
- Mast, J.M.; Kuppusamy, P. Hyperoxygenation as a Therapeutic Supplement for Treatment of Triple Negative Breast Cancer. Front. Oncol. 2018, 8, 527. [Google Scholar] [CrossRef]
- Sachdeva, S.; Gupta, M. Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm. J. 2013, 21, 245–253. [Google Scholar] [CrossRef]
- Liu, H.; Xia, Y. Beneficial and detrimental role of adenosine signaling in diseases and therapy. J. Appl. Physiol. 2015, 119, 1173–1182. [Google Scholar] [CrossRef]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef]
- Kobayashi, S.; Zimmermann, H.; Millhorn, D.E. Chronic Hypoxia Enhances Adenosine Release in Rat PC12 Cells by Altering Adenosine Metabolism and Membrane Transport. J. Neurochem. 2000, 74, 621–632. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Belikoff, B.; Schreiber, T.; Sethumadhavan, S.; Abbott, R.; Philbrook, P.; Thayer, M.; Shujia, D.; et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. Klin. Wochenschr. 2014, 92, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Sitkovsky, M.V. Antihypoxic oxygenation agents with respiratory hyperoxia to improve cancer immunotherapy. J. Clin. Investig. 2020, 130, 5629–5637. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell. Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Sundström, P.; Stenstad, H.; Langenes, V.; Ahlmanner, F.; Theander, L.; Ndah, T.G.; Fredin, K.; Börjesson, L.; Gustavsson, B.; Bastid, J.; et al. Regulatory T Cells from Colon Cancer Patients Inhibit Effector T-cell Migration through an Adenosine-Dependent Mechanism. Cancer Immunol. Res. 2016, 4, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Clambey, E.T.; McNamee, E.N.; Westrich, J.A.; Glover, L.E.; Campbell, E.L.; Jedlicka, P.; de Zoeten, E.F.; Cambier, J.C.; Stenmark, K.R.; Colgan, S.P.; et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA 2012, 109, E2784–E2793. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Ardebili, S.M.; Moornani, M.B.; Masjedi, A.; Atyabi, F.; Kiani, M.; Namdar, A.; Karpisheh, V.; Izadi, S.; Baradaran, B.; et al. Silencing of HIF-1α/CD73 axis by siRNA-loaded TAT-chitosan-spion nanoparticles robustly blocks cancer cell progression. Eur. J. Pharmacol. 2020, 882, 173235. [Google Scholar] [CrossRef]
- Khadge, S.; Cole, K.; Talmadge, J.E. Myeloid derived suppressor cells and the release of micro-metastases from dormancy. Clin. Exp. Metastasis 2021, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.-I.; Cheng, P.; Cho, H.-I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, Q.; Shao, N.; Shan, Z.; Cheang, T.; Zhang, Z.; Su, Q.; Wang, S.; Li, Y. Respiratory hyperoxia reverses immuno-suppression by regulating myeloid-derived suppressor cells and PD-L1 expression in a triple-negative breast cancer mouse model. Am. J. Cancer Res. 2019, 9, 529–545. [Google Scholar] [PubMed]
- Hanidziar, D.; Nakahori, Y.; Cahill, L.; Gallo, D.; Keegan, J.W.; Nguyen, J.P.; Otterbein, L.E.; Lederer, J.A.; Robson, S.C. Characterization of pulmonary immune responses to hyperoxia by high-dimensional mass cytometry analyses. Sci. Rep. 2020, 10, 4677. [Google Scholar] [CrossRef]
- Souto, J.C.; Vila, L.; Brú, A. Polymorphonuclear neutrophils and cancer: Intense and sustained neutrophilia as a treatment against solid tumors. Med. Res. Rev. 2009, 31, 311–363. [Google Scholar] [CrossRef]
- Lerman, I.; Hammes, S.R. Neutrophil elastase in the tumor microenvironment. Steroids 2018, 133, 96–101. [Google Scholar] [CrossRef]
- Mahiddine, K.; Blaisdell, A.; Ma, S.; Créquer-Grandhomme, A.; Lowell, C.A.; Erlebacher, A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J. Clin. Investig. 2019, 130, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Martins, J.D.S.; Bos, S.A.; Courties, G.; Rickelt, S.; Severe, N.; Baryawno, N.; Faget, J.; et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 2017, 358, eaal5081. [Google Scholar] [CrossRef]
- Ni, Y.-N.; Wang, Y.-M.; Liang, B.-M.; Liang, Z.-A. The effect of hyperoxia on mortality in critically ill patients: A systematic review and meta analysis. BMC Pulm. Med. 2019, 19, 53. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Dias-Freitas, F.; Coimbra, C.M.; Roncon-Albuquerque, R. Molecular mechanisms underlying hyperoxia acute lung injury. Respir. Med. 2016, 119, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ristescu, A.I.; Tiron, C.E.; Tiron, A.; Grigoras, I. Exploring Hyperoxia Effects in Cancer—From Perioperative Clinical Data to Potential Molecular Mechanisms. Biomedicines 2021, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.H.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Fang, F.; Xu, F. Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-κB-dependent mechanism. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1399–1410. [Google Scholar] [PubMed]
- Zou, D.; Li, J.; Fan, Q.; Zheng, X.; Deng, J.; Wang, S. Reactive oxygen and nitrogen species induce cell apoptosis via a mitochondria-dependent pathway in hyperoxia lung injury. J. Cell. Biochem. 2019, 120, 4837–4850. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Basabe, A.; Strati, F.; Facciotti, F. License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 3909. [Google Scholar] [CrossRef]
- Nowak-Machen, M.; Schmelzle, M.; Hanidziar, D.; Junger, W.; Exley, M.; Otterbein, L.; Wu, Y.; Csizmadia, E.; Doherty, G.; Sitkovsky, M.; et al. Pulmonary Natural Killer T Cells Play an Essential Role in Mediating Hyperoxic Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Singer, M.; Young, P.J.; Laffey, J.G.; Asfar, P.; Taccone, F.S.; Skrifvars, M.B.; Meyhoff, C.S.; Radermacher, P. Dangers of hyperoxia. Crit. Care 2021, 25, 440. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.; Haudek-Prinz, V.; Slany, A.; Grillari, J.; Micksche, M.; Gerner, C. Proteome profiling in IL-1β and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS ONE 2017, 12, e0179065. [Google Scholar] [CrossRef] [PubMed]
- Sirotkovic-Skerlev, M.; Ana, K.; Bradic, L.; Cacev, T. Protumor effects of proinflammatory mediators in breast cancer. Pero-Dicium Biol. 2012, 114, 489–496. [Google Scholar]
- Chen, L.; Huang, C.-F.; Li, Y.-C.; Deng, W.-W.; Mao, L.; Wu, L.; Zhang, W.-F.; Zhang, L.; Sun, Z.-J. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 2018, 75, 2045–2058. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Kawabata-Iwakawa, R.; Rokudai, S.; Toyoda, M.; Nishiyama, M.; Chikamatsu, K. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2015, 64, 1407–1417. [Google Scholar] [CrossRef]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J.; Yokota, S.J.; Bankert, R.B. Characterization of Human Lung Tumor-Associated Fibroblasts and Their Ability to Modulate the Activation of Tumor-Associated T Cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Hooper, S.; Nye, E.; Williamson, P.; Harrington, K.; Sahai, E. Autocrine IL-1β-TRAF6 signalling promotes squamous cell carcinoma invasion through paracrine TNFα signalling to carcinoma-associated fibroblasts. Oncogene 2013, 32, 747–758. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8, 8633–8647. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Bunt, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation Induces Myeloid-Derived Suppressor Cells that Facilitate Tumor Progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Avraham, I.; Dor, Y.; Bachar-Lustig, E.; Itin, A.; Yung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. VEGF-Induced Adult Neovascularization: Recruitment, Retention, and Role of Accessory Cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Ferrara, N. Developmental and Pathological Angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Blythe, T.; Ourradi, K.; Jarrett, C.; Welsh, G.I.; Bates, D.O.; Millar, A.B. Effects of hypoxia and hyperoxia on the differential expression of VEGF-A isoforms and receptors in Idiopathic Pulmonary Fibrosis (IPF). Respir. Res. 2018, 19, 9. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Dinarello, C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010, 29, 317–329. [Google Scholar] [CrossRef]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The Role of IL-1β in the Early Tumor Cell–Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef]
- Stasinopoulos, I.; O’Brien, D.R.; Bhujwalla, Z.M. Inflammation, but not hypoxia, mediated HIF-1α activation depends on COX-2. Cancer Biol. Ther. 2009, 8, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Koh, Y.; Kim, D.-W.; Ahn, Y.-O.; Kim, T.M.; Han, S.-W.; Oh, D.-Y.; Lee, S.-H.; Im, S.-A.; Heo, D.S.; et al. Clinical Implications of VEGF, TGF-beta1, and IL-1beta in Patients with Advanced Non-small Cell Lung Cancer. Cancer Res. Treat. 2013, 45, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; Solimando, A.G.; Krebs, M.; Leone, P.; Susca, N.; Brunetti, O.; Racanelli, V.; Vacca, A.; Silvestris, N. Anti-angiogenesis and Immunotherapy: Novel Paradigms to Envision Tailored Approaches in Renal Cell-Carcinoma. J. Clin. Med. 2020, 9, 1594. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Solimando, A.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Andrés-León, E.; Núñez-Torres, R.; Rojas, A.M. miARma-Seq: A comprehensive tool for miRNA, mRNA and circRNA analysis. Sci. Rep. 2016, 6, 25749. [Google Scholar] [CrossRef]
- Andrés-León, E.; Rojas, A.M. miARma-Seq, a comprehensive pipeline for the simultaneous study and integration of miRNA and mRNA expression data. Methods 2019, 152, 31–40. [Google Scholar] [CrossRef]
- Babraham Bioinformatics-FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 27 April 2022).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Nikolayeva, O.; Robinson, M.D. EdgeR for differential rna-seq and ChIP-seq analysis: An application to stem cell biology. In Stem Cell Transcriptional Networks: Methods and Protocols; Kidder, B.L., Ed.; Humana Press: New York, NY, USA, 2014; pp. 45–79. [Google Scholar] [CrossRef]
- Reeb, P.D.; Bramardi, S.J.; Steibel, J.P. Assessing Dissimilarity Measures for Sample-Based Hierarchical Clustering of RNA Sequencing Data Using Plasmode Datasets. PLoS ONE 2015, 10, e0132310. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Campos, A.B.; Zamudio-Martinez, E.; Delgado-Bellido, D.; Fernández-Cortés, M.; Montuenga, L.M.; Oliver, F.J.; Garcia-Diaz, A. Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System. Cancers 2022, 14, 2740. https://doi.org/10.3390/cancers14112740
Herrera-Campos AB, Zamudio-Martinez E, Delgado-Bellido D, Fernández-Cortés M, Montuenga LM, Oliver FJ, Garcia-Diaz A. Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System. Cancers. 2022; 14(11):2740. https://doi.org/10.3390/cancers14112740
Chicago/Turabian StyleHerrera-Campos, Ana Belén, Esteban Zamudio-Martinez, Daniel Delgado-Bellido, Mónica Fernández-Cortés, Luis M. Montuenga, F. Javier Oliver, and Angel Garcia-Diaz. 2022. "Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System" Cancers 14, no. 11: 2740. https://doi.org/10.3390/cancers14112740
APA StyleHerrera-Campos, A. B., Zamudio-Martinez, E., Delgado-Bellido, D., Fernández-Cortés, M., Montuenga, L. M., Oliver, F. J., & Garcia-Diaz, A. (2022). Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System. Cancers, 14(11), 2740. https://doi.org/10.3390/cancers14112740






