1. Introduction
Expression of the F-actin-bundling protein Fascin-1 (Fscn1) is tightly regulated at the transcriptional level [
1,
2] and, under homeostatic conditions, is largely confined to neuronal and glial cells, as well as some endothelial cell populations [
3]. Fscn1 was demonstrated to be required for the growth and stabilization of axons in the case of neuronal cells [
4] and to support the formation and turnover of filopodial extensions in other cell types [
3]. Interestingly, de novo expression of Fscn1 was reported early for Epstein–Barr virus-infected B cells [
5] and subsequently for human T-lymphotropic virus type 1-infected T cells [
6]. In addition, immortalized tumor cell lines [
7] were reported to express Fscn1 and have frequently been used to as cell models to delineate the interaction of Fscn1 with other cytoskeletal proteins, such as cofilin-1 [
8,
9] and Daam1 (disheveled-associated activator of morphogenesis 1) [
10,
11], among others [
12].
Fscn1 may also be expressed de novo by tumor cells [
13,
14]. In response to the expression of Fscn1, tumor cells were reported to display increased migratory activity, thereby enhancing the invasive and metastatic properties of the tumor [
15]. Accordingly, a number of studies have correlated poor prognosis to the extent of Fscn1 expression [
16]. Besides its structural properties, Fscn1 was also shown to actively translocate from the cytoplasm into the nucleus of tumor cells and to positively regulate expression of pro-tumorigenic genes, such as the amino-acid transporter solute carrier family 3 member 2 [
17] and to promote pro-tumorigenic canonical wingless signaling via activation of activation of focal adhesion kinase [
18]. To date, several Fscn1 inhibitors that block interaction of Fscn1 with F-actin in order to inhibit tumor metastasis have been developed and successfully evaluated in vitro and in preclinical models [
19,
20,
21]. More recently, a clinical phase I trial demonstrated that oral application of a Fscn1 inhibitor was well-tolerated and demonstrated antimetastatic activity, with increased progression-free survival in a number of patients [
22]. Based on these results, a phase 2A clinical trial enrolling ovarian cancer patients to be treated with the Fscn1 inhibitor alone or in combination with a checkpoint inhibitor is planned.
Aside from constitutive expression of Fscn1 in some cell types and its de novo expression in virus-infected and malignant cells, we and others have previously shown that Fscn1 is strongly upregulated in stimulated dendritic cells (DCs) [
23,
24]. DCs are scattered throughout the body and constantly internalize extracellular material [
25]. A fraction of DCs migrates into lymph nodes and the spleen to present derived oligopeptide antigens to T cells. Under homeostatic conditions, DCs induce peripheral T-cell tolerance towards self and harmless environmental antigens due to the absence of T-cell costimulatory signals. However, in response to pathogen-derived and endogenous danger signals, DCs are activated, upregulate surface expression of antigen-presenting receptors and costimulators and migrate to secondary lymphoid organs in elevated numbers to induce T-effector cells [
26]. In that regard, activated DCs are the most potent antigen-presenting cell population and are the main inducers of primary immune responses directed, for example, against malignant cells [
27].
We have previously shown that stimulation-induced Fscn1 in DCs is responsible for the formation of dendritic protrusions [
23,
24]. Dendritic protrusions may contribute to the migratory activity of activated DCs, as deduced from impaired migration of Hela cells in response to Fscn1 inhibition [
7]. Subsequently, Yamakita and colleagues demonstrated that DCs of Fascin-1 knockout mice displayed attenuated migratory activity in vivo [
28].
Previously, it was shown that inhibition of Fscn1 in stimulated DCs using antisense oligonucleotides attenuated their T-cell stimulatory capacity, although surface expression of major histocompatibility complex (MHC)II and costimulators remained unaltered [
29]. We demonstrated that Fscn1 colocalized with F-actin and that this complex accumulated within the immunological synapse (IS) formed between DCs and antigen-specific T cells [
30]. In the case of DCs, which lacked the costimulators CD80 and CD86, IS formation was weak and accompanied by strongly attenuated Fscn1/F-actin accumulation. Accordingly, Elizondo and colleagues reported that CD40-deficient DCs displayed attenuated Fscn1 expression in response to stimulation, which was associated with impaired DC/T-cell interactions and T-cell hypoproliferation [
31]. Forced overexpression of Fscn1 rescued the attenuated T-cell stimulatory capacity of CD40-deficient DCs.
In light of the important role of DCs in the induction of (antitumor) T-cell responses and the well-established role of Fscn1 for the functional activity of activated DC in migration and T-cell activation, the intent of this investigation is to elucidate the impact of Fscn1 inhibitors developed for tumor therapy with DCs. Treatment of bone-marrow-derived DCs (BMDCs) with two structurally distinct Fscn1 inhibitors, NP-G2-044 and BDP-13176, interfered with the acquisition of a stimulation-induced mature immune phenotype, which was correlated with the applied dose (NP-G2-044 > BDP-13176). Interestingly, both inhibitors evoked PD-L2 expression in BMDCs. Of note, NP-G2-044 also attenuated antigen uptake by unstimulated BMDCs. Furthermore, both inhibitors diminished Fscn1 expression in stimulated BMDCs and attenuated both their interaction with antigen-specific CD4+ T cells and their T-cell stimulatory capacity, which was also apparent when applying Fscn1 inhibitors directly to DC/T-cell cocultures. However, both Fscn1 inhibitors also differentially affected T-cell activation by agonistic antibodies, suggesting unspecified off-target effects. Altogether, our results indicate that Fscn1 inhibitors developed for tumor therapy may also interfere with adaptive antitumor immune responses on several levels.
2. Materials and Methods
2.1. Materials
Fscn1 inhibitors NP-G2-044 (Selleckchem, Houston, TX, USA) and BDP-13176 (MedChemExpress, Monmouth Junction, NJ, USA) were reconstituted in DMSO (Roth, Karlsruhe, Germany). APC-eFl70-labeled anti-CD11c (clone N418), FITC-MHCI (28148), eFl450-MHCII (M5/114.15.2), APC-CD40 (1C10), PerCP-eFl710-CD80 (16-10A1), PE/Cy7-CD86 (GL-1), PE/TexasRed-CD274/PD-L1 (10F.9G2), PE-CD273/PD-L2 (Ty25), eFl506-CD3 (145-2C11), SB600-CD11b (M1/70), SB702-CD19 (eBio103), PE-NK1.1 (PK136), PE-eFl610-Ly6G (1A8-L6g), eFl780-FVD and eFl450-FVD used for flow cytometric analysis were purchased from BD Biosciences (Franklin Lakes, NJ, USA), BioLegend (San Diego, CA, USA) or ThermoFisher (Waltham, MA, USA). Unlabeled mouse anti-human Fscn1 antibody (clone 55K2; Sigma-Aldrich, Deisenhofen, Germany), a corresponding isotype control antibody (mouse IgG1, clone MOPC-21, BioLegend), secondary AF488-labeled IgG goat anti-mouse antibody (948492), AF647-labeled anti-CD11c antibody (clone N418), Hoechst (nuclear staining) and Alexa Fluor 555 phalloidin (F-actin) (all from ThermoFisher) were used for confocal laser scanning analysis (CLSM).
2.2. Mice
C57BL/6 mice, as well as OT-I [
32] and OT-II [
33] mice on C57BL/6 background, were bred and maintained in the Central Animal Facility of the Johannes Gutenberg-University Mainz under specific pathogen-free conditions on a standard diet according to the guidelines of the regional animal care committee. The “Principles of Laboratory Animal Care” (NIH publication no. 85-23, revised 1985) were followed. Mice (6–12 weeks) were sacrificed for organ retrieval according to § 4(3) TierSchG.
2.3. Cell Culture
Spleens were mechanically disrupted using a pestle and a 40 µM cell strainer (Greiner Bio-One, Frickenhausen, Germany) to obtain a single-cell suspension. Spleen cells (2 × 106/500 µL) were cultured in FACS tubes overnight in medium (IMDM, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin (all from Sigma-Aldrich, Deisenhofen, Germany) and 50 µM ß-mercaptoethanol (Roth) containing 5% FBS (PAN-Biotech, Aidenbach, Germany)). Bone marrow cells (2 × 105/mL) were seeded in 12-well suspension culture plates (Greiner Bio-One) in culture medium supplemented with recombinant murine GM-CSF (10 ng/mL; Miltenyi, Bergisch Gladbach, Germany). Culture media were replenished on days 3 and 6 of culture.
2.4. Confocal Laser Scanning Microscopy (CLSM)
BMDCs (days 6–7 of culture) were incubated overnight with Fscn1 inhibitors, harvested and resuspended (106/mL) in staining buffer. Cells were cytospun (4000 rpm, 5 min, room temperate) onto microscope slides (Superfrost Plus; VWR, Darmstadt, Germany) using a Cytospin 3 (Thermo Fisher, Waltham, MA, USA). Samples were incubated with pre-cooled methanol (Carl Roth) for 10 min for permeabilization of cell membranes and washed 2 times with PBS. Cytospins were incubated with PBS/2% bovine serum albumin (Sigma-Aldrich, Deisenhofen, Germany) plus 2.4-G2 antibody (1:50) for 10 min in a humified chamber at room temperature to block unspecified binding sites. Afterwards, samples were incubated with Fscn1-specific (diluted 1:50 in PBS/2% FBS) or isotype control (1:50 in PBS/2% FBS) for 20 min at room temperature in a humidified chamber. Samples were washed with PBS and incubated with secondary anti-mouse (1:400 in PBS/2% FBS), CD11c-specific antibody (1:50 in PBS/2% FBS) and phalloidin (1:150 in PBS/2%FBS) for 20 min at 4 °C in a humified chamber. After washing 2 times (PBS), samples were incubated with Hoechst dye (1 µg/mL) for 5 min and washed with purified water. In control settings, cytospins were left untreated or were incubated with one agent only. Finally, cytospins were covered with fluorescence mounting medium (DAKO; Agilent, Santa Clara, CA, USA).
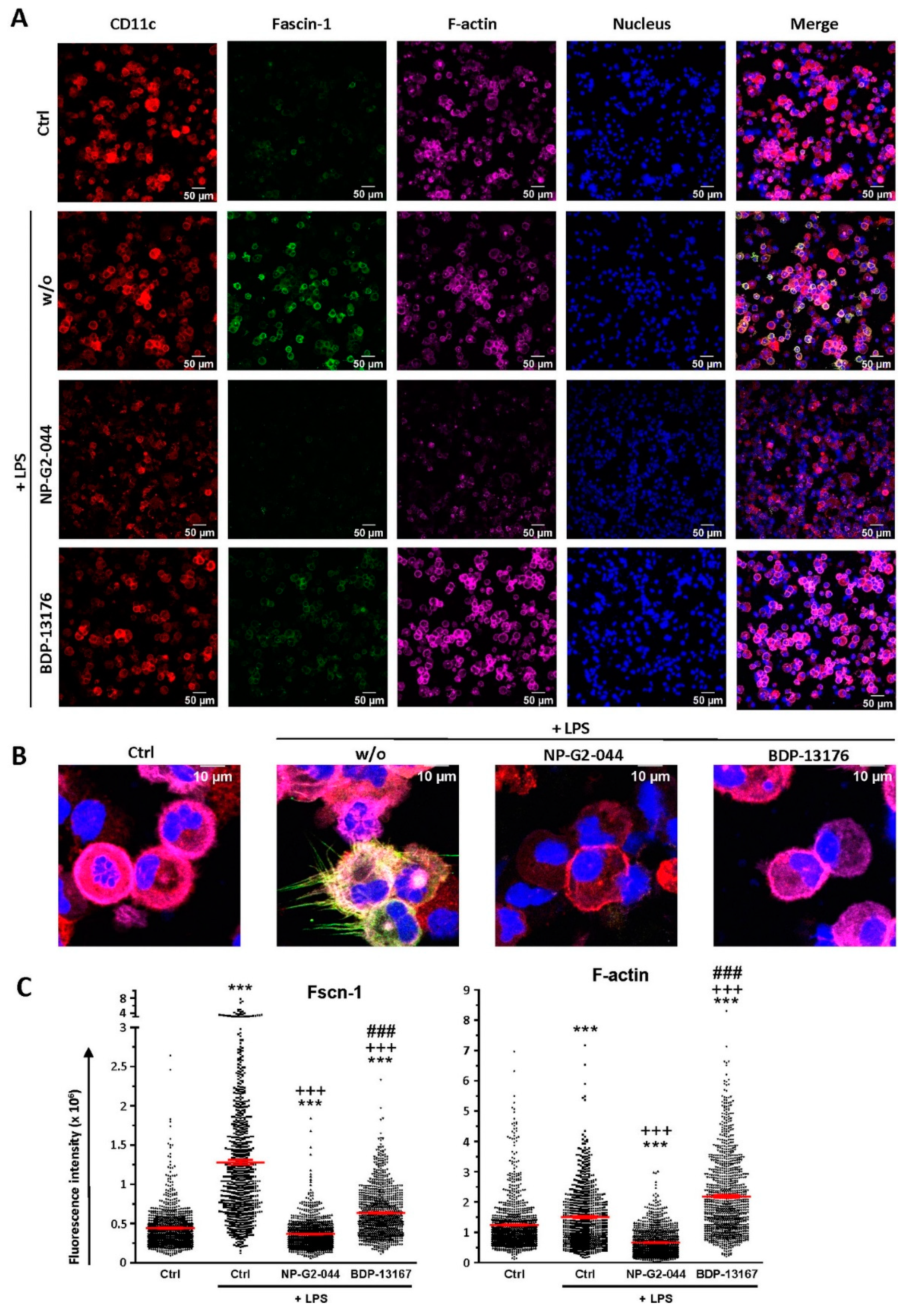
Samples were imaged on a Leica SP8 confocal microscope (Mannheim, Germany) with a 20/0.75 NA air objective with exposure from a 405 nm laser for transmission images and for Hoechst excitation (emission and detection within a spectral window of 415 nm to 530 nm), with 488 nm laser exposure for AF 488 (Fscn1) excitation (emission and detection within a spectral window of 499 nm to 581 nm), 552 nm laser exposure for phalloidin plus 555 (F-Actin) excitation (emission and detection within a spectral window of 562 nm to 632 nm) and with 638 nm laser exposure for AF 647 (CD11c) excitation (emission and detection within a spectral window of 647 nm to 795 nm). The images were acquired at a factor of at least 2.3 times less than the calculated confocal resolution at a scan rate of 400 lines/min and with 2× averaging for 581 µm × 581 µm images, with a pixel size of 0.11 µm (or 5296 × 5296 pixels). All images used in comparison were prepared and acquired under the same conditions. Control images acquired of single stained cells or of unstained cells revealed that under these detection and imaging schemes and under the aforementioned conditions for imaging cross-talk fluorescence, background fluorescence and background autofluorescence were either non-existent or below statistical significance. The images shown in the figures were smoothed with the standard Leica smoothing algorithm. In some cases, the Hoechst signal was processed by lowering the upper threshold by as much as 20% in order to create homogenous nuclear images and homogenous cell nuclear borders for cell border determination. In some cases, the CD11c signal had up to a 10% cutoff and/or a 10% threshold applied in order to create a homogeneous cell border.
Once the cell borders were created, the original fluorescence images were then used for quantification. The fluorescence ratios were calculated from the total sum of each spectral fluorescence signal intensity for the defined regions of each cell. The nuclear and cell-bounded regions were obtained with automated batch processing analysis with Imaris software version 9.3.1 (Bitplane, Zurich, Switzerland) According to the cell biology package with the nuclear boundary obtained from the edge of the Hoechst image and with the cell boundary obtained from the edge of the CD11c image and the algorithm, there can be only one nucleus per detected cell. Cells without a visible nucleus or cell boundary were rejected from the analysis.
2.5. Surface Markers
Differentially treated spleen cells and BMDCs were washed in staining buffer (PBS, 1% FBS, 0.5 mM EDTA) and were incubated with rat anti-mouse CD16/CD32 antibody (clone 2.4G2; 15 min, room temperature) to block antibody binding to Fcγ receptors. Then, samples were incubated with fluorescence-labeled antibodies (20 min, 4 °C) and washed with PBS. Afterwards, samples were incubated with FVD to discriminate live/dead cells. Samples were measured using an Attune NxT flow cytometer (Thermo Fisher, Waltham, MA, USA), and data were analyzed using Attune NxT software (Thermo Fisher, Waltham, MA, USA).
2.6. Antigen Uptake and Processing
The capacity of BMDCs to internalize and process antigens was monitored by applying 25 µg/mL OVA-AF647 (uptake) and OVA-DQ (processing), respectively, to BMDCs differentiated in 12-well plates. Samples were incubated at 37 °C for 1 h. In parallel settings, replicate samples were preincubated on ice for 30 min prior to administration of OVA derivatives, followed by incubation on ice for 1 h as a control to differentiate temperature-insensitive binding and temperature-sensitive internalization. Then, samples were harvested, and CD11c-specific antibody was applied. Samples were subjected to flow cytometric analysis in order to delineate the frequencies of BMDCs internalization and OVA processing.
2.7. Cytokines
Supernatants of spleen cells and BMDCs differentially treated overnight with Fscn1 inhibitors, as well as those of DC/T-cell cocultures, were used to measure cytokine contents by flow cytometry (Cytometric Bead Array; BD, Heidelberg, Germany), followed by analysis using FCAP ArrayTM software v.1 (BD, Heidelberg, Germany).
2.8. DC/T-Cell Interaction
BMDCs were incubated with 5 µg/mL of endotoxin-free ovalbumine (OVA; Merck, Darmstadt, Germany). After 3 h, Fscn1 inhibitors were applied at different concentrations as indicated. LPS (100 ng/mL; Merck Millipore, Burlington, MA, USA) was added 1 h later. The next day, samples were harvested and washed, and BMDCs (106/mL) were resuspended in PBS. Splenic OVA-responsive CD4+ T cells (OT-II) were immunomagnetically sorted (Miltenyi Biotec, Bergisch-Gladbach, Germany) and resuspended in PBS (106 T cells/mL). BMDC (CellTrace Violet) and T cells (carboxyfluorescein succinimidyl ester, CFSE) were labeled with the corresponding fluorescent dyes (5 µM each; both from Thermo Fisher, Waltham, MA, USA) for 30 min at 37 °C. After washing, BMDCs (2 × 105/mL) and T cells (106/mL) were resuspended in culture medium, and 150 µL of each cell suspension was applied to 8-well ibiTreat µ-slides (ibidi, Gräfeling, Germany).
BMDC/T-cell cultures were kept in an OkoLabs environmental incubator (H-301K environmental chamber, Oko Touch, Oko Pump, T-Control and CO2 control, Ottaviano, Italy) on a microscope table. DC/T-cell interaction was monitored by CLSM using a Leica TCS SP8 (Mannheim, Germany) equipped with a 20/0.75 NA objective (405 nm and 488 nm excitation, respectively, each approximately 150 µW; emission windows: 415–478 nm and 498–578 nm) and with scanning differential interference contrast transmission imaging in a 580 µm × 580 µm frame format (400 lines/s, 1.14 µm/pixel (512 × 512 pixels per frame)) and with two times averaging per line (frame acquisition of every 2 min per selected position within the chamber) over the 8 h observation period.
Image sequences were imported into Imaris version 9.3.1 (Bitplane, Zurich, Switzerland). DCs and T cells were detected automatically by fluorescence with whole-cell spot and whole-cell surface analysis (differed in control analysis by less than 1%). Whole-cell spot automated analysis was applied to all images (estimated cell diameter: 16 µm). Automated tracking was performed for all image sequences within Imaris using the autoregressive motion algorithm, with a maximum average distance of 60 µm to 300 µm per step and with zero step gap applied.
For T-cell/DC interaction analysis, a smoothed boundary around the DCs was created and expanded by the length of the average T-cell diameter. A T cell was considered to be in contact with a DC if the center of the T cell’s fluorescence was within the boundary of a DC. The average number of T cells per DC was determined for each DC and was tracked and reported as the average per image and per DC for the entire track length of each tracked DC. Cell velocity was determined by dividing each ‘x, y step’ by 2 min. Acceleration (or cell acceleration) was determined by subtracting a step speed from the previous step speed and dividing by 120 s (or 2 min). The displacement length was determined by subtracting the initial x, y position from the final x, y position to determine the difference in the vector length for each track over 8 h of acquisition. The track length added each absolute x, y vector step for an entire track over 8 h of acquisition. The mean track speed was acquired by averaging the speed of the steps for individual tracks. Results of cell tracking were verified using Fiji (plugin: TrackMate) [
34].
2.9. T-Cell Proliferation
BMDCs were incubated with OVA, and 3 h later, Fscn1 inhibitors were applied at different concentrations as indicated. In parallel assays, LPS (1 µg/mL) was added 1h later. On the next day, samples were harvested, washed and resuspended in culture medium w/o GM-CSF. BMDCs were (2 × 104/200 µL) seeded in wells (triplicates) of 96-well plates (Greiner Bio-One) and were serially titrated (1:2). Splenic OVA peptide-specific CD8+ (OT-I) and CD4+ T cells (OT-I) were immunomagnetically enriched as recommended by the manufacturer and were added (each 5 × 104 cells/100 µL) to serially diluted BMDCs. In some experiments, T cells were polyclonally stimulated by applying beads conjugated with agonistic anti-CD3 and anti-CD28 antibodies (Dynabeads Mouse T-Activator CD3/CD28; Thermo Fisher, Waltham, MA, USA) as recommended by the manufacturer. After 3–4 days of culture, 3H-thymidine (0.5 μCi/well) was added to the cocultures for 16–18 h to assess T-cell proliferation. To this end, cell lysates were transferred onto glass fiber filter mats (Harvester 96; TomTec, Hamden, CT, USA). Genomically incorporated radioactivity was monitored using a microplate β-counter (1450 MicroBeta Trilux; Perkin Elmer, Waltham, MA, USA).
2.10. Statistical Analysis
Statistical analysis was performed using GraphPad Prism software v5.0 (GraphPad Software Inc., San Diego, CA, USA). Results were expressed as mean ± standard error of the mean (SEM). Differences among groups were tested by one-way ANOVA, followed by post hoc Tukey’s test, assuming significance at p < 0.05.
4. Discussion
The actin-bundling protein Fscn1 has attracted attention as a target protein in cancer therapy due to its requirement for tumor metastasis [
15]. Accordingly, a number of pharmacological Fscn1 inhibitors have been developed, which impaired tumor cell migration in vitro by inhibiting the interaction of Fscn1 with F-actin [
37,
38] and accordingly attenuated tumor growth in preclinical mouse models [
19]. To date, one clinical phase I trial that employed the Fscn1 inhibitor NP-G2-044 has been conducted and demonstrated efficacy in the treatment of various types of advanced and metastatic treatment-refractory tumors [
22]. Consequently, a subsequent clinical trial proposal dedicated to determining the outcome of NP-G2-044 monotherapy and coapplied with a PD-(L)1-blocking antibody has been submitted (NCT05023486).
Besides de novo expression by malignant cells, Fscn1 expression is largely confined to neuronal cells, glial cells, some endothelial cells at low levels [
3] and stimulated DCs, as shown by us and others [
23,
24]. Similar to tumor cells, DCs require Fscn1 to exert migratory activity [
28] but also for their interaction with T cells [
30] in order to mount antigen-specific T-cell responses [
29]. In light of the importance of the patient immune system for the induction of antitumor responses, as well as other adaptive immune responses against pathogens, we investigated the potential effects of Fscn1 inhibitors on the DC immunophenotype and functions. We employed two distinct Fscn1 inhibitors, NP-G2-044 [
37] and BDP-13176 [
38]. Both Fscn1 inhibitors have been identified by screening of compound libraries for their activity to bind Fscn1 in order to block its F-actin cross-linking activity in a cell-free environment. However, only NP-G2-044 was further tested with regard to its inhibitory activity on tumor cell motility, and subsequently in tumor models [
19,
39]. We comparatively assessed the effects of these two structurally distinct inhibitors on DCs and T cells to delineate which effects were common and thereby most probably consequences of Fscn1 inhibition, as well as to which extent both inhibitors evoked distinct and thereby, most probably, Fscn1 inhibition-independent, inhibitor-specific, off-target effects.
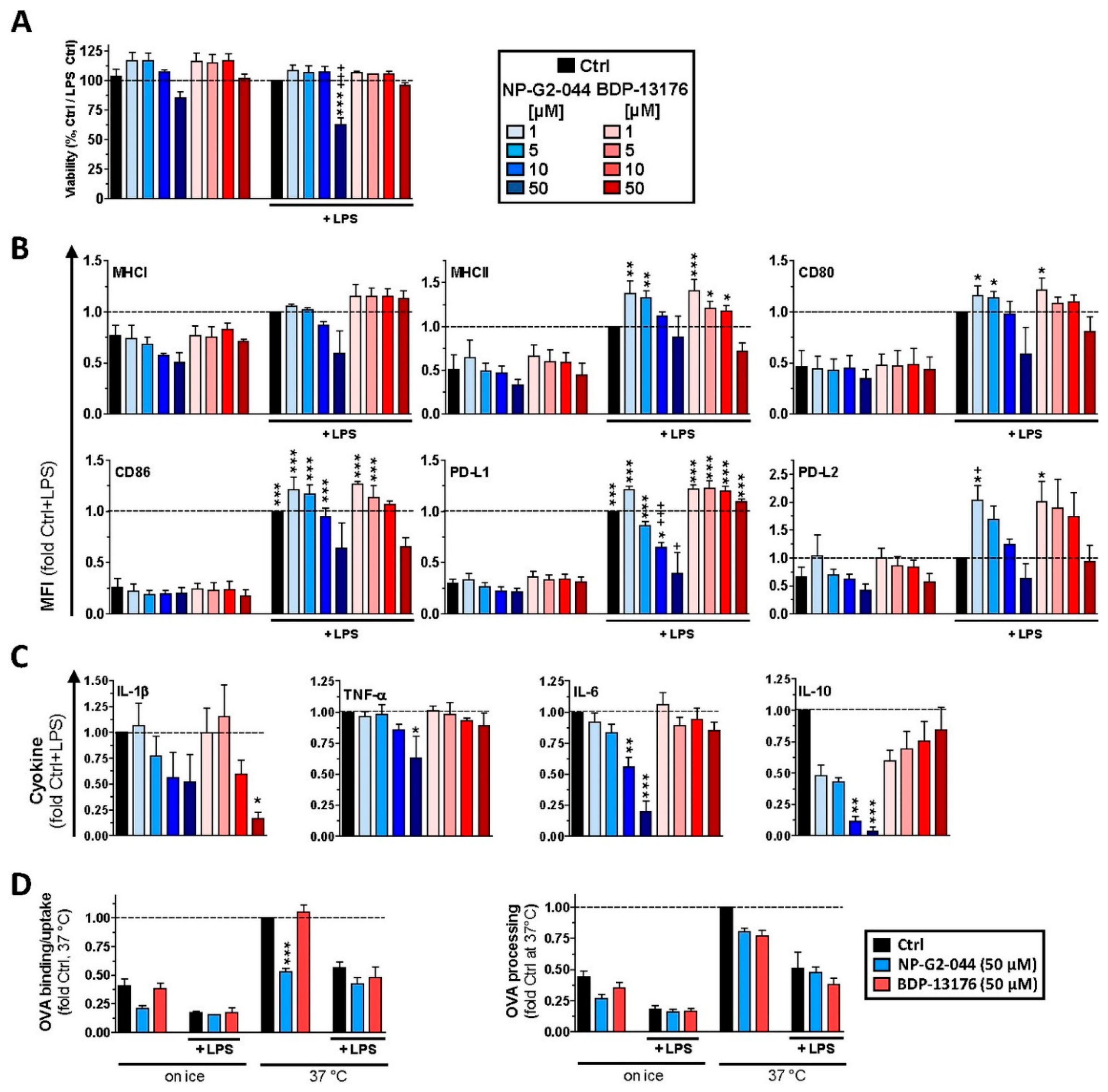
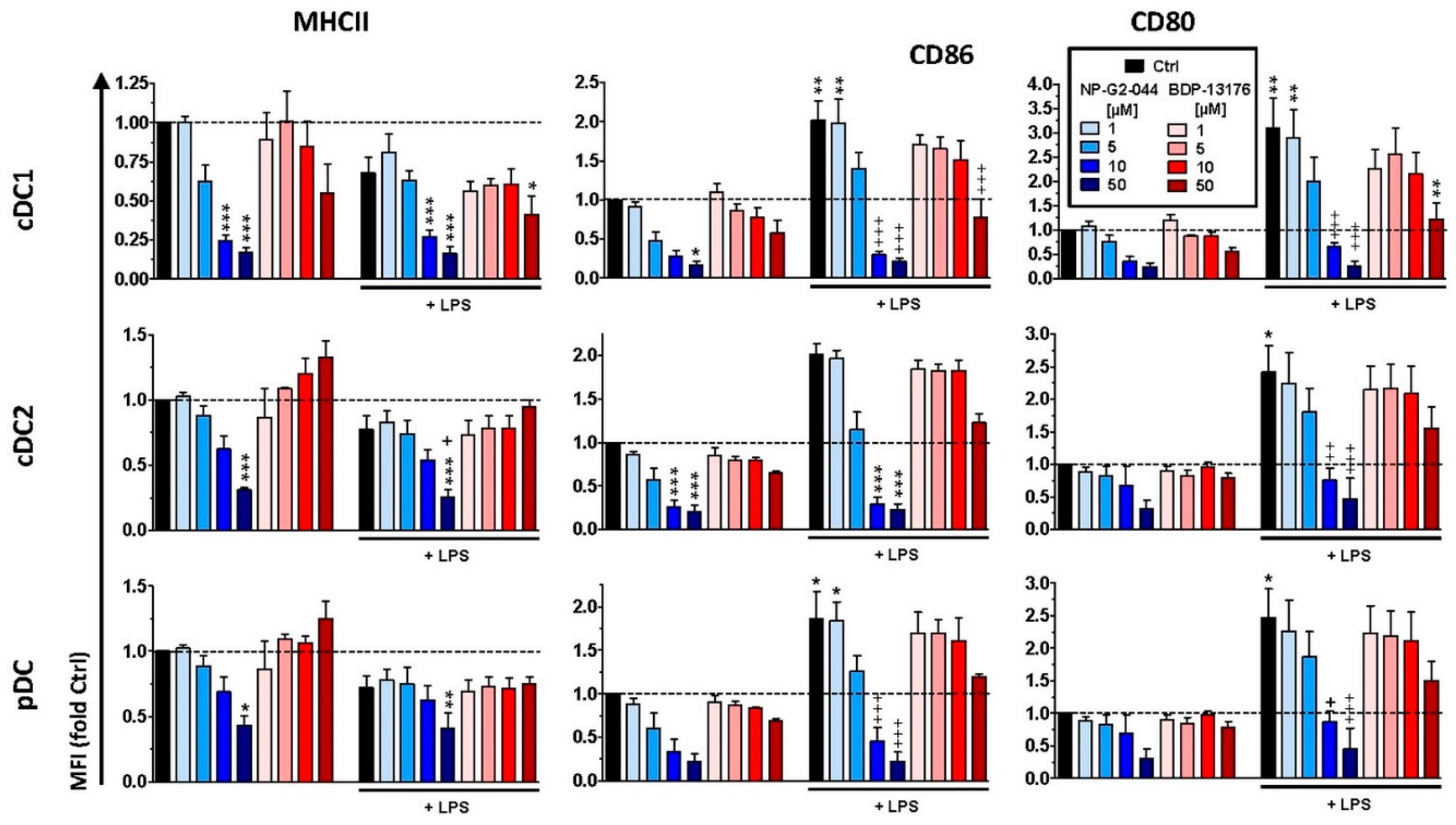
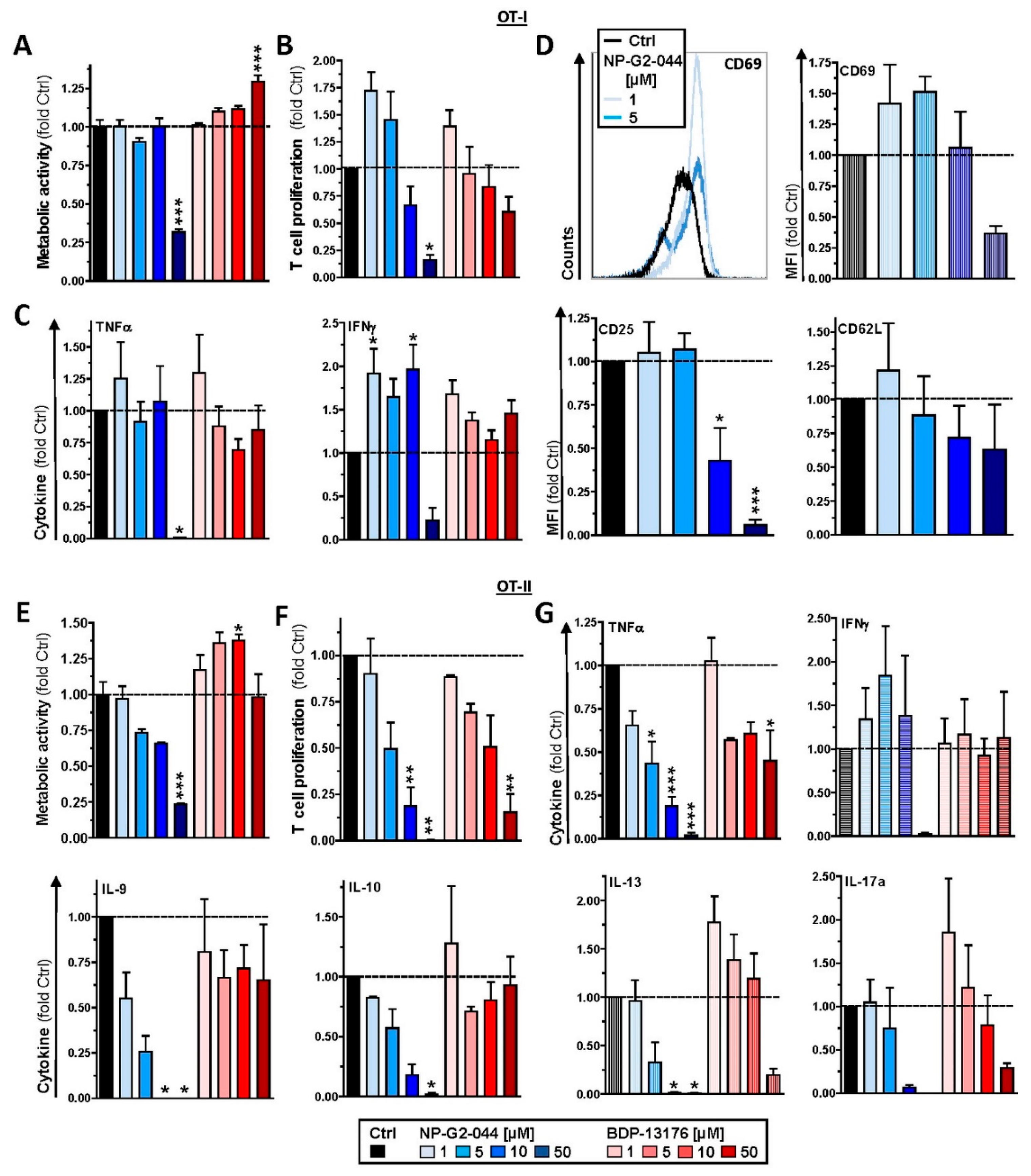
We observed strong downregulation of Fscn1 in BMDCs cotreated with either Fscn1 inhibitor in the course of stimulation, which suggests that blockade of Fscn1 interaction with F-actin promoted its turnover. We are not aware of similar findings obtained concerning tumor cells treated with Fscn1 inhibitors. Somewhat surprisingly, however, in the case of treatment with NP-G2-044, diminished Fscn1 concentrations were accompanied by an overall decrease in F-actin, whereas in the case of BDP-13176 treatment, F-actin levels considerably increased. To the best of our knowledge, major effects of Fscn1 inhibition or knockdown on F-actin contents have not been described to date.
Fscn1 protein knockdown in stimulated DCs as conferred by either Fscn1 inhibitor was associated with the absence of the formation of dendritic protrusions, irrespective of F-actin contents. This observation is in agreement with the results of our previous studies, which demonstrated that Fscn1-specific inhibitory oligonucleotides inhibited the formation of dendritic processes [
23,
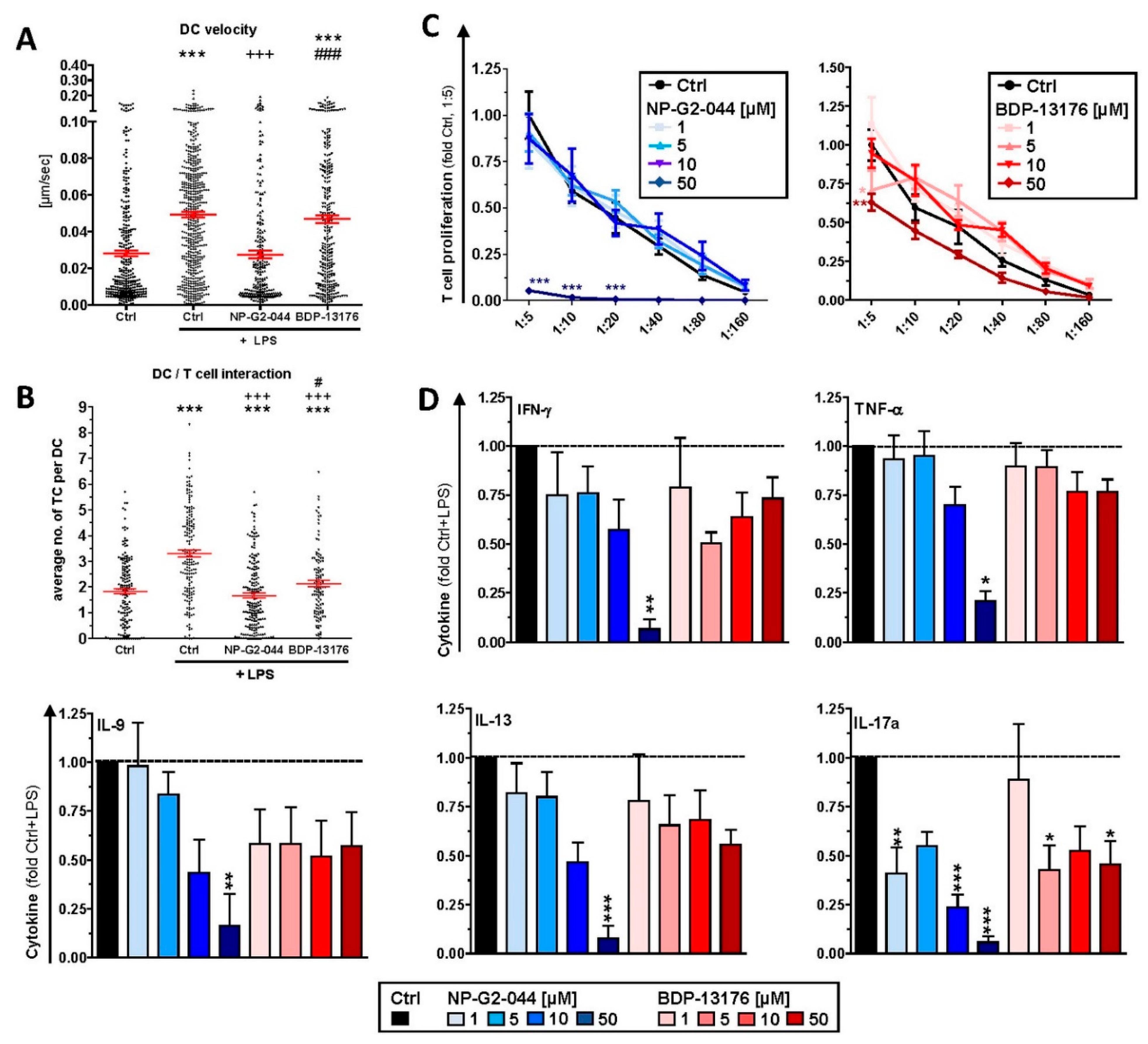
24]. Our observation of attenuated motility of stimulated DCs pretreated with either Fscn1 inhibitor in terms of velocity in cocultures with antigen-specific CD4
+ T cells supports the role of Fscn1 in the migratory activity of DCs [
28,
39].
In these DC/T-cell coculture experiments, we also observed reduced interaction of CD4
+ T cells with DCs stimulated in the presence of Fscn1 inhibitors. In line with these observations, such DCs exerted less proliferation of CD8
+ and CD4
+ T cells. We previously showed that Fscn1 accumulated within the IS between DCs and CD4
+ T cells [
30]. Akbar and colleagues reported on attenuated T-cell stimulatory activity of DCs pretreated with Fscn1 siRNA [
29]. We and others have demonstrated that dynamic reorganization of F-actin within the IS on the DC side upon contact with a T cell is necessary for proper T-cell stimulation [
30,
40]. Therefore, our results confirm that functional impairment of Fscn1 interferes with DC-mediated T-cell activation. However, we also showed that both Fscn1 inhibitors inhibited the expression of MHCII and costimulators (CD80 and CD86) in bone-marrow-derived DCs used for most subsequent experiments and in primary splenic DC subpopulations (pDC and cDC1/2). Interestingly, at low to intermediate concentrations, both inhibitors promoted expression of PD-L2, which exerts coinhibitory effects by triggering PD-1 in T cells [
41]. Therefore, we cannot rule out that, besides disturbed reorganization of F-actin, impaired expression of MHCII and costimulators may also contribute to the attenuated T cell stimulatory activity of corresponding pretreated DCs. Our observation of impaired expression of DC activation markers is in contrast to the results of a previous study showing that knockdown of Fscn1 in BMDCs by shRNA exerted no effect on the expression of MHCII and costimulators but was sufficient to attenuate T-cell stimulation [
29]. Therefore, further studies are necessary to elucidate whether Fscn1 is implicated in expressional control of either gene or whether both inhibitors inhibit MHCII and costimulatory expression in DC via off-target effects. In agreement with the observation that Fscn1 inhibitors interfered with the acquisition of a mature phenotype of stimulated DC and their impaired interaction with antigen-specific T cells, they exerted less T-cell stimulatory activity.
Altogether, our results suggest that in vivo treatment with Fscn1 inhibitors for tumor therapy could affect the DC immunophenotype and DC/T-cell interactions. This issue has been addressed most recently in a study by Wang and colleagues [
39]. Treatment of tumor-burdened mice with NP-G2-044 increased cDC1 and cDC2 frequencies within the tumor microenvironment (TME). As delineated for cDC1, this effect was strongly elevated upon cotreatment with a PD-1-blocking antibody. Under the latter condition, total DCs retrieved from the TME also displayed stronger expression of costimulatory receptors (CD40, CD80 and CD86) as compared to tumors derived from untreated mice and in response to a single treatment. Furthermore, Wang and colleagues demonstrated an inhibitory effect of NP-G2-044 on the migratory capacity of cDC1-like cells in vitro (MuTu cell line [
42]. Therefore, the authors concluded that Fscn1 inhibition resulted in a migratory arrest of mature DCs in the TME.
Cotreatment of tumor-burdened mice with NP-G2-044 and a PD-1-blocking antibody resulted in slower tumor growth and longer overall survival [
39]. The corresponding TME contained higher frequencies of CD4
+ and CD8
+ T cells, and a higher frequency of the latter displayed an effector phenotype as compared to mice that received a single treatment with either antitumor agent.
Based on these results the authors hypothesized that mature DCs trapped within the TME as consequence of NP-G2-044 treatment may stimulate tumor antigen-specific T cells in local tertiary lymphoid structures (TLS), resulting in antitumor T-cell responses.
Some of our results are in contrast to these conclusions. First, we consider it conceivable that systemic application of the Fscn1 inhibitor (applied by gavage) could inhibit the motility of stimulated DCs throughout the body. In light of the limited life span of stimulated DCs [
43], it is therefore conclusive that effective systemic Fscn1 inhibition would counteract DC accumulation within the TME. Secondly, T-cell activation requires presentation of internalized and processed antigens. Wang and colleagues demonstrated that DC-like MuTu cells treated with NP-G2-044 (3–10 µM) displayed an elevated increase in the uptake of the model antigen bovine serum albumin, as well as of dextran [
39]. In contrast, we observed an inhibitory effect of this Fscn1 inhibitor (but not of BDP-13176) on the internalization of OVA by DC. However, due to the very low expression level of Fscn1 by unstimulated DCs, we cannot rule out that the results obtained for unstimulated DCs treated with NP-G2-044 is the consequence of an Fscn1-independent off-target effect. Likewise, it is not clear at to which extent unstimulated MuTu cells express Fscn1. Thirdly, our findings of attenuated interaction of Fscn1 inhibitor-pretreated DCs with T cells and the impaired T-cell stimulatory capacity of the former, both in the case of DC pretreatment, as well as when applied to DC/T-cell cocultures, do not support the hypothesis of enhanced T-cell stimulation by mature DCs trapped in the TME. Here, we also observed that levels of Tc1/Th1-associated cytokines (IFN-γ, TNF-γ), which are beneficial for antitumor immune responses [
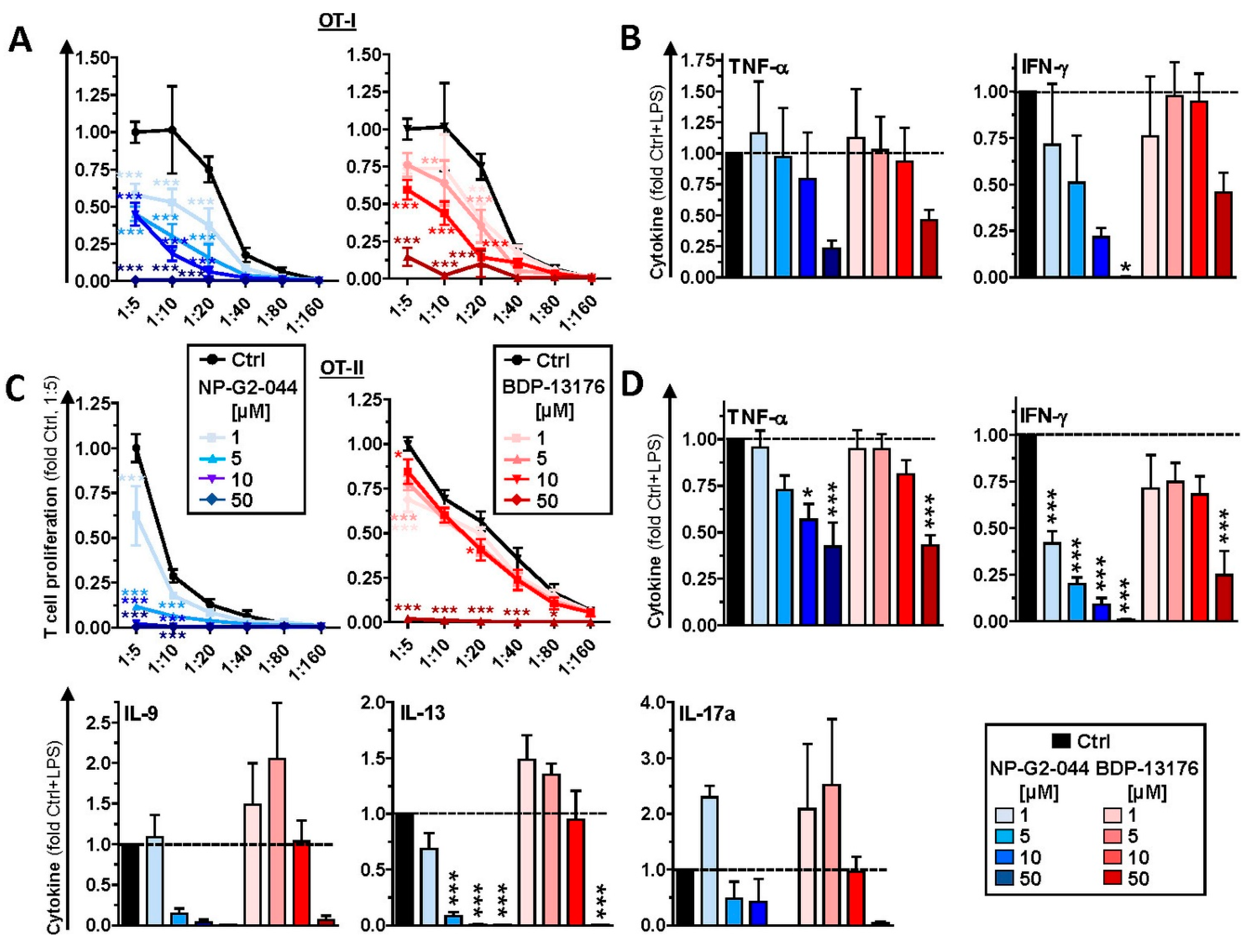
44], were generally attenuated in a largely Fscn1 inhibitor dose-dependent manner in corresponding DC/T-cell cocultures.
Altogether, these issues raise the question of whether NP-G2-044 may confer antitumor activity in vivo by alternative mechanisms. In this regard, it is noteworthy that within the TME, besides DCs tumor-associated macrophages (TAMs) also expressed Fscn1 at a high level [
39]. In light of the important role of TAMs in conferring tumor progression, e.g., by neoangiogenesis, and in the release of anti-inflammatory mediators, such as IL-10, to promote tumor immune evasion [
45], it is possible that inhibition of Fscn1 in TAMs may contribute to inhibition of tumor growth. Additional studies should elucidate the functional role of Fscn1 for TAMs and the consequences of its inhibition for these immunoregulatory cells.
Furthermore, the finding of elevated numbers of activated T cells within the TME of NP-G2-044-treated mice [
39] may also be explained by our observation that NP-G2-044 but not BDP-13176 acted as a coactivator of polyclonally stimulated CD8
+ T cells with regard to IFN-γ production. This result shows that NP-G2-044 may exert pronounced off-target effects on Fscn1-deficient (immune) cells via yet unknown molecular mechanisms.
Along this line, Wang and colleagues reported that besides DCs, other types of innate immune cells (neutrophils, monocytes and NK cells), which do not express Fscn1, were significantly enriched in the TME of NP-G2-044 treated mice. It remains possible that these cell populations may contribute to the observed pronounced antitumor effects.
As outlined above, the finding of tumoricidal activity of NP-G2-044, especially in combination with blockade of the PD-1/PD-L1 axis, could also be due to off-target effects of this Fscn1 inhibitor on non-DCs. It remains possible that each Fscn1 inhibitor may evoke distinct off-target effects, as evidenced by the inhibitory versus stimulatory effect of NP-G2-044 and BDP-13176 on F-actin levels, respectively, as well as the coactivating potential of NP-G2-044 in CD8
+ T cells. Furthermore, we also noted that treatment of DC/T-cell cocultures with BDP-13176 but not NP-G2-044 at low to intermediate concentrations favored production Th2- (IL-13) and Th9- (IL-9) [
46] associated cytokines, whereas NP-G2-044 at the lowest concentration upregulated Th17-associated IL-17 [
47] production.
Altogether, our results confirm the necessity of performing comparative, in-depth in vivo studies employing distinct Fscn1 inhibitors and subsequent ex vivo analysis to delineate, in detail, by which mechanisms these may inhibit tumor growth. These mechanisms comprise inhibition of Fscn1-dependent tumor cell migration [
19,
20,
21,
48] and Fscn1-dependent gene expression in tumor cells [
18,
49,
50,
51], which may impact the characteristics of the TME, e.g., via soluble mediators. However, as shown in this study, Fscn1 inhibitors may also affect the immunophenotype and function of DCs and exert pronounced off-target effects on immune cells in an inhibitor-specific manner, as shown here for DCs and T cells.